Abstract
Background
Randomized controlled trials are the gold standard for evaluating therapy; however, controversy exists regarding the applicability of such results to daily practice, as patients are often pre-selected and may not reflect real-world clinical settings. We studied the eligibility criteria for 3102 “real-life” patients with stable coronary artery disease (SCAD) according to the ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) trial exclusion criteria. The aim of our analysis was to estimate the percentage of real-life patients who would have met the exclusion criteria for the ISCHEMIA trial.
Methods
We analyzed 3102 patients with SCAD referred to the Silesian Center for Heart Disease who underwent both coronary angiography and stent implantation between January 2006 and December 2011. The patients were divided into two groups. Group A was composed of patients with SCAD who would have been excluded from the ongoing ISCHEMIA trial, whereas group B represented the remaining patients.
Results
A total of 1900 (61.3 %) patients met at least one of the exclusion criteria. The most frequent exclusion criterion noted was revascularization within the previous 12 months (938 patients; 49.4 %), followed by unacceptable level of angina symptoms (532 patients; 28 %), low ejection fraction (467 patients; 24.6 %), and acute coronary syndrome within the previous 2 months (456 patients; 24 %). Patients from our cohort who would have been excluded from the ISCHEMIA trial were older, had more comorbidities, and experienced worse long-term outcomes.
Conclusions
The ISCHEMIA trial exclusion criteria ruled out the majority of the patients with SCAD undergoing percutaneous coronary intervention in “real life”. Our cohort of patients who would have been excluded from the ISCHEMIA trial had more comorbidities and experienced significantly worse long-term outcomes than patients who did not meet the ISCHEMIA trial exclusion criteria.
Trial registration
ClinicalTrials.gov NCT01471522.
Electronic supplementary material
The online version of this article (doi:10.1186/s13063-015-0934-4) contains supplementary material, which is available to authorized users.
Keywords: ISCHEMIA trial, Revascularization versus optimal medical treatment, Stable coronary artery disease
Background
Stable coronary artery disease (SCAD) remains one of the most common indications for referral to a cardiac catheterization laboratory [1]. Primary treatment goals in patients with SCAD include the prevention of acute coronary syndrome and the relief of ischemia. Percutaneous coronary intervention and coronary artery bypass grafting are established methods of improving cardiac symptoms [2, 3].
The benefits of revascularization remain unclear. The COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial demonstrated no difference in mortality between patients with SCAD who were treated invasively and those who treated using optimal medical therapy [4]. Although the COURAGE trial was composed of a wider spectrum of patients than previous studies [5–11], there were concerns regarding its design, related to potential selection biases [12]. A meta-analysis by Boden et al. [4] demonstrated that, in patients with SCAD, percutaneous coronary intervention did not offer any benefit in terms of mortality, incidence of myocardial infarction or need for subsequent revascularization over optimal medical therapy; however, a more recent meta-analysis by Windecker et al. [13] provided evidence regarding improved survival with the use of new-generation drug-eluting stents as opposed to balloon angioplasty, bare metal stents or early-generation drug-eluting stents.
Numerous trials have compared optimal medical therapy with revascularization for periods of up to 30 days [14–17], but all of them included cohorts selected via randomization. Therefore, the results of those studies may not be representative for the entire population of patients undergoing percutaneous coronary intervention in real life, particularly among subgroups of patients with a high baseline cardiovascular risk who are excluded from most randomized trials [17]. In a study that included low risk patients with SCAD, the use of an invasive strategy worsened the prognosis of myocardial infarction, stroke and cardiovascular death, as did the use of repetitive revascularization [18] and other techniques, suggesting modest benefits [19–21]. Therefore, selection bias and risk burden are crucial in establishing the suitability of invasive revascularization in a broad spectrum of patients with SCAD.
The purpose of the ongoing ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) trial is to determine the best management strategy for high-risk patients with stable ischemic heart disease and proven ischemia, using different diagnostic modalities. The primary aim of the ISCHEMIA trial is to test the hypothesis that the use of an invasive strategy, followed by revascularization plus optimal medical therapy, in patients with either moderate or severe ischemia inducible on stress imaging, is superior to a conservative strategy (optimal medical therapy only) [22].
In this analysis, we studied the eligibility criteria of 3102 consecutive patients with SCAD who underwent stent implantation, according to the exclusion criteria of the ISCHEMIA trial, to determine what percentage of real-world patients would be excluded from the ISCHEMIA trial. In addition, we characterized both the risk profiles and the long-term outcomes of patients who did not fulfill the exclusion criteria of the ISCHEMIA trial.
Methods
We analyzed a cohort of 3502 patients with SCAD who were referred to the Silesian Center for Heart Disease (Zabrze, Poland) and underwent both coronary angiography and stent implantation between January 2006 and December 2011.
We screened all patients who underwent coronary angiography but were discharged with diagnosis other than SCAD (ICD10 I25.0 or I25.2) [23]. The screening was performed to identify patients admitted because of angina symptoms but discharged with another diagnosis (for example, cardiogenic shock) owing to in-hospital complications. Data regarding patients’ clinical and demographic characteristics, as well as their symptoms on admission, were taken from an electronic database containing data from structured medical charts. This database has been used to store information regarding patients’ medical histories at our institution since 2006. Patients’ echocardiography, angiography and laboratory test results were collected from the medical history database. All patients admitted to our center signed consent forms for data collection and processing and provided phone contact as part of our admission procedure. Patients who did not consent to phone contact were excluded from this analysis. All patients fulfilled the ISCHEMIA inclusion age criterion. The youngest analyzed patient was 29 years old. Out of a group of 3502 patients, 400 patients were excluded from further analysis due to incomplete data. A further study was conducted on a group of 3102 patients with complete clinical data. The study was approved by the ethics committee at the regional medical chamber (Ethics Committee of Silesian Medical Chamber, Katowice; Resolution number 34/2011 from 21 November 2011).
Follow-up data
Information regarding survival was based on patients’ Polish National Health Fund insurance status, which may be verified electronically, as this national health insurance is mandatory for all Polish citizens; patients who were insured were considered to be alive. We attempted to contact the relatives of any uninsured patients, as well as the relevant local registry office, to obtain patients’ dates of death. Complete follow-up data were available for 3086 (99.5 %) patients. The median follow-up duration was 3.5 years. During the observation period, 366 deaths were reported.
ISCHEMIA trial exclusion criteria
We analyzed the exclusion criteria using an ISCHEMIA study protocol available online. The exclusion criteria that we applied to the cohort are given in Fig. 1. Regarding the ISCHEMIA trial protocol, we did not note any restrictions pertaining to patients who underwent a previous valve replacement procedure. Therefore, we considered an implanted heart valve to be an exclusion criterion. The patients were divided into two groups. Group A was composed of patients with SCAD who would have been excluded from the ongoing ISCHEMIA trial, whereas group B represented the remaining patients who did not meet the ISCHEMIA exclusion criteria.
Fig. 1.
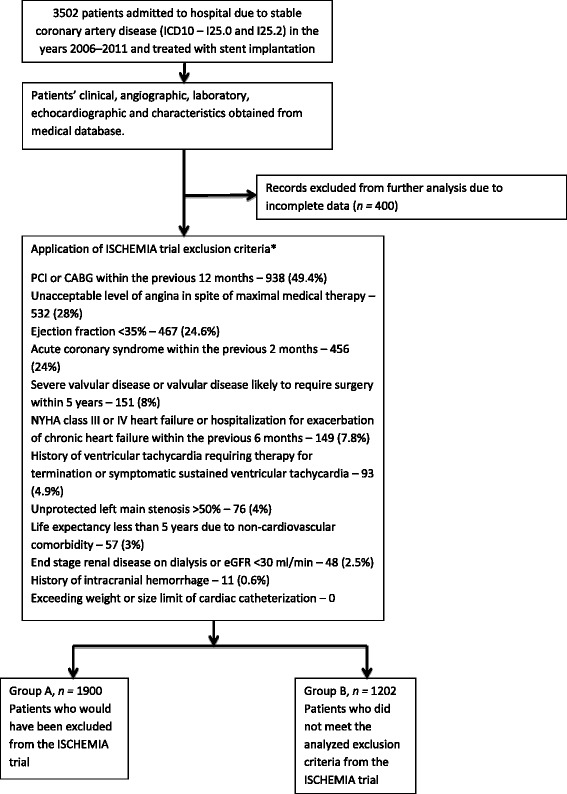
Scheme of the study. Owing to retrospective type of analysis or insufficient data, the following criteria were not applicable: finding of non-obstructive coronary artery disease, unsuitable coronary anatomy, pregnancy, patients with an estimated glomerular filtration rate of 30–59 who were likely to have significant unprotected left main stenosis, inability to comply with the ISCHEMIA protocol, very dissatisfied with medical treatment, ischemic stroke within 6-months, history of non-compliance
Statistical analysis
Continuous variables are presented as medians and interquartile ranges. Categorical variables are presented as percentages. Continuous variables were compared using either the t test or the Mann–Whitney U test, where appropriate. Long-term prognoses were analyzed in both groups using the Kaplan–Meier method, with log-rank testing. All reported values of P are two-sided. The analyses were performed using Statistica, version 7.1 (StatSoft, Inc., Tulsa, OK, USA), and Number Crunching Statistical Systems, version 8 (NCSS; Kaysville, UT, USA).
Results
A total of 1900 (61.3 %) patients met at least one of the exclusion criteria pertaining to the ISCHEMIA trial. The most frequent exclusion criterion noted was revascularization within the previous 12 months (938 patients; 49.4 %), followed by unacceptable level of angina symptoms (532 patients; 28 %), low ejection fraction (467 patients; 24.6 %) and acute coronary syndrome within the previous 2 months (456 patients; 24 %) (Fig. 1). A total of 802 (44.2 %) patients fulfilled more than one exclusion criterion. The basic clinical characteristics of our cohort and the patients who met the exclusion criteria (group A), as well as those of the remaining patients (group B), are listed in Table 1. The patients who would have been excluded from the ISCHEMIA study (group A) were older and had more comorbidities, including myocardial infarction, previous revascularization, diabetes, hypertension, heart failure, and lower ejection fraction. These patients exhibited lower hemoglobin levels (median 8.5 mmol versus 8.8 mmol, P < 0.001) and hematocrits (median 41 % versus 42 %, p < 0.001), as well as lower estimated glomerular filtration rates (median 84.0 ml/min/ 1.73 m2) versus 85.2 ml/min/ 1.73 m2), P < 0.001) and higher creatinine levels (median 80.1 mmol/l vs. 77.7 mmol/l, P < 0.001) (Table 2). Group A also exhibited a higher incidence of multivessel disease (median 22.1 % versus 18.1 %, P = 0.01) (Table 3). Additionally, the patients who met at least one of the exclusion criteria pertaining to the ISCHEMIA trial had worse long-term prognosis. During the follow-up period, 79 (6.6 %) patients in group B and 287 (15.1 %) patients in group A, respectively, died. The mortality for the whole analyzed group was 11.8 % (366 patients). Figure 2 shows the Kaplan–Meier survival curves for cohorts who met the exclusion criteria for the ISCHEMIA trial (group A), as well as the data pertaining to the patients who would have been eligible for the ISCHEMIA trial (group B). Multivariate analysis of independent predictors of mortality in the analyzed group was presented in additiona material (Additional file 1).
Table 1.
Baseline clinical characteristics, expressed as percentages or as medians and interquartile ranges
| Variable | All patients | Group A | Group B | P | |
|---|---|---|---|---|---|
| (Patients who met ISCHEMIA exclusion criteria) | (Patients who did not meet ISCHEMIA exclusion criteria) | (A vs. B) | |||
| n = 3102 | n = 1900 | n = 1202 | |||
| Median age, years | 64 (57–71) | 64 (57–72) | 64 (57–70) | 0.11 | |
| Men | 2187 (70.5) | 1354 (71.3) | 833 (69.3) | 0.26 | |
| Previous myocardial infarction | 1786 (57.6) | 1335 (70.3) | 451 (37.5) | <0.001 | |
| Previous percutaneous coronary intervention | 1451 (46.9) | 1166 (61.4) | 285 (23.7) | <0.001 | |
| Previous coronary artery bypass graft | 407 (13.1) | 253 (13.3) | 154 (12.8) | 0.7 | |
| Diabetes | 1118 (36.1) | 785 (41.3) | 333 (27.8) | <0.001 | |
| Diabetes treatment | Diet only | 238 (7.7) | 1176 (9.3) | 62 (5.2) | <0.001 |
| Insulin | 433 (14.0) | 284 (14.9) | 149 (12.4) | ||
| Oral drugs | 447 (14.4) | 325 (17.1) | 670 (55.7) | ||
| Hypertension | 2217 (71.5) | 1301 (68.5) | 916 (76.2) | <0.001 | |
| Hypercholesterolemia | 1752 (56.5) | 1082 (57) | 670 (55.7) | 0.53 | |
| Current smoker | 1140 (36.9) | 719 (37.8) | 421 (35) | 0.09 | |
| Past smoker | 331 (10.7) | 187 (9.8) | 144 (12) | 0.09 | |
| Obesity | 1035 (33.4) | 626 (33) | 409 (34) | 0.56 | |
| Family history of premature myocardial infarction (<55 years) | 256 (9.3) | 155 (8.3) | 131 (10.9) | 0.01 | |
| Systolic blood pressure (mmHg) | 130 (120–140) | 130 (120–140) | 130 (120–140) | <0.001 | |
| Diastolic blood pressure (mmHg) | 80 (70–85) | 80 (70–85) | 80 (70–85) | 0.03 | |
| Heart rate (min−1) | 70 (63–76) | 70 (63–77) | 70 (64–76) | 0.32 | |
| Heart failure | 568 (18.3) | 496 (26.1) | 72 (6) | <0.001 | |
| Ejection fraction (%) | 48 (40–55) | 45 (35–50) | 50 (46–55) | <0.001 | |
| Mitral valve regurgitation, severe | 75 (2.5) | 75 (4.1) | 0 | <0.001 | |
| Mitral valve stenosis, severe | 4 (0.1) | 4 (0.2) | 0 | ||
| Aortic stenosis, severe | 24 (0.8) | 24 (1.3) | 0 | ||
| Aortic regurgitation, severe | 7 (0.2) | 7 (0.4) | 0 | <0.001 | |
| Aortic valve insufficiency, combined | 5 (0.2) | 5 (0.3) | 0 | ||
| Bicuspid aortic valve | 6 (0.2) | 6 (0.3) | 0 | ||
| Tricuspid regurgitation, severe | 26 (0.9) | 26 (1.4) | 0 | <0.001 | |
Table 2.
Laboratory data, expressed as medians and interquartile ranges
| Variable | All patients | Group A | Group B | P |
|---|---|---|---|---|
| (Patients who met ISCHEMIA exclusion criteria) | (Patients who did not meet ISCHEMIA exclusion criteria) | (A vs. B) | ||
| n = 3102 | n = 1900 | n = 1202 | ||
| Red blood cell count (106/µL) | 4.5 (4.2–4.8) | 4.4 (4.1–4.8) | 4.6 (4.3–4.9) | <0.001 |
| Hemoglobin (mmol/l) | 8.6 (8–9.2) | 8.5 (7.8–9) | 8.8 (8.2–9.4) | <0.001 |
| Hematocrit (%) | 41 (38–45) | 41 (38–45) | 42 (39–45) | <0.001 |
| White blood cells (103/mm3) | 7.2 (6.1–8.4) | 7.1 (6–8.4) | 7.2 (6.1–8.5) | 0.49 |
| Platelets (103/mm3) | 200 (163–240) | 199 (163–241) | 200 (164–235) | 0.68 |
| Creatinine (mmol/l) | 79.2 (67.2–93.5) | 80.1 (68.5–96) | 77.7 (66–90) | <0.001 |
| Estimated glomerular filtration rate (ml/min 1.73 m2)) | 83.3 (67.8–99.7) | 82 (62.6–99.3) | 85.2 (68.3–100.1) | <0.001 |
Table 3.
Angiographic characteristics and procedural complications, expressed as percentages or as median and interquartile ranges
| Variable | All patients | Group A | Group B | P | |
|---|---|---|---|---|---|
| (Patients who met ISCHEMIA exclusion criteria) | (Patients who did not meet ISCHEMIA exclusion criteria) | (A vs. B) | |||
| n = 3102 | n = 1900 | n = 1202 | |||
| Angiographic characteristics | |||||
| Multivessel disease | 636 (20.5) | 419 (22.1) | 217 (18.1) | 0.01 | |
| Significant stenosis; left main coronary artery | 157 (5.1) | 129 (6.8) | 28 (2.3) | <0.001 | |
| Significant stenosis – left anterior descending or diagonal | 1781 (57.4) | 1092 (57.5) | 689 (57.3) | 0.94 | |
| Significant stenosis – circumflex or obtuse marginal | 1620 (52.2) | 579 (48.2) | 1041 (54.8) | <0.001 | |
| Significant stenosis – right coronary artery | 1746 (56.3) | 1045 (55) | 701 (58.3) | 0.07 | |
| Number of vessels, percutaneous coronary intervention | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0.005 | |
| Number of stents | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.005 | |
| Implanted stent | bare metal stent | 1785 (57.5) | 1087 (57.2) | 698 (58.1) | |
| drug-eluting stent | 1211 (39) | 743 (39.1) | 468 (38.9) | 0.57 | |
| bare metal stent and drug-eluting stent | 106 (3.5) | 70 (3.7) | 36 (3) | ||
| Complications | |||||
| Myocardial infarction | 40 (1.3) | 27 (1.4) | 13 (1.1) | 0.51 | |
| Stroke or transient ischemia attack | 10 (0.3) | 9 (0.5) | 1 (0.1) | 0.1 | |
| Bleeding | 52 (1.7) | 29 (1.5) | 23 (1.9) | 0.47 | |
| Blood transfusion | 30 (1.0) | 22 (1.2) | 8 (0.7) | 0.19 | |
| Dissection | 159 (5.1) | 108 (5.7) | 51 (4.2) | 0.08 | |
| Repeat of percutaneous transluminal coronary angioplasty | 9 (0.3) | 5 (0.3) | 4 (0.3) | 0.74 | |
| Urgent coronary artery bypass graft | 2 (0.1) | 1 (0.1) | 1 (0.1) | 1.0 | |
| Sudden cardiac arrest | 30 (1) | 16 (0.8) | 14 (1.2) | 0.45 | |
| Mortality | 366 (11.9) | 287 (15.1) | 79 (6.6) | <0.001 | |
Fig. 2.
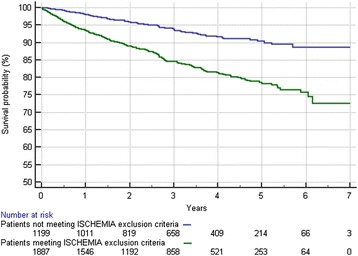
Kaplan–Meier survival plots of patients meeting the exclusion criteria for the ISCHEMIA trial (group A) and patients who would have been eligible for the ISCHEMIA trial (group B)
Discussion
In patients with SCAD, invasive treatment has been shown to improve coronary symptoms compared with optimal medical therapy alone; however, revascularization does not offer any benefits in terms of death, myocardial infarction or the need for subsequent revascularization [2–5].
Randomized controlled trials are the gold standard for evaluating therapeutic efficacy; however, there is controversy regarding the applicability of such findings to daily practice. For example, it has been reported that as many as 50 % of patients with myocardial infarction in the real world might not be represented in randomized clinical trials [24].
Both selection bias and risk burden are crucial in determining the utility of revascularization in a heterogeneous group of patients with symptomatic SCAD. We aimed to answer the question regarding how much of our population with SCAD who underwent stent implantation would have been excluded from the ISCHEMIA trial.
Based on our analysis, it should be noted that the results of the ISCHEMIA trial may not be extrapolated to a wide spectrum of patients, including subgroups of patients with moderately impaired left ventricular function, heart failure, previous myocardial infarction and revascularization, or severe angina symptoms. We want to stress the importance of registries as valuable data sources. Registries include data from all-comers, or the real-world population; therefore, an assessment of the potential benefits of different treatment modalities on a wider spectrum of patients is possible. However, there is no better way to establish proper management strategies for high-risk patients than randomized controlled trials.
Out of 938 patients excluded owing to prior revascularization within 12 months, 789 underwent percutaneous coronary intervention as part of a staged revascularization after myocardial infarction. The second part of revascularization is performed after the acute phase of myocardial infarction and symptoms are consistent with symptoms reported by patients suffering from SCAD only; percutaneous coronary intervention is performed only if the lesion is significant. Therefore, we are convinced that analysis of this subgroup is essential.
Study limitations
It is important to note that the results of this study represent only a single-center experience. Despite the fact that a structured medical interview regarding patients’ detailed medical histories and symptoms on admission has been mandatory for an attending physicians since 2006, this was a retrospective observational study with several intrinsic limitations. Moreover, as we are a cardiology referral center in Poland, a larger proportion of high-risk patients may have been cared for at our center than in other centers. However, the application of the current guidelines and treatment methods is also higher than average in Poland.
Conclusions
Our single-center comprehensive analysis has raised concerns regarding the fact that the ISCHEMIA trial does not represent a real-world heterogeneous group of patients with SCAD. The majority of our patients who met exclusion criteria as defined in the ISCHEMIA trial had undergone prior percutaneous coronary intervention or coronary artery bypass graft within the previous 12 months, or had a low ejection fraction, and therefore had a worse long-term prognosis. New randomized trials may be necessary, to determine the benefits of revascularization among high-risk patients with SCAD.
Acknowledgements
We would like to thank Mr. Andrzej Szafranek for help in managing the patients’ electronic history database. We would like to thank Małgorzata Gonera, Marcin Gawlita, Roch Pakuła and Paweł Przybyło for entering data regarding clinical and angiographic characteristics into electronic database.
Abbreviations
- COURAGE
Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation
- ISCHEMIA
International Study of Comparative Health Effectiveness with Medical and Invasive Approaches
- SCAD
stable coronary artery disease
Additional file
Multivariate analysis of independent predictors of mortality in the analyzed group. To identify predictors of long-term outcome, Cox regression models were utilized to evaluate the association between clinical laboratory electrocardiographic and angiographic variables and mortality. The stepwise selection of model building was used, with P = 0.1 for a confounder to stay in the model. (PDF 276 kb)
Footnotes
Competing interests
Professor Lech Poloński is one of the investigators in the ISCHEMIA trial, Dr. Jarosław Wasilewski and Dr. Anna Kurek are involved in recruitment of patients for the ISCHEMIA trial. None of the authors received funds regarding analysis of the data or the preparation of the manuscript. The authors have no other conflict of interest to declare.
Authors’ contributions
JW designed the research plan, participated in organizing the study, and drafted the manuscript. LP conceived the study, participated in its design and coordination, and helped to draft the manuscript. AL participated in the study design and helped to draft the manuscript. TO participated in the study design, performed the statistical analysis, and helped to draft the manuscript. RR, KB, and AK participated in data acquisition and analysis of and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Jarosław Wasilewski, Email: jaroswasilewski@gmail.com.
Lech Poloński, Email: scchs@sum.edu.pl.
Andrzej Lekston, Email: alekstonhemo@sccs.pl.
Tadeusz Osadnik, Email: tadeuszosa@wp.pl.
Rafał Reguła, Email: r.regula@gmail.com.
Kamil Bujak, Email: kamil_bujak@o2.pl.
Anna Kurek, Email: ania_kurek@o2.pl.
References
- 1.Lenzen MJ, Boersma E, Bertrand ME, Maier W, Moris C, Piscione F, et al. Management and outcome of patients with established coronary artery disease: the Euro Heart Survey on coronary revascularization. Eur Heart J. 2005;26(12):1169–79. doi: 10.1093/eurheartj/ehi238. [DOI] [PubMed] [Google Scholar]
- 2.Silber S, Albertsson P, Avilés FF, Camici PG, Colombo A, Hamm C, et al. Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur Heart J. 2005;26(8):804–47. doi: 10.1093/eurheartj/ehi138. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness criteria for coronary revascularization: a report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology: Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. Circulation. 2009;119(9):1330–52. doi: 10.1161/CIRCULATIONAHA.108.191768. [DOI] [PubMed] [Google Scholar]
- 4.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 5.Dakik HA, Kleiman NS, Farmer JA, He ZX, Wendt JA, Pratt CM, et al. Intensive medical therapy versus coronary angioplasty for suppression of myocardial ischemia in survivors of acute myocardial infarction : a prospective, randomized pilot study. Circulation. 1998;98(19):2017–23. doi: 10.1161/01.CIR.98.19.2017. [DOI] [PubMed] [Google Scholar]
- 6.Folland ED, Hartigan PM, Parisi AF. Percutaneous transluminal coronary angioplasty versus medical therapy for stable angina pectoris: outcomes for patients with double-vessel versus single-vessel coronary artery disease in a Veterans Affairs Cooperative randomized trial. Veterans Affairs ACME Investigators. J Am Coll Cardiol. 1997;29(7):1505–11. doi: 10.1016/S0735-1097(97)00097-1. [DOI] [PubMed] [Google Scholar]
- 7.Hartigan PM, Giacomini JC, Folland ED, Parisi AF. Two- to three-year follow-up of patients with single-vessel coronary artery disease randomized to PTCA or medical therapy (results of a VA cooperative study). Veterans Affairs Cooperative Studies Program ACME Investigators. Angioplasty Compared to Medicine. Am J Cardiol. 1998;82(12):1445–50. doi: 10.1016/S0002-9149(98)00685-7. [DOI] [PubMed] [Google Scholar]
- 8.Hueb WA, Bellotti G, de Oliveira SA, Arie S, de Albuquerque CP, Jatene AD, et al. The Medicine, Angioplasty or Surgery Study (MASS): a prospective, randomized trial of medical therapy, balloon angioplasty or bypass surgery for single proximal left anterior descending artery stenoses. J Am Coll Cardiol. 1995;26(7):1600–5. doi: 10.1016/0735-1097(95)00384-3. [DOI] [PubMed] [Google Scholar]
- 9.Hueb W, Soares PR, Gersh BJ, César LA, Luz PL, Puig LB, et al. The medicine, angioplasty, or surgery study (MASS-II): a randomized, controlled clinical trial of three therapeutic strategies for multivessel coronary artery disease: one-year results. J Am Coll Cardiol. 2004;43(10):1743–51. doi: 10.1016/j.jacc.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 10.Parisi AF, Folland ED, Edward D, Hartigan PM. A comparison of angioplasty with medical therapy in the treatment of single-vessel coronary artery disease. N Engl J Med. 1992;326:10–6. doi: 10.1056/NEJM199201023260102. [DOI] [PubMed] [Google Scholar]
- 11.Pursnani S, Korley F, Gopaul R, Kanade P, Chandra N, Shaw RE, et al. Percutaneous coronary intervention versus optimal medical therapy in stable coronary artery disease: a systematic review and meta-analysis of randomized clinical trials. Circ Cardiovasc Interv. 2012;5(4):476–90. doi: 10.1161/CIRCINTERVENTIONS.112.970954. [DOI] [PubMed] [Google Scholar]
- 12.Kereiakes DJ. Interpreting the COURAGE trial. PCI is no better than medical therapy for stable angina? Cleve Clin J Med. 2007;74(9):637–42. doi: 10.3949/ccjm.74.9.637. [DOI] [PubMed] [Google Scholar]
- 13.Windecker S, Stortecky S, Stefanini GG, da Costa BR, Rutjes AW, Di Nisio M, et al. Revascularisation versus medical treatment in patients with stable coronary artery disease: network meta-analysis. BMJ. 2014;348:g3859–9. doi: 10.1136/bmj.g3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92(3):657–71. doi: 10.1161/01.CIR.92.3.657. [DOI] [PubMed] [Google Scholar]
- 15.Madsen JK, Nielsen TT, Grande P, Eriksen UH, Saunamäki K, Thayssen P, et al. Revascularization compared to medical treatment in patients with silent vs. symptomatic residual ischemia after thrombolyzed myocardial infarction – the DANAMI study. Cardiology. 2007;108(4):243–51. doi: 10.1159/000096951. [DOI] [PubMed] [Google Scholar]
- 16.Mathur VS, Guinn GA. Prospective randomized study of the surgical therapy of stable angina. Cardiovasc Clin. 1977;8(2):131–44. [PubMed] [Google Scholar]
- 17.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360(24):2503–15. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komarov AL, Iliushenko TA, Shakhmatova OO, Deev AD, Samko AN, Panchenko EP. Comparative efficacy of conservative and invasive treatment of patients with stable form of ischemic heart disease (according to results of five year prospective study) Kardiologiia. 2012;52(8):4–14. [PubMed] [Google Scholar]
- 19.Bangalore S, Pursnani S, Kumar S, Bagos PG. Percutaneous coronary intervention versus optimal medical therapy for prevention of spontaneous myocardial infarction in subjects with stable ischemic heart disease. Circulation. 2013;127:769–81. doi: 10.1161/CIRCULATIONAHA.112.131961. [DOI] [PubMed] [Google Scholar]
- 20.Schömig A, Mehilli J, de Waha A, Seyfarth M, Pache J, Kastrati A. A meta-analysis of 17 randomized trials of a percutaneous coronary intervention-based strategy in patients with stable coronary artery disease. J Am Coll Cardiol. 2008;52:894–904. doi: 10.1016/j.jacc.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 21.Jeremias A, Kaul S, Rosengart TK, Gruberg L, Brown DL. The impact of revascularization on mortality in patients with nonacute coronary artery disease. Am J Med. 2009;122:152–61. doi: 10.1016/j.amjmed.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 22.ClinicalTrials.gov. International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA). https://clinicaltrials.gov/ct2/show/NCT01471522. Accessed 20 Dec 2014.
- 23.World Health Organization. International Classification of Diseases (ICD) http://www.who.int/classifications/icd/en/ (2011). Accessed 20 Dec 2014.
- 24.Zeymer U, Senges J. Why do we need prospective registries in patients with acute myocardial infarction? Eur Heart J. 2003;24(18):1611–2. doi: 10.1016/S0195-668X(03)00435-4. [DOI] [PubMed] [Google Scholar]


