Abstract
Inhalation anthrax is a rare but acute infectious disease following adsorption of Bacillus anthracis spores through the lungs. The disease has a high fatality rate if untreated, but early and correct diagnosis has a significant impact on case patient recovery. The early symptoms of inhalation anthrax are, however, non-specific and current anthrax diagnostics are primarily dependent upon culture and confirmatory real-time PCR. Consequently, there may be a significant delay in diagnosis and targeted treatment. Rapid, culture-independent diagnostic tests are therefore needed, particularly in the context of a large scale emergency response.
The aim of this study was to evaluate the ability of monoclonal antibodies to detect anthrax toxin proteins that are secreted early in the course of B. anthracis infection using a time-resolved fluorescence (TRF) immunoassay. We selected monoclonal antibodies that could detect protective antigen (PA), as PA83 and also PA63 and LF in the lethal toxin complex. The assay reliable detection limit (RDL) was 6.63 × 10−6 μM (0.551 ng/ml) for PA83 and 2.51 × 10−5 μM (1.58 ng/ml) for PA63. Despite variable precision and accuracy of the assay, PA was detected in 9 out of 10 sera samples from anthrax confirmed case patients with cutaneous (n=7), inhalation (n=2), and gastrointestinal (n=1) disease. Anthrax Immune Globulin (AIG), which has been used in treatment of clinical anthrax, interfered with detection of PA. This study demonstrates a culture-independent method of diagnosing anthrax through use of monoclonal antibodies to detect PA and LF in the lethal toxin complex.
Keywords: Bacillus anthracis, protective antigen, anthrax toxin, europium, immunoassays, time resolved fluorescence
1. Introduction
Bacillus anthracis is an aerobic spore-forming gram-positive bacterium that is the causative agent of anthrax. Anthrax in humans can manifest in four different forms: cutaneous, gastrointestinal, inhalation or injection (Logan et al., 2011; Palmateer et al., 2013). Cutaneous anthrax is the most common form of the disease, accounting for 99% of cases worldwide but with a low fatality if treatment is available (Centers for Disease and Prevention, 2001; Logan et al., 2011). Ingestion of B. anthracis can result in either oropharangeal or gastrointestinal disease, with a variable mortality rate depending on how quickly treatment is started (Logan et al., 2011). Inhalation anthrax is rare but has a high mortality rate (89%) if not diagnosed early and treated promptly (Logan et al., 2011). In 2001 anthrax spores were intentionally released in mailed letters in the United States, resulting in 22 cases (Logan et al., 2011). The mortality rate of inhalation anthrax was as high as 89% before 2001, but with advanced treatment and supportive care, the mortality rate was only 45% in 2001 (Jernigan et al., 2002). Injection anthrax is a more recent type of infection associated with intravenous drug users (Palmateer et al., 2013). Symptoms of injection anthrax is similar to cutaneous, but the infection may be in deeper tissues such as muscle and it can go systemic quickly (CDC, 2013).
Toxins released by B. anthracis play a major role in establishing and maintaining infection. Anthrax toxins consist of protective antigen (PA), lethal factor (LF), and edema factor (EF). Native PA is produced as a 83-kDa protein (PA83) that binds to host cell receptors, is cleaved and activated by cellular proteases to release a 20-kDa segment, leaving PA63 to form an oligomeric complex at the cell membrane (Young and Collier, 2007; Kintzer et al., 2009). The PA63 complex binds up to four LF and EF molecules to form lethal toxin (LTx; PA63 + LF) or edema toxin (ETx; PA63 + EF) which may then be internalized into the cell to cause a cascade of cytotoxic effects (Young and Collier, 2007).
Anthrax is diagnosed by a variety of methods including: staining of specimens to visualize the organism, culture, PCR, and serology (Logan et al., 2011). Other methodologies for diagnosing anthrax have been reported in the literature and include those that detect anthrax toxins instead of the organism itself (Kobiler et al., 2006; Boyer et al., 2007; Rossi et al., 2008; Tang et al., 2009; Oh et al., 2011; Dragan et al., 2012). Anthrax toxins are secreted early during the course of infection and therefore provide a more timely diagnosis than the use of immunoserology, which requires the production of antibodies by the immune system, or culture, which may take several days and requires appropriate laboratory facilities (Logan et al., 2011). Tang et al. previously described an immunoassay using both polyclonal and monoclonal antibodies in time-resolved fluorescence (TRF) immunoassay, a method that utilizes a high fluorescent nanoparticle (europium), to detect PA in sera to aid in diagnosis of anthrax (Tang et al., 2009).
The aim of this study was to evaluate antigen-specific monoclonal antibodies for use in culture independent assays capable of detecting PA83, PA63 and LTx in the early and convalescent stages of infection following treatment with antibiotics and immunotherapy. TRF was chosen to evaluate our collection monoclonal antibodies because of its higher sensitivity compared to ELISA.
2. Materials and methods
2.1. Materials
Purified recombinant native protective antigen [83 kDa (1 μM=83μg/ml); PA83] was obtained from BEI Resources (Manassas, VA). Activated protective antigen [63 kDa (1 μM=63μg/ml); PA63] and recombinant lethal factor [90 kDa (1 μM=90 μg/ml); LF] were obtained from List Biological Laboratories (Campbell, CA). Dissociation-enhanced lanthanide fluorescent immunoassay (DELFIA) buffer, wash concentrate, enhancement solution, Streptavidin Microtitration 96-well plates, Platewash, Plateshake, and Victor™ X4 Multilabel Plate Reader were from Perkin-Elmer Life Sciences (Shelton, CT). Lethal toxin (LTx) was prepared by combining PA63 and LF in a 7:4 ratio. Anthrax Immune Globulin (AIG), an investigational product for anthrax treatment consisting primarily of anti-PA antibody, was previously acquired from Cangene (Winnipeg, MB, Canada).
2.2. Monoclonal Antibody Preparation, Selection, and Labeling
Mouse monoclonal anti-PA IgG antibodies (1 μM=150 μg/ml) were prepared in the Division of Scientific Resources at CDC as previously described (Boyer et al., 2007). Monoclonal anti-PA IgG AVR1046 was selected as the detector antibody (Li et al., 2008) and monoclonal anti-LF antibody, AVR1674, was selected for experiments for detection of LF in the LTx complex (Boyer et al., 2007). AVR1046 and AVR1674 were europium (Eu) labeled (Eu-AVR1046 and Eu-AVR1674) by Perkin-Elmer BioSignal Inc. (Montreal, QC, Canada). Four anti-PA mouse monoclonal antibodies were tested and AVR1162 was selected as the capture antibody based on its ability to bind PA83 and PA63 without competing with AVR1046, the detector antibody (data not shown). AVR1162 was biotinylated (Bio-AVR1162) in the Division of Scientific Resources at CDC with EZ-Link® Sulfo-NHS-Biotin (Pierce Biotechnology, Rockford, IL) following the manufacturer’s instructions.
2.3. Human Sera
Serum from 10 healthy human donors that had not been previously diagnosed with anthrax or received the anthrax vaccine was obtained from Tennessee Blood Services (Memphis, TN) for use in assay development. Sera were confirmed non-reactive for anti-PA IgG by ELISA before spiking experiments (Quinn et al., 2002; Semenova et al., 2012).
Serum samples from 10 healthy unvaccinated volunteers enrolled in an anthrax vaccine human clinical trial (Marano et al., 2008) and 10 patients confirmed with anthrax were tested to determine performance on clinical samples. Of the 10 human patients confirmed with disease, seven of the cases were cutaneous anthrax with sera samples drawn between 1 and 8 days after symptoms onset (Boyer et al., 2011b). Two of the sera were from inhalation anthrax cases and the samples were collected two to eight days after symptoms onset. The sample from the patient with gastrointestinal anthrax was drawn 11 days after symptoms onset. All samples were taken prior to treatment with AIG. Methods for detection of LF and anti-PA IgG have been previously described (Boyer et al., 2011a; Semenova et al., 2012).
Use of human serum used in the study were approved by the Centers for Disease Control and Prevention (CDC) Human Subjects Institutional Review Board (IRB).
2.4. Optimization of LTx complex detection assay
Ratios of capture and detector antibody pairs for PA were optimized using standard checkerboard titrations. Capture antibody Bio-AVR1162 was tested at concentrations ranging from 0.03 μg/mL to 32.0 μg/mL and the detector antibody Eu-AVR1046 was tested between 0.0078 μg/mL to 16.0 μg/mL. Based on the results of the experiment, the following assay procedure was used for all subsequent experiments. DELFIA Streptavidin Microtitration 96-well plate was washed with one cycle on the DELFIA Platewash. The capture antibody (24.0 μg/mL Bio-AVR1162) was added to the plate and then incubated at room temperature for 2.5 hours with shaking on the DELFIA Plateshake and then washed for two cycles. Test samples and controls were added in duplicate to the plate and then incubated for 1 hour at room temperature with shaking and then washed for 10 cycles. The detector antibody (1.0 μg/mL Eu-AVR1046) was added to the plate, incubated at room temperature for 1 hour with shaking and then washed for 10 cycles. After washing 200 μl DELFIA enhancement solution was added to each well and the plate was incubated for 10 minutes at room temperature on low on the DELFIA Plateshake and then read on the Victor™ X4 Multilabel Plate Reader to determine the counts per second (CPS).
To determine if the formation LTx would interfere with PA63 detection and if LF could be detected in the LTx complex, pooled sera from 10 donors was spiked with 7:4 ratio of PA63 to LF (0.5 μM PA63 and 0.286 μM LF). The master dilution mix was then serially transferred in 2-fold dilutions and pipetted into six rows on a DELFIA Streptavidin Microtitration 96-well plate in the sample addition step. For the first two rows 1.0 μg/mL Eu-AVR1046 was used as the detector antibody as described in 2.4. For the next two, 2.0 μg/mL Eu-AVR1674 was used to detect LF present in LTx. The last two rows used a both 1.0 μg/mL Eu-AVR1046 and 2.0 μg/mL Eu-AVR1674 to determine if the combination of these antibodies could provide an additive effect in detecting anthrax toxin. As a control, PA63 only was serially diluted in duplicate on the plate and probed with 1.0 μg/mL Eu-AVR1046. This experiment was repeated independently seven times. The Student’s t-test was used to compare the different test conditions, a p-value≤0.05 was considered significant.
2.5. Limit of detection and quantification
Serum from each of 10 donors was pooled and spiked with 0.88 μM of either PA83 or PA63. The master dilution of the spiked sera was then serially transferred in 2-fold dilutions and tested in duplicate in 16 independent experiments for both PA83 and PA63. The TRF assay method was followed as described in section 2.4.
The measured counts per second were fit to a 5-Parameter Logistic (5-PL) model (Gottschalk and Dunn, 2005) after being log10 transformed:
Where Y is the fluorescence measured in counts per second and Conc is the concentration of the reference standard in μM. The A parameter corresponds to the lower asymptote of the curve, the B parameter corresponds to the upper asymptote, the C parameter determines the midpoint concentration of the curve, the D parameter determines the slope of the curve at the midpoint, and the H parameter represents the degree of symmetry between the upper and lower halves of the curve. The curves displayed significant asymmetry between the upper and lower asymptotes, requiring the use of the 5-PL model rather than the symmetrical 4-PL. The log10 transformation homogenized the variance across the range of the assay, and allowed the data to be fit without requiring additional weighting.
The observed reference standard data were fit to the 5-PL model using non-linear ordinary least squares in SAS® version 9.3 (SAS Institute Inc, Cary, NC). Sample concentrations, run in duplicate, were calculated by interpolating the mean of the duplicates to the standard curve using the measured sample fluorescence in counts per second.
The limit of detection was calculated as the minimum detectable concentration (MDC) and the limit of quantification was calculated as the reliable detection limit (RDL) (O'Connell et al., 1993; Quinn et al., 2002). The MDC is the concentration of PA83 or PA63 at which the five-PL-log fit of the standard curve data crosses the upper 95% confidence limit of the lower asymptote. The RDL is the concentration of PA83 or PA63 at which the lower 95% confidence limit of the 5-PL-log fit of the standard curve crosses the upper 95% confidence limit of the lower asymptote.
2.6. Precision and Accuracy for PA83 and PA63 Detection
Acceptable performance characteristics were based on published data for anti-PA IgG ELISAs (Semenova et al., 2012). Precision and accuracy of PA83 and PA63 detection were determined with TRF by spiking a known amount of PA into pooled sera and then calculating the measured concentration using a standard curve. The standard curve was produced by spiking pooled sera from 10 donors with 0.15 μM of PA83 or 0.75 μM of PA63. The master dilution of the spiked sera was then serially transferred in 3-fold dilutions to make a 14 point dilution series in pooled sera to ensure coverage of the dynamic range of the assay. Six dilution points, including points above and below the MDC and RDL, were run in duplicate on 10 independent runs on different days. The concentration of PA83 or PA63 in spiked sera samples was determined by interpolating the sample responses to the standard 5-PL curve fit as described in section 2.5.
Precision is a measurement of the degree of repeatability of an assay using standard operating procedures (FDA, 1996; FDA, 2001). Precision was expressed as the percent coefficient of variation (CV). An acceptable inter-assay precision would be a CV of ≤20%. Accuracy is a measure of the exactness of the assay and it was expressed as a percent error between the assay-determined concentration and the known concentration for that serum (FDA, 1996; FDA, 2001). The percent error was calculated as the absolute value of [(observed − expected)/expected] × 100. An acceptable percent error was considered to be ≤25%.
Dilutional linearity was calculated from the same raw data as accuracy and precision by linear regression of the observed concentrations against the spiked concentrations on log10 scale. Linearity was evaluated by the slope, intercept and r-squared coefficient of the linear regression fit line. For this assay, acceptable linearity was considered to be 0.8 ≤ slope ≤ 1.2, −0.5 ≤ intercept ≤ 0.5, and r2 ≥ 0.85 (FDA, 2001).
2.7. Detection of PA83, PA63, and LTx clinical samples
The TRF assay method was followed for detection of PA only as described in section 2.4 with PA83 as the standard. Undiluted sera samples from patients were tested in duplicate on a plate with 50 μl in each well. Concentration of PA in serum sample was determined by interpolating the sample responses to the standard 5-PL curve fit as described in section 2.5
2.8. Detection of PA63 and LTx in the presence of AIG
AIG is an adjunctive therapy for treatment of systemic anthrax and could impact the ability of the assay to detect PA. The effect of AIG on PA detection was evaluated by spiking AIG into sera with PA63 with and without LF. The standard curve was made by spiking pooled sera with PA63 at 0.75 μM or with LTx (7:4 ratio of PA63 to LF (0.75 μM PA63 and 0.43 μM LF)). The master dilution mixes were then serially transferred in 3-fold dilutions to make a 10 point dilution series. PA63 was tested at two different concentrations 0.03 μM and 0.003 μM with and without LF (0.0171 μM or 0.00171 μM, respectively). The anti-PA IgG concentration present in AIG was determined by the ELISA (Semenova et al., 2012). AIG was added in a 50 molar excess of the PA63 concentration, 1.5 μM for PA63 at 0.03 μM or 0.15 μM for PA63 at 0.003 μM of the PA63, and then serially transferred in a 3-fold dilution to make a 12 point dilution. Once the 12 point dilution of AIG was made PA63 or PA63 with LF was added to the appropriate tubes. Standard curves and experimental samples were run in duplicate on the plate. The IC50 of AIG was determined by plotting the observed CPS against the AIG concentration and fitting to a 4-parameter logistic (4-PL) model. The 4-PL was chosen instead of the 5-PL for this fit because there was insufficient resolution of the lower end of the curve to converge the 5-PL model.
3. Results
3.1. Monoclonal antibody selection and assay optimization
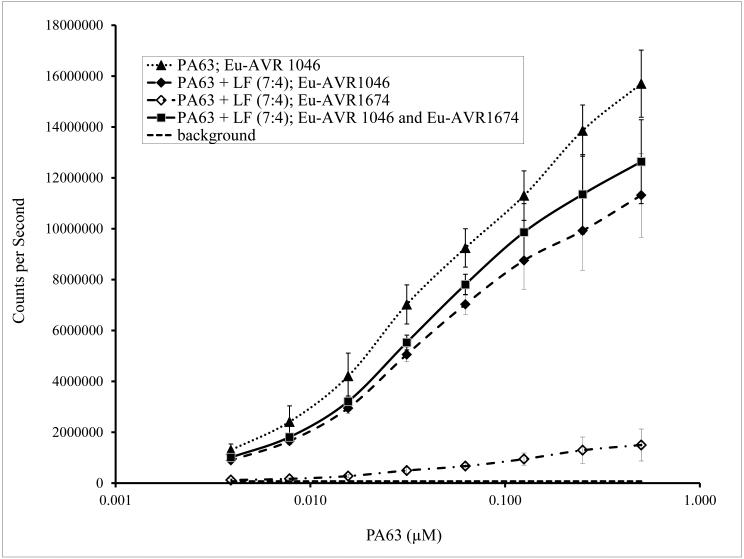
Monoclonal anti-PA antibodies AVR1046 and AVR1162 to PA83 and PA 63 were screened and selected on the basis of non-competitive binding to PA (data not shown). The anti-PA antibody pair on the TRF platform was able to detect PA63 in the presence of LF. Although there was a statistically significant reduction in signal point estimates (p < 0.01) the signal to background ratio for detection of PA63 was 150 at the lowest concentration of analyte evaluated (Fig. 1). When combining Eu-AVR1046 and Eu-AVR1674 to detect both PA63 and LF, there was an increase in signal compared to Eu-AVR1046 alone as the detector antibody. The increase was not statistically significant (p > 0.5).
Fig. 1.
Detection of PA63 in the presence of LF. Results of detection a 7:4 ratio of PA63 to LF (0.5 μM PA63 and 0.286 μM LF) spiked in pooled sera from 10 donors. The master dilution mix was then serially transferred in 2-fold dilutions to make an 8 point dilution series in pooled sera. PA63 without LF with the equivalent 8 point dilution series was used as a control. Eu-AVR1046 was used as the detector antibody as described in 2.4. The captured toxin was detected using 1.0 μg/mL Eu-AVR1046 and/or 2.0 μg/mL Eu-AVR1674. The curve is the mean ± standard error (SE) of 7 duplicate runs.
3.2. Limit of detection and quantification
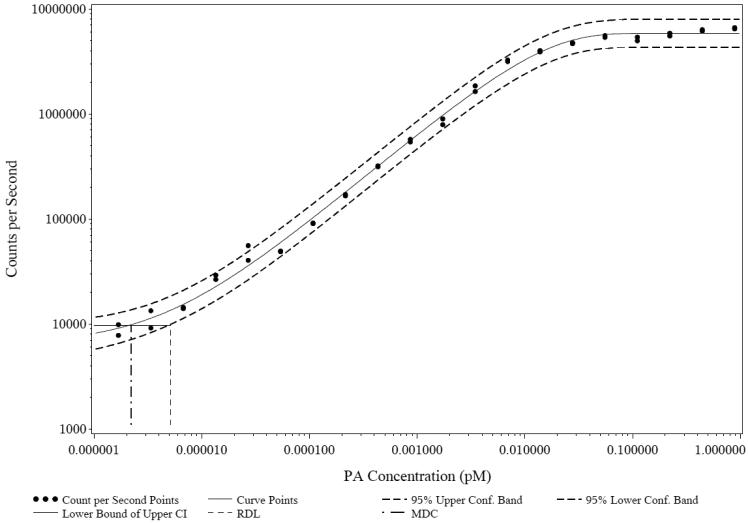
As described in section 2.5, the MDC and RDL were the calculated lower limits of detection (LOD) and quantification (LOQ), respectively, for this assay. MDC and RDL were calculated using the 95% confidence intervals of a five-parameter logistic-log transformation of the standard curve (Table 1). The MDC and RDL values for PA83 detection were 2.69 × 10−6 μM and 6.63 × 10−6 μM, respectively (Fig 2). For PA63 detection the MDC and RDL were and 9.17 × 10−6 μM and 2.51 × 10−5 μM, respectively (Table 1). The lower limit of detection of PA83 versus PA63 is indicated in the lower asymptote of PA83 detection curve (Fig 3).
Table 1.
Limit of detection calculated as the minimum detectable concentration (MDC) and reliable detection limit (RDL) for PA83 or PA63 in pooled human sera.
| PA83 ( n=16) |
PA63 ( n=16) |
|
|---|---|---|
| MDC ± SD (μM) |
2.69 × 10−6 ± 2.25 × 10−6 | 9.17 × 10−6 ± 8.91 × 10−6 |
| CV (%) | 83.8 | 97.2 |
| RDL ± SD (μM) |
6.63 × 10−6 ± 6.30 × 10−6 | 2.51 × 10−5 ± 2.73 × 10−5 |
| CV (%) | 94.9 | 108.6 |
Fig. 2.
Graphic representation of minimum detectable concentration (MDC) and reliable detection limit (RDL). Figure shows the mean result of one run in duplicate of PA83 dilution series from 0.0000017 μM to 0.884 μM along with the 95% confidence interval and MDC and RDL.
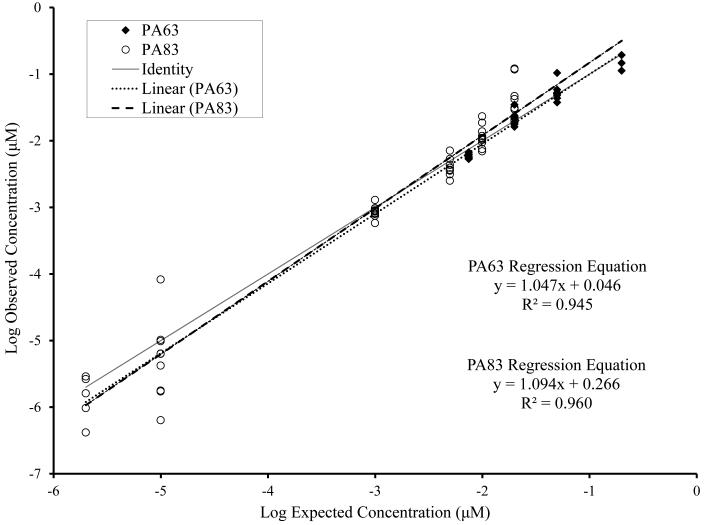
Fig. 3.
Dilutional linearity of the time-resolved fluorescence (TRF) assay for PA using PA83 and PA63. Dilutional linearity determines the ability of an assay to obtain a result that is proportional to the concentration of the PA that is in the sample. Six samples with a range of concentration from 0.000002 to 0.02 μM for PA83 and 0.000009 to 0.02 μM for PA63 were used in the experiments. Data points below the lower limit of quantification for PA63 are not shown.
3.3. Precision and accuracy for PA83 and PA63 detection
The precision of the assay was variable with CV values ranging from 20.8% to 189.7% for PA83 and from 8.2% to 133.2% for PA63 (Table 2). The average CV for PA63 was lower at 45.0% compared to PA83 at 69.4%, but did not meet the acceptance criterion of ≤20%. The accuracy of the assay ranged from 4.7% to 156.2% for PA83 with 4 of 6 spiked concentrations having a percent error meeting the acceptance criterion of 25% (Table 2). The accuracy for PA63 was similar with a range from 9.0% to 58.4% with 4 of 5 concentrations meeting the acceptance criterion (Table 2). PA83 demonstrated better dilutional linearity than PA63, with calculated r2 of 0.960 and 0.945, respectively. In addition, the two lowest concentrations of PA63 spiked into sera were censored in order to maintain linearity of the data (Fig. 3).
Table 2.
Assessment of assay accuracy and precision for detecting PA83 and PA63. Assay was repeated in duplicate 10 times (n) and for each the number of runs with a measurable results (nm), expected mean (μM) the observed mean (μM), standard deviation (St. dev), coefficient of variation (CV), and percent error (% error).a
| Toxin | Expected mean (μM) |
n m /n | Observed mean (μM) |
St. dev | CV (%) |
% Error |
|---|---|---|---|---|---|---|
| PA83 | 0 | 3/10 | 0.0000028 | 0.0000023 | 81.7 | ND |
| 0.000002 | 6/10 | 0.0000019 | 0.0000011 | 55.9 | 4.7 | |
| 0.00001 | 9/10 | 0.000014 | 0.000026 | 189.7 | 37.7 | |
| 0.001 | 10/10 | 0.0009 | 0.0002 | 20.8 | 11.8 | |
| 0.005 | 10/10 | 0.0041 | 0.0013 | 31.8 | 18.4 | |
| 0.01 | 10/10 | 0.012 | 0.005 | 41.2 | 23.5 | |
| 0.02 | 9/10 | 0.051 | 0.04 | 77.2 | 156.2 | |
| PA63 | 0 | 0/10 | ND | ND | ND | ND |
| 0.000009 | 0/10 | ND | ND | ND | ND | |
| 0.00003 | 3/10 | 0.00012 | 0.00002 | 133.2 | 58.4 | |
| 0.008 | 10/10 | 0.0057 | 0.0005 | 8.2 | 23.4 | |
| 0.02 | 10/10 | 0.022 | 0.005 | 23.9 | 9.0 | |
| 0.05 | 10/10 | 0.055 | 0.018 | 32.7 | 10.5 | |
| 0.2 | 3/10 | 0.15 | 0.04 | 27.0 | 24.5 |
ND = not determined
3.4. Detection of PA in clinical samples
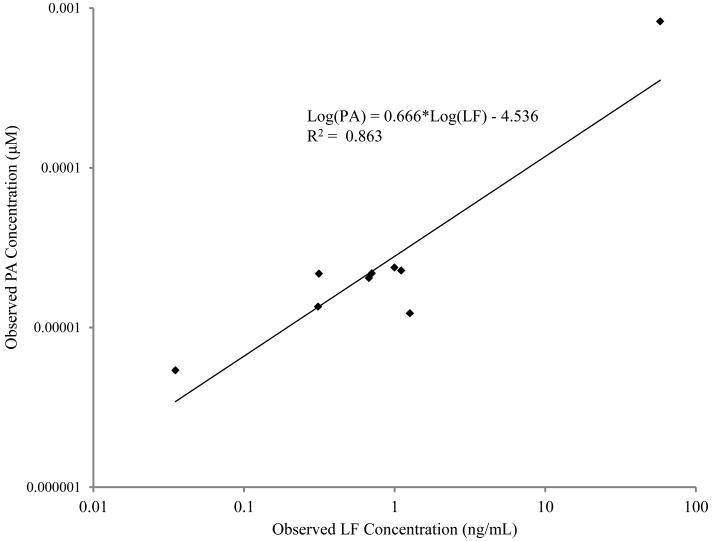
PA concentrations were above the LOQ with a CV of <20% for 9 of 10 of the confirmed anthrax patients tested while none of the samples from healthy controls had PA levels greater or equal to the LOQ (Table 3). For the cutaneous anthrax cases the measured PA levels ranged from 1.02 ng/ml to 68.77 ng/ml (1.23 × 10−5 μM to 4.85 × 10−5 μM) (Table 3). The one sample that had PA levels less than the LOQ (6.63 × 10−6 μM) was from the only cutaneous case that had reportable levels of anti-PA IgG. The presence of endogenous anti-PA may negatively affect the ability of the assay to detect PA (Table 3). There was no direct correlation between the time from onset of clinical signs and the amount of PA that was detected. The highest PA level was in the single patient with a sample taken one day after symptoms onset. For the inhalation and gastrointestinal anthrax cases the measured PA was greater than the LOQ in all samples tested (Table 3). The measured PA from sera of a patient with gastrointestinal anthrax and one of the inhalation cases were within the same range as was seen with the cutaneous cases, but both of these samples were drawn more than a week after onset of symptoms and the patient with inhalation anthrax had been on antimicrobial therapy for 6 days (Table 3). The highest levels of PA that were measured in all cases was from a sample taken only two days after the onset of symptoms and prior to antimicrobial treatment in a patient with inhalation anthrax (68.73 ng/ml) (Table 3). In contrast to the other inhalation case and the gastrointestinal case, this patient did not have anti-PA IgG levels above the LLOQ. A linear correlation between the measured amount of LF and PA was found with a goodness of fit (mean R2) of 0.863 (Fig. 4).
Table 3.
Performance of TRF assay in detecting PA as PA83, PA63, and toxin complex in clinical samples. Results of testing 100 μl sera from ten confirmed clinical cases (cutaneous, inhalation, and gastrointestinal) and ten healthy persons. Concentration was determined using a PA83 standard curve.a
| Clinical Form | Time from Onset to Sera Collection (Days) |
Lethal Factorb
(ng/ml) |
anti-PA IgGc
(μg/ml) |
TRF PA detection | |
|---|---|---|---|---|---|
| Concenetration (ng/ml)d |
Coefficient of Variation (CV) (%) |
||||
| Cutaneouse | 8 | 0.996 | <LLOQ | 1.97 | 15.7 |
| Cutaneouse | 8 | 1.264 | <LLOQ | 1.02 | 14.8 |
| Cutaneouse | 7 | 0.310 | <LLOQ | 1.12 | 2.2 |
| Cutaneouse | 6 | 0.675 | <LLOQ | 1.70 | 17.3 |
| Cutaneouse | 1 | <LOD | <LLOQ | 4.03 | 5.3 |
| Cutaneouse | 3 | 1.105 | <LLOQ | 1.89 | 1.3 |
| Cutaneouse | 5 | 0.035 | 4.0 | <LLOQ | NA |
| Inhalation | 8 | 0.705 | 7.5 | 1.81 | 8.2 |
| Inhalation | 2 | 57.985 | <LLOQ | 68.73 | 2.2 |
| Gastrointestinal | 11 | 0.314 | 6.9 | 1.80 | 8.8 |
| Healthy control | NA | NA | <LLOQ | <LLOQ | NA |
| Healthy control | NA | NA | <LLOQ | <LLOQ | NA |
| Healthy control | NA | NA | <LLOQ | <LLOQ | NA |
| Healthy control | NA | NA | <LLOQ | <LLOQ | NA |
| Healthy control | NA | NA | <LLOQ | <LLOQ | NA |
| Healthy control | NA | NA | <LLOQ | <LLOQ | NA |
| Healthy control | NA | NA | <LLOQ | <LLOQ | NA |
| Healthy control | NA | NA | <LLOQ | <LLOQ | NA |
| Healthy control | NA | NA | <LLOQ | <LLOQ | NA |
| Healthy control | NA | NA | <LLOQ | <LLOQ | NA |
NA = not applicable
Measured using mass spectrometry with a limit of detection (LOD) for lethal factor of <0.005 ng/ml (Boyer et al.,2007; Boyer et al., 2011a).
Measured using ELISA with a lower limit of quantification (LLOQ) for anti-PA IgG of ≤3.7 μg/ml (Semenova, 2012).
The limit of quantification (LOQ), based on the RDL, for PA is ≤0.551 ng/ml.
Lethal factor and anti-PA IgG data from Boyer et al., 2011b.
f 37.5 μl per well instead of 50 μl of sera was tested, result was divided by 0.75 to adjust for this volume difference.
Fig. 4.
Comparative analysis of observed PA concentrations and LF concentrations in clinical cases. PA concentrations (μM) for a panel of 10 sera samples collected from human anthrax clinical cases were compared to previously measured LF concentrations (ng/ml) (Boyer et al., 2011b).
3.5. Detection of PA63 and LTx in the presence of AIG
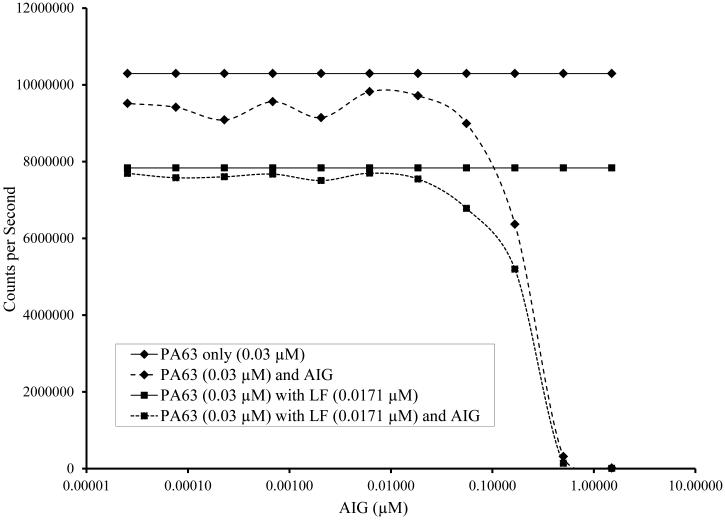
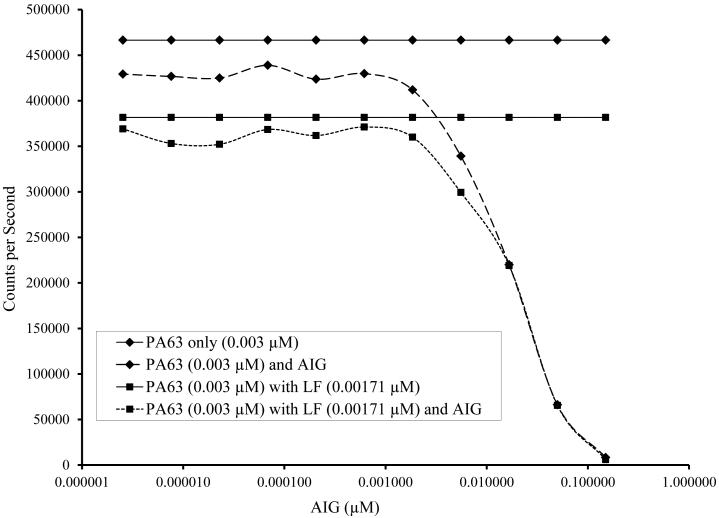
AIG was found to interfere with detection of PA63 and LTx. When the ratio of AIG to PA approached 1.1 to 3.6 (Figure 5) there was a 10% reduction in signal. A 50% reduction in signal was seen when AIG:PA ratio was 5.4:1 when the (0.03 μM PA) and 6.8:1 (0.003 μM PA).
Fig. 5.
Detection of PA63 and lethal toxin (PA63 + LF) in the presence of anthrax immunoglobulin (AIG) at a concentration of (A) 0.03 μM PA63 (with and without 0.0171 μM LF) and (B) 0.03 μM PA63 (with and without 0.00171 μM LF). AIG contains anti-PA antibodies and is used in treatment of patients with anthrax. A 50-fold molar excess of AIG was serially transferred in 3-fold dilutions to make an 11 point dilution series in sera starting at a maximum concentration of 1.5 μM. After the dilutions were completed, PA63 with and without LF was added. The 4PL fit of Counts per Second versus the anti-PA IgG concentration (μM) is shown and IC50 and IC90 are given.
4. Discussion
Early diagnosis and treatment of anthrax increases the likelihood of survival and recovery of the patient. Anthrax toxins are secreted early in the course of disease prior to detectable bacteremia or immune response and therefore provide a more timely diagnosis compared to serology or culture dependent methodologies. In rhesus macaque models of inhalation anthrax the onset of LF toxemia also precedes the detection of vegetative cell markers such as poly-D-glutamate capsule (Boyer et al., 2009). In the current study a europium based, time resolved fluorescence immunoassay (TRF) was used for the initial evaluation a library of anti-PA murine monoclonal antibodies that could also be used on other platforms to detect PA in spiked sera and sera from clinical anthrax patients. An antibody pair for capture (AVR1162) and detection (AVR1046) of PA were selected for their non-competitive binding to both PA83 and PA63 even in the presence of LF.
An assay for diagnosing anthrax through detection of PA must be able to detect both PA83 and PA63, and it must be able to detect them in the presence of LF or EF. LF and EF are the proteolytic enzymes that are translocated by the PA63 oligomer to the host cell cytosol where they can then cause cytotoxic effects (Young and Collier, 2007). The binding of LF and/or EF to PA63 with sub-nanomolar affinity (Young and Collier, 2007) could potentially interfere with the ability of an assay to detect PA in clinical samples. It has been determined that three to four LF and/or EF can bind the PA63 complex (Young and Collier, 2007; Kintzer et al., 2009). In order to ensure that PA63 was saturated with LF, we spiked a ratio of seven PA63 with four LF in sera. Due to the commonalities of LF and EF interactions with the PA63 oligomer, we did not separately determine the effect of EF on the assay. The assay was able to detect PA63 in the presence of LF although there was a statistically significant decrease (p < 0.01) in signal. Thesignal to background ratio was still acceptably high at 150. The anti-LF mAb, AVR1674 did bind the LF as part of the LTx complex, but the increase in signal was not statistically significant. The ability to detect PA in the presence of LF is critical since the toxin complex would form in clinical samples from anthrax patients.
The calculated lower limit of quantification (LOQ), based on the reliable detection limit (RDL), in human sera was 6.63 × 10−6 μM (0.551 ng/ml) for PA83 and 2.51 × 10−5 μM (0.158 ng/ml) for PA63 with a calculated lower limit of detection (LOD), based on the MDC, of 2.66 × 10−6 μM (0.223 ng/ml) for PA83 and 9.17 × 10−6 μM (0.5578 ng/ml) for PA63. A previous study that used TRF reported the ability to detect PA in spiked mouse plasma at levels as low as 0.02 ng/ml, although this was not consistently reproducible at levels as high as 0.04 ng/ml (Tang et al., 2009). The LOD was tenfold lower for Tang et al. (2009), although it was calculated using a linear fit while the current study used non-linear 5-PL model. Differences in LOD could be due to the additional steps that they used to increase sensitivity while our method was based on a more traditional ELISA type assay so that we could evaluate performance of the mAbs for use on this and other platforms. Other assays for PA detection have included non-TRF fluorescence based assays (Oh et al., 2011; Dragan et al., 2012), electrochemiluminescence (Kobiler et al., 2006; Rossi et al., 2008) and traditional ELISAs (Kobiler et al., 2006; Mabry et al., 2006). Limits of detection of these assay ranged from 0.0001– 83 ng/mL, with the ELISA assays performing in the middle of the range. As with the Tang et al (2009) study, a limitation to comparing the performance of these assays is the variability in how the LOD was determined.
The precision of the TRF assay for detecting PA83 and PA63 was not optimal, with an average CV of 69.4% for PA83 and 45.0% for PA63. Only one dilution point had a CV < 20% for PA63 and none for PA83. CV values this high are not desirable for quantitative analysis of clinical specimens, although we did see much better CV values when testing sera specimens from anthrax patients. For accuracy of detecting both PA83 and PA63 the % error was below 25%, which is acceptable for qualitative detection of an analyte. As mentioned previously, we found a lower RDL for PA83 detection compared to PA63 at 6.634 pM versus 25.107 pM, respectively. The ability to detect PA83 at lower concentrations than PA63 was also reflected in the accuracy experiments (Table 2, Fig. 3). Acceptable dilutional linearity for quantification of PA63 was only observed for four of the six spiked concentrations and the goodness of fit (mean R2) was lower for PA63 compared to PA83 (Fig. 3). PA83 is activated by cleavage of a 20-kDa amino-terminal polypeptide (PA20) by a variety of serum and cellular proteases (Young and Collier, 2007). The conformational differences between PA63 after the loss of PA20 from PA83 could alter the ability of our capture and detector antibodies to bind, therefore lowering the detection limit. Variability in monoclonal antibody affinity for PA83 and PA63 has been demonstrated previously (Moayeri et al., 2007). The limitations of precision and accuracy on this platform may therefore limit the application of this approach to a qualitative ‘yes/no’ diagnostic test. In the context of high-throughput testing during an emergency, or for a point of care/point of need diagnostic setting, this is not necessarily a significant disadvantage, on condition that the diagnostic sensitivity and specificity remain high and the analytic sensitivity with precision is in the clinically relevant range.
To determine the performance of the assay in detecting PA as PA83, PA63, and LTx when testing clinical samples, sera samples from 10 case patients with confirmed anthrax were tested; 7 from patients with cutaneous anthrax; two from inhalation anthrax; and one from gastrointestinal anthrax. None of the patients had received AIG before samples were collected. PA levels above the LOQ were detected in all samples except for one, a sample drawn 8 days after onset of cutaneous anthrax in the only cutaneous patient with detectable anti-PA IgG. There was a positive correlation between PA and LF concentrations measured in sera from these patients (R2=0.854, Fig. 4). The sera from one of the inhalation anthrax cases estimated PA levels at greater than 6.0 × 10−4 μM or 68.73 ng/ml. This was the only non-cutaneous case tested that had a serum sample available that was drawn close to onset of symptoms.
In the other eight patients tested, PA levels in sera were all less than 4.1 ng/ml. Six of the eight case patients with lower PA levels were diagnosed with cutaneous anthrax. The lower PA levels in these patients are likely due to the fact that cutaneous anthrax is generally a local infection and thus toxins are not as abundant in the blood system. Reports in the literature support this, with maximum LF levels in sera higher in an inhalation case (203.0 ng/ml) compared to 26 cutaneous cases (1.264 ng/ml) (Walsh et al., 2007; Boyer et al., 2011b). This perspective is also supported by immunologic data; persons with cutaneous anthrax had lower peak anti-PA IgG response with fewer memory B cells compared to patients with inhalation anthrax and it was proposed that this was due to the infection remaining locally (Quinn et al., 2004). The other two patients with low serum PA levels were diagnosed with either gastrointestinal or inhalation anthrax. The low PA levels in these patients were likely due to the timing of specimen collection. The serum samples tested from these patients were not drawn until more than one week after onset of symptoms and these patients had already begun to mount an immune response, evidenced by detectable anti-PA IgG antibody found in the sera. In addition, the patient with inhalation anthrax with low levels of PA had received appropriate antimicrobial therapy for anthrax beginning 6 days before the specimen was collected.
Another potential concern for detection of PA in clinical samples is if the patient had previously been treated with an immunotherapeutic such as Anthrax Immune Globulin (AIG, Cangene). AIG has been used for treatment of persons with anthrax under a FDA Emergency Investigational New Drug (E-IND) Protocol in the United States and other countries (Stern et al., 2008). AIG is derived from plasma from donors with antibody against PA due to anthrax vaccination and these anti-PA antibodies could compete with the antibodies used in the assay for binding to PA. In this study we found that the presence of AIG in spiked sera did interfere with the ability to detect PA63 and LTx. AIG caused a 50% decrease in signal when the concentration was around five to seven times greater than the PA63 concentration. The current therapeutic adult dose of AIG for treatment of anthrax, based on available data to date, is 420 U AIG or about 500 mg anti-PA IgG antibody. Using average human blood volume calculations (Feldschuh and Enson, 1977), this dose would be equivalent to about 94.0 μg/mL anti-PA IgG antibody in an adult male and 109.4 μg/mL anti-PA IgG antibody in an adult female. Based on our lower AIG:PA ratio of 5.4 to 1, the therapeutic anti-PA IgG antibody concentration would cause a 50% reduction in signal at PA concentrations just over 1.0 μM, a concentration much higher than the levels of PA detected in the patients with anthrax tested in this study (highest detected concentration was 6.20 × 10−4 μM). Actual measured maximum concentration of anti-PA IgG antibody levels in patients treated with AIG ranged between 104.7 to 804.0 μg/ml, with values being much higher than the target concentration (Walsh et al., 2007)(unpublished data). These data suggest AIG will interfere in the ability of the assay to detect and measure PA levels. In patients that have been treated with AIG however, LF is still detectable up to seven days after treatment (Boyer et al., 2011b). Adding an additional assay for detection of LF and/or EF would be helpful in cases in which the patient has already been treated with AIG and monitoring of toxins levels is useful for patient management.
In this study, an anti-LF monoclonal antibody and an anti-PA monoclonal antibody pair were selected that can detect PA83, PA63, LF and LTx in serum. The ability to target multiple epitopes in the toxin complex using combinations of anti-PA and anti-LF antibodies is an important facet of developing immunodiagnostic assays because the toxin conformation in serum may change depending on the stage of the infection. These data provide a basis for simple and efficient diagnostics for early stage anthrax – a significant advantage over the high sensitivity and specificity but high complexity approaches such as mass spectrometry. These antibodies are currently being evaluated for development of culture independent point-of-care assays to provide rapid, inexpensive and low complexity diagnosis of symptomatic anthrax.
Acknowledgments
The authors would like to thank Hanan Dababneh, Panagiotis Maniatis, Maribel Gallegos Candela, Renato C. Lins, and John R. Barr for assistance with sample testing and reagent acquisition; Todd Parker and Elke Saile for technical assistance; and Yon Yu for review of the manuscript.
Footnotes
Required Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry. Names of vendors or manufacturers are provided as examples of available product sources; inclusion does not imply endorsement of the vendors, manufacturers or products by the Centers for Disease Control and Prevention, the U.S. Department of Health and Human Services.
References
- Boyer AE, Gallegos-Candela M, Lins RC, Kuklenyik Z, Woolfitt A, Moura H, Kalb S, Quinn CP, Barr JR. Quantitative mass spectrometry for bacterial protein toxins--a sensitive, specific, high-throughput tool for detection and diagnosis. Molecules. 2011a;16:2391–413. doi: 10.3390/molecules16032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer AE, Quinn CP, Beesley CA, Gallegos-Candela M, Marston CK, Cronin LX, Lins RC, Stoddard RA, Li H, Schiffer J, Hossain MJ, Chakraborty A, Rahman M, Luby SP, Shieh WJ, Zaki S, Barr JR, Hoffmaster AR. Lethal factor toxemia and anti-protective antigen antibody activity in naturally acquired cutaneous anthrax. J Infect Dis. 2011b;204:1321–7. doi: 10.1093/infdis/jir543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer AE, Quinn CP, Hoffmaster AR, Kozel TR, Saile E, Marston CK, Percival A, Plikaytis BD, Woolfitt AR, Gallegos M, Sabourin P, McWilliams LG, Pirkle JL, Barr JR. Kinetics of lethal factor and poly-D-glutamic acid antigenemia during inhalation anthrax in rhesus macaques. Infect Immun. 2009;77:3432–41. doi: 10.1128/IAI.00346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer AE, Quinn CP, Woolfitt AR, Pirkle JL, McWilliams LG, Stamey KL, Bagarozzi DA, Hart JC, Jr., Barr JR. Detection and quantification of anthrax lethal factor in serum by mass spectrometry. Analytical chemistry. 2007;79:8463–70. doi: 10.1021/ac701741s. [DOI] [PubMed] [Google Scholar]
- CDC 2013 Injection Anthrax. 2014 [Google Scholar]
- Centers for Disease, C. and Prevention Human anthrax associated with an epizootic among livestock--North Dakota, 2000. MMWR Morb Mortal Wkly Rep. 2001;50:677–80. [PubMed] [Google Scholar]
- Dragan AI, Albrecht MT, Pavlovic R, Keane-Myers AM, Geddes CD. Ultra-fast pg/ml anthrax toxin (protective antigen) detection assay based on microwave-accelerated metal-enhanced fluorescence. Analytical biochemistry. 2012;425:54–61. doi: 10.1016/j.ab.2012.02.040. [DOI] [PubMed] [Google Scholar]
- FDA . 1996 Guidance for Industry: Q2B Validation of Analytical Procedures: Methodology. U.S. Department of Health and Human Services; Rockville, MD: C.f.D.E.a. Research. [Google Scholar]
- FDA . 2001 Guidance for Industry: Bioanalytical Method Validation. U.S. Department of Health and Human Services; Rockville, MD: p. 22. C.f.D. Evaluation (Ed.) [Google Scholar]
- Feldschuh J, Enson Y. Prediction of the normal blood volume. Relation of blood volume to body habitus. Circulation. 1977;56:605–12. doi: 10.1161/01.cir.56.4.605. [DOI] [PubMed] [Google Scholar]
- Gottschalk PG, Dunn JR. The five-parameter logistic: a characterization and comparison with the four-parameter logistic. Analytical biochemistry. 2005;343:54–65. doi: 10.1016/j.ab.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Jernigan DB, Raghunathan PL, Bell BP, Brechner R, Bresnitz EA, Butler JC, Cetron M, Cohen M, Doyle T, Fischer M, Greene C, Griffith KS, Guarner J, Hadler JL, Hayslett JA, Meyer R, Petersen LR, Phillips M, Pinner R, Popovic T, Quinn CP, Reefhuis J, Reissman D, Rosenstein N, Schuchat A, Shieh WJ, Siegal L, Swerdlow DL, Tenover FC, Traeger M, Ward JW, Weisfuse I, Wiersma S, Yeskey K, Zaki S, Ashford DA, Perkins BA, Ostroff S, Hughes J, Fleming D, Koplan JP, Gerberding JL, National Anthrax Epidemiologic Investigation, T. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis. 2002;8:1019–28. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintzer AF, Thoren KL, Sterling HJ, Dong KC, Feld GK, Tang II, Zhang TT, Williams ER, Berger JM, Krantz BA. The protective antigen component of anthrax toxin forms functional octameric complexes. Journal of molecular biology. 2009;392:614–29. doi: 10.1016/j.jmb.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobiler D, Weiss S, Levy H, Fisher M, Mechaly A, Pass A, Altboum Z. Protective antigen as a correlative marker for anthrax in animal models. Infect Immun. 2006;74:5871–6. doi: 10.1128/IAI.00792-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Soroka SD, Taylor TH, Jr., Stamey KL, Stinson KW, Freeman AE, Abramson DR, Desai R, Cronin LX, Oxford JW, Caba J, Pleatman C, Pathak S, Schmidt DS, Semenova VA, Martin SK, Wilkins PP, Quinn CP. Standardized, mathematical model-based and validated in vitro analysis of anthrax lethal toxin neutralization. Journal of immunological methods. 2008;333:89–106. doi: 10.1016/j.jim.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Logan NA, Hoffmaster AR, Shadomy SV, Stauffer KE. Bacillus and other aerobic endospore-forming bacteria. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. Manual of Clinical Microbiology. 10th Vol. 1. ASM Press; Washington, D.C.: 2011. [Google Scholar]
- Mabry R, Brasky K, Geiger R, Carrion R, Jr., Hubbard GB, Leppla S, Patterson JL, Georgiou G, Iverson BL. Detection of anthrax toxin in the serum of animals infected with Bacillus anthracis by using engineered immunoassays. Clin Vaccine Immunol. 2006;13:671–7. doi: 10.1128/CVI.00023-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marano N, Plikaytis BD, Martin SW, Rose C, Semenova VA, Martin SK, Freeman AE, Li H, Mulligan MJ, Parker SD, Babcock J, Keitel W, El Sahly H, Poland GA, Jacobson RM, Keyserling HL, Soroka SD, Fox SP, Stamper JL, McNeil MM, Perkins BA, Messonnier N, Quinn CP, Anthrax Vaccine Research Program Working, G. Effects of a reduced dose schedule and intramuscular administration of anthrax vaccine adsorbed on immunogenicity and safety at 7 months: a randomized trial. JAMA : the journal of the American Medical Association. 2008;300:1532–43. doi: 10.1001/jama.300.13.1532. [DOI] [PubMed] [Google Scholar]
- Moayeri M, Wiggins JF, Leppla SH. Anthrax protective antigen cleavage and clearance from the blood of mice and rats. Infect Immun. 2007;75:5175–84. doi: 10.1128/IAI.00719-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell MA, Belanger BA, Haaland PD. Calibration and assay development using the four-parameter logistic model. Chemometr Intel Lab. 1993;20:97–114. [Google Scholar]
- Oh BN, Lee S, Park HY, Baeg JO, Yoon MY, Kim J. Sensitive fluorescence assay of anthrax protective antigen with two new DNA aptamers and their binding properties. The Analyst. 2011;136:3384–8. doi: 10.1039/c0an00978d. [DOI] [PubMed] [Google Scholar]
- Palmateer NE, Hope VD, Roy K, Marongiu A, White JM, Grant KA, Ramsay CN, Goldberg DJ, Ncube F. Infections with spore-forming bacteria in persons who inject drugs, 2000-2009. Emerg Infect Dis. 2013;19:29–34. doi: 10.3201/eid1901.120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CP, Dull PM, Semenova V, Li H, Crotty S, Taylor TH, Steward-Clark E, Stamey KL, Schmidt DS, Stinson KW, Freeman AE, Elie CM, Martin SK, Greene C, Aubert RD, Glidewell J, Perkins BA, Ahmed R, Stephens DS. Immune responses to Bacillus anthracis protective antigen in patients with bioterrorism-related cutaneous or inhalation anthrax. J Infect Dis. 2004;190:1228–36. doi: 10.1086/423937. [DOI] [PubMed] [Google Scholar]
- Quinn CP, Semenova VA, Elie CM, Romero-Steiner S, Greene C, Li H, Stamey K, Steward-Clark E, Schmidt DS, Mothershed E, Pruckler J, Schwartz S, Benson RF, Helsel LO, Holder PF, Johnson SE, Kellum M, Messmer T, Thacker WL, Besser L, Plikaytis BD, Taylor TH, Jr., Freeman AE, Wallace KJ, Dull P, Sejvar J, Bruce E, Moreno R, Schuchat A, Lingappa JR, Martin SK, Walls J, Bronsdon M, Carlone GM, Bajani-Ari M, Ashford DA, Stephens DS, Perkins BA. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg Infect Dis. 2002;8:1103–10. doi: 10.3201/eid0810.020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi CA, Ulrich M, Norris S, Reed DS, Pitt LM, Leffel EK. Identification of a surrogate marker for infection in the African green monkey model of inhalation anthrax. Infect Immun. 2008;76:5790–801. doi: 10.1128/IAI.00520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova VA, Schiffer J, Steward-Clark E, Soroka S, Schmidt DS, Brawner MM, Lyde F, Thompson R, Brown N, Foster L, Fox S, Patel N, Freeman AE, Quinn CP. Validation and long term performance characteristics of a quantitative enzyme linked immunosorbent assay (ELISA) for human anti-PA IgG. Journal of immunological methods. 2012;376:97–107. doi: 10.1016/j.jim.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Stern EJ, Uhde KB, Shadomy SV, Messonnier N. Conference report on public health and clinical guidelines for anthrax. Emerg Infect Dis. 2008;14 doi: 10.3201/eid1404.070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Moayeri M, Chen Z, Harma H, Zhao J, Hu H, Purcell RH, Leppla SH, Hewlett IK. Detection of anthrax toxin by an ultrasensitive immunoassay using europium nanoparticles. Clin Vaccine Immunol. 2009;16:408–13. doi: 10.1128/CVI.00412-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JJ, Pesik N, Quinn CP, Urdaneta V, Dykewicz CA, Boyer AE, Guarner J, Wilkins P, Norville KJ, Barr JR, Zaki SR, Patel JB, Reagan SP, Pirkle JL, Treadwell TA, Messonnier NR, Rotz LD, Meyer RF, Stephens DS. A case of naturally acquired inhalation anthrax: clinical care and analyses of anti-protective antigen immunoglobulin G and lethal factor. Clin Infect Dis. 2007;44:968–71. doi: 10.1086/512372. [DOI] [PubMed] [Google Scholar]
- Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annual review of biochemistry. 2007;76:243–65. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]