Abstract
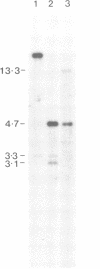
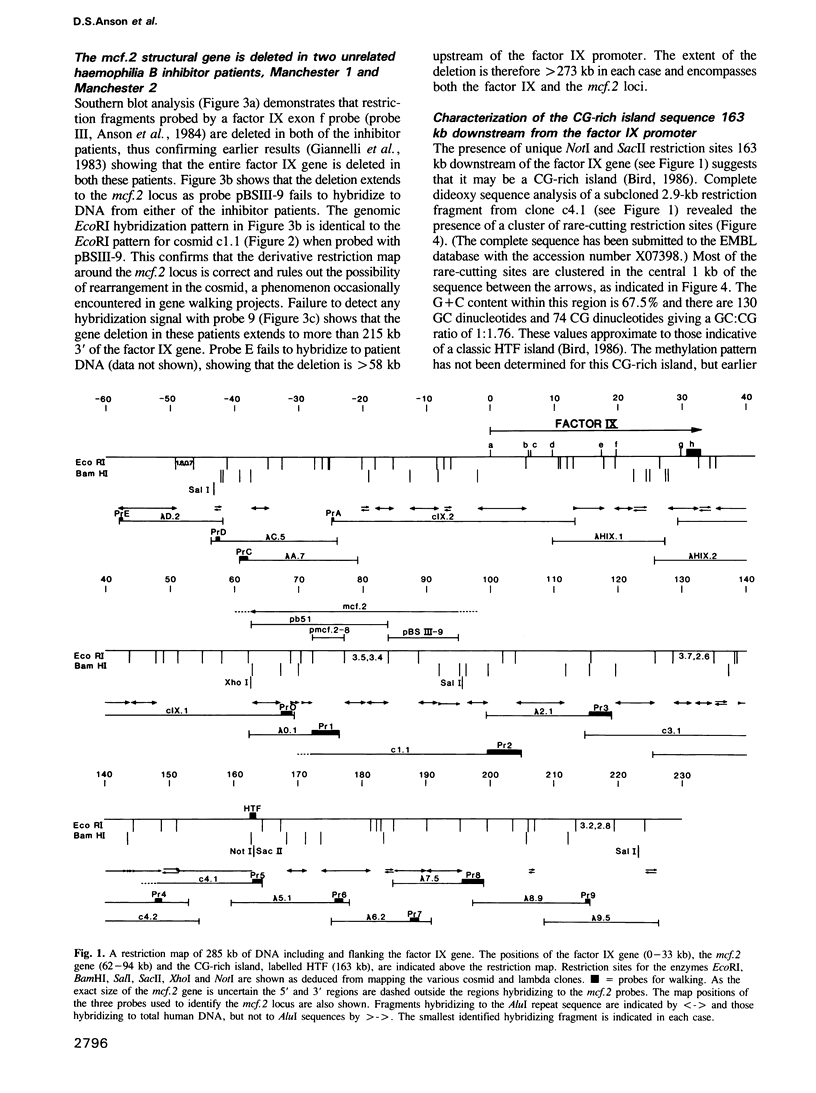
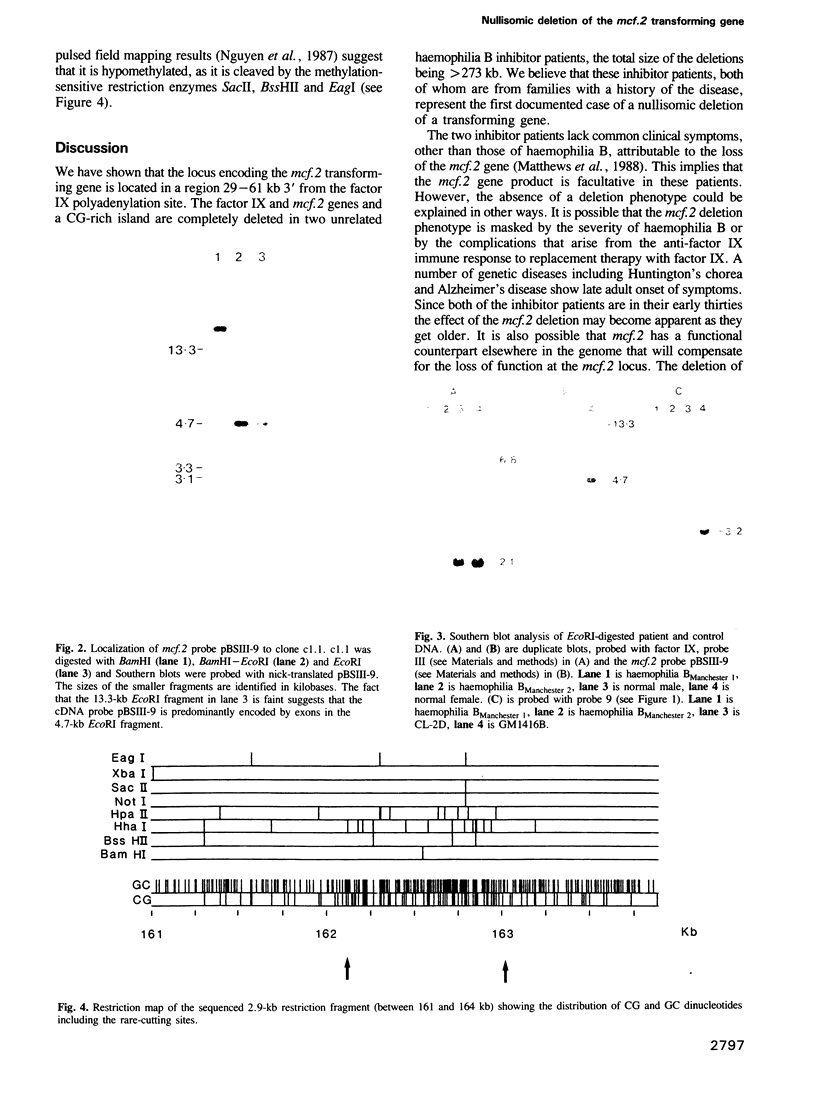
The mcf.2 transforming gene sequence has been located to the region between 29 and 61 kb 3' of the factor IX gene. Two unrelated haemophilia B patients who raise antibodies to infused factor IX ('inhibitors') have deletions in excess of 273 kb encompassing the factor IX and mcf.2 genes and a CG-rich island. We believe these patients show the first nullisomic deletion of a transforming gene to be reported. No clinical condition can be attributed to the loss of the mcf.2 gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson D. S., Choo K. H., Rees D. J., Giannelli F., Gould K., Huddleston J. A., Brownlee G. G. The gene structure of human anti-haemophilic factor IX. EMBO J. 1984 May;3(5):1053–1060. doi: 10.1002/j.1460-2075.1984.tb01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bernardi F., del Senno L., Barbieri R., Buzzoni D., Gambari R., Marchetti G., Conconi F., Panicucci F., Positano M., Pitruzzello S. Gene deletion in an Italian haemophilia B subject. J Med Genet. 1985 Aug;22(4):305–307. doi: 10.1136/jmg.22.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Yoshitake S., Chance P. F., Bray G. L., Thompson A. R., Scott C. R., Kurachi K. An intragenic deletion of the factor IX gene in a family with hemophilia B. J Clin Invest. 1985 Dec;76(6):2161–2164. doi: 10.1172/JCI112222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings D. E. A general theory of carcinogenesis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3324–3328. doi: 10.1073/pnas.70.12.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano O., Birnbaum D., Edlund L., Fogh J., Wigler M. New human transforming genes detected by a tumorigenicity assay. Mol Cell Biol. 1984 Sep;4(9):1695–1705. doi: 10.1128/mcb.4.9.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischel-Ghodsian N., Higgs D. R., Beyer E. C. Function of a new globin gene. Nature. 1987 Oct 1;329(6138):397–397. doi: 10.1038/329397b0. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Fuhrman S. A., Deininger P. L., LaPorte P., Friedmann T., Geiduschek E. P. Analysis of transcription of the human Alu family ubiquitous repeating element by eukaryotic RNA polymerase III. Nucleic Acids Res. 1981 Dec 11;9(23):6439–6456. doi: 10.1093/nar/9.23.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli F., Choo K. H., Rees D. J., Boyd Y., Rizza C. R., Brownlee G. G. Gene deletions in patients with haemophilia B and anti-factor IX antibodies. Nature. 1983 May 12;303(5913):181–182. doi: 10.1038/303181a0. [DOI] [PubMed] [Google Scholar]
- Goss S. J., Harris H. Gene transfer by means of cell fusion I. Statistical mapping of the human X-chromosome by analysis of radiation-induced gene segregation. J Cell Sci. 1977 Jun;25:17–37. doi: 10.1242/jcs.25.1.17. [DOI] [PubMed] [Google Scholar]
- Grosveld F. G., Dahl H. H., de Boer E., Flavell R. A. Isolation of beta-globin-related genes from a human cosmid library. Gene. 1981 Apr;13(3):227–237. doi: 10.1016/0378-1119(81)90028-7. [DOI] [PubMed] [Google Scholar]
- Hassan H. J., Leonardi A., Guerriero R., Chelucci C., Cianetti L., Ciavarella N., Ranieri P., Pilolli D., Peschle C. Hemophilia B with inhibitor: molecular analysis of the subtotal deletion of the factor IX gene. Blood. 1985 Sep;66(3):728–730. [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott V., Rees D. J., Cheng Z., Brownlee G. G. Randomly picked cosmid clones overlap the pyrB and oriC gap in the physical map of the E. coli chromosome. Nucleic Acids Res. 1988 Mar 25;16(6):2601–2612. doi: 10.1093/nar/16.6.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Nguyen C., Pontarotti P., Birnbaum D., Chimini G., Rey J. A., Mattei J. F., Jordan B. R. Large scale physical mapping in the q27 region of the human X chromosome: the coagulation factor IX gene and the mcf.2 transforming sequence are separated by at most 270 kilobase pairs and are surrounded by several 'HTF islands'. EMBO J. 1987 Nov;6(11):3285–3289. doi: 10.1002/j.1460-2075.1987.tb02647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Mattei M. G., Oberlè I., Planche J., Imbert J., Pelassy C., Birg F., Birnbaum D. Localization of the mcf.2 transforming sequence to the X chromosome. EMBO J. 1987 May;6(5):1301–1307. doi: 10.1002/j.1460-2075.1987.tb02368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordenskjöld M., Lundberg C. Recessive cancer genes and chromosomal mechanisms in tumorigenesis. Ann Clin Res. 1986;18(5-6):307–313. [PubMed] [Google Scholar]
- Peake I. R., Furlong B. L., Bloom A. L. Carrier detection by direct gene analysis in a family with haemophilia B (factor IX deficiency). Lancet. 1984 Feb 4;1(8371):242–243. doi: 10.1016/s0140-6736(84)90123-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theillet C., Lidereau R., Escot C., Hutzell P., Brunet M., Gest J., Schlom J., Callahan R. Loss of a c-H-ras-1 allele and aggressive human primary breast carcinomas. Cancer Res. 1986 Sep;46(9):4776–4781. [PubMed] [Google Scholar]
- Wyman A. R., Wolfe L. B., Botstein D. Propagation of some human DNA sequences in bacteriophage lambda vectors requires mutant Escherichia coli hosts. Proc Natl Acad Sci U S A. 1985 May;82(9):2880–2884. doi: 10.1073/pnas.82.9.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota J., Tsunetsugu-Yokota Y., Battifora H., Le Fevre C., Cline M. J. Alterations of myc, myb, and rasHa proto-oncogenes in cancers are frequent and show clinical correlation. Science. 1986 Jan 17;231(4735):261–265. doi: 10.1126/science.3941898. [DOI] [PubMed] [Google Scholar]