Abstract
Objective
To determine MR and CT imaging features of low grade fibromyxoid sarcoma (LGFMS) in children.
Materials and methods
We retrospectively analyzed images of 11 pediatric patients with diagnosis of LGFMS from a phase III clinical trial of non-rhabdomyosarcoma soft tissue sarcoma (Children’s Oncology Group Protocol ARST0332). MRI and CT were performed in 10 and 4 patients, respectively. Location, size, margin, and composition on imaging were correlated to pathology.
Results
Tumors were located in extremities in 9 patients and one each in tongue and lung. Tumors were deep in 7 patients and superficial in 4. All tumors were well defined, solitary, and non-metastatic at presentation. Tumors were complex solid-cystic in 8 patients and completely solid in 3. On T1W images, all tumors had at least some areas hypointense to muscles, and 6 had a “split-fat” sign. On STIR/T2W images, 8 tumors had areas hypointense to adjacent muscle and 8 tumors had fluid signal intensity. On post-contrast MR studies, 8 tumors had thick enhancing internal septations, and 3 had peripheral nodular gyriform enhancement. Correlating to pathology, areas with hypointense signal intensity on both T1 and T2W images are likely related to fibrous component; areas with fluid signal intensity on T2W images are likely related to myxoid component. On CT imaging, all 4 tumors were hypodense to muscle and one tumor showed punctate calcific foci.
Conclusion
LGFMS is hypodense to muscle on CT imaging. MR imaging may identify both fibrous and myxoid components of this rare pediatric soft tissue sarcoma.
Introduction
Low grade fibromyxoid sarcoma (LGFMS) is a rare soft tissue sarcoma characterized by relatively benign histology with fibrous paucicellular and myxoid hypervascular zones (1). In the short term (< 5 years), LGFMS has an indolent clinical behavior but also a tendency for late local recurrence and metastasis (2). It is more common in young and middle aged adults but has increasingly been recognized in the pediatric population. Although in most cases it is localized to deep soft tissues of extremities, these tumors can be seen in superficial subcutaneous tissues. The latter location is more commonly seen in children relative to adults. On cytogenetic studies, these tumors have a t(7;16)(q34;p11) translocation (1). Cross-sectional imagaing, such as computed tomography (CT) and magnetic resonance imaging (MRI), has played a crucial role in the detection, evaluation of local extent, and staging of soft tissue tumors.
The Children’s Oncology Group (COG) recently completed a 5-year multi-institutional trial (ARST0332) to investigate a risk-based strategy for therapy of patents with non-rhadomyosarcoma soft tissue sarcoma (NRSTS) under 30 years of age. Of the 551 eligible patients enrolled, 18 subjects were diagnosed histologically to have LGFMS. Due to the rarity of this tumor, there is a paucity of literature describing its imaging features. The purpose of our study was to determine whether the CT and MR imaging features of LGFMS may suggest this diagnosis prior to biopsy in pediatric patients.
Material and Methods
The study cohort comprised patients with LGFMS who were enrolled on COG ARST0332 between February 2007 and February 2012. The protocol was HIPAA compliant and approved by Institutional Review Boards at each participating institution. All subjects and/or their guardians signed informed consent or assent as appropriate. All tumors were reviewed centrally by two expert pediatric pathologists (CC, DP) to confirm the histologic subtypes of NRSTS. Of the 18 subjects with LGFMS, 11 underwent MR and/or CT imaging of the primary tumor prior to tumor resection. MR and CT images of these 11 subjects were reviewed retrospectively by a study radiologist central reviewer (SCK) and a pediatric radiology fellow (SK). MRI imaging was obtained in 10 subjects (Table 1), three of whom also underwent contrast-enhanced multidetector CT with coronal and sagittal reconstructions. One subject underwent only CT imaging of the primary tumor. Demographics, clinical data, and pathology reports were reviewed and pathology findings were correlated with imaging findings to the extent possible. Clinical and imaging evidence for local recurrence and metastasis were sought during a 5-year planned follow-up period.
Table 1.
Combined MR and CT imaging Features in 11 Pediatric Low-grade Fibromyxoid Sarcomas
| Features | Findings | Number (%) |
|---|---|---|
| Shape | Round to oval | 11 (11/11, 100%) |
| Morphology | Complex solid cystic | 8 (8/11, 73%) |
| Solid | 3 (3/11, 27%) | |
| Location | Intramuscular | 6 (6/11, 55%) |
| Subcutaneous | 4 (4/11, 36%) | |
| Deep (lung) | 1 (1/11, 9 %) | |
| Lower extremity | 5 (5/11, 45%) | |
| Upper extremity | 4 (4/11, 36%) | |
| Tongue muscles | 1 (1/11, 9%) | |
| Diameter (cm) | Range | 2–12 |
| Median | 6 | |
| Margin | Circumferentially Well defined | 9 (9/11, 82%) |
| Relatively well defined | 1 (1/11, 9 %) | |
| Infiltrating | 1 (1/11, 9 %) | |
| Involvement of adjacent structures | Bone | 1 (1/11, 9 %) |
| Skin | 1 (1/11, 9 %) | |
| Vessels | 2 (2/11, 18 %) |
MR imaging
The standard MR protocol included precontrast T1-weighted and fat-saturated T2-weighted or STIR axial and coronal, precontrast T1-weighted fat-saturation axial, and postcontrast fat-saturation T1-weighted axial and coronal sequences. Additional sagittal images were acquired in some subjects for better demonstration of tumor extent.
On MR images, the radiologist reviewers (CSK and SK) assessed the anatomic location, size, shape, margin, homogeneity, and signal intensity patterns on T1 and STIR /T2 weighted fat-saturated sequences, contrast enhancement patterns on T1 weighted fat-saturated sequences, peritumoral edema, hemorrhage, necrosis, and depth of involvement (skin, muscle, bone, joint, and neurovascular invasion). Tumor dimensions were measured in the cephalocaudal, transverse, and anteroposterior planes.
Four terms were used to visually classify the tumor margin:
“Circumferentially well-defined” when we were able to appreciate well demarcated margins in over 90 percent of tumor surface;
“Relatively well- defined” with well demarcated margins seen in 70 to 90 percent of tumor surface;
“Discrete mass with poorly-defined margins” where well demarcated margins were seen in less than 70 percent of tumor surface and there was no infiltration of surrounding structures;
“Infiltrating margins” when tumor was clearly seen invading adjacent structures.
Tumor signal homogeneity was classified subjectively on both T1- and T2-weighted images as:
“Homogenous” when more than 95 percent of the tumor volume showed uniform signal intensity;
“Mildly inhomogeneous” when 75–95 percent of tumor volume showed uniform signal intensity;
“Moderately inhomogeneous” when 50–75 percent of the tumor volume showed uniform signal intensity;
“Complex” when less than 50 percent of the tumor volume showed uniform signal intensity.
On T1-weighted MR images, we classified signal intensity of tumor components into four categories:
“Hypointense” in areas where tumor had signal intensity less than adjacent muscles;
“Isointense” in areas with signal intensity the same as muscles;
Areas with signal intensity brighter than muscles but less bright than subcutaneous fat;
Areas with signal intensity equal to fat.
On STIR or T2-weighted fat-saturated images, we classified the signal intensity of tumor components into 4 categories:
“Hypointense” in areas with signal intensity less than adjacent muscles;
“Isointense” in areas with signal intensity the same as muscles;
Areas brighter than muscles but less bright than fluid;
Areas with signal intensity as bright as fluid.
A “cystic” mass was defined as having homogeneous hypointense signal on T1W images and homogeneous hyperintense signal, similar to fluid on T2W images, with no enhancing component on post-contrast images. A “complex cystic” mass was defined as having predominantly cystic signal with some enhancing solid components or internal septations. A “solid” mass was defined as having heterogeneous (predominantly iso- to hypointense) signals on T1W images and heterogeneous (iso- to hyperintense, but less than fluid) signal on T2W images with enhancement on post-contrast images.
We also evaluated contrast enhancement in tumors by comparing pre- and post-contrast T1-weighted fat-saturated images. Tumors with peritumoral regions having T1 hypointense and T2 hyperintense signals were designated to exhibit peritumoral edema. Areas having T1 hyperintense signal and T2 hyperintense or T2 hypointense signal that did not suppress on fat-saturated images were characterized as internal hemorrhage. Non-enhancing areas within the tumor were characterized as “necrosis.” We also evaluated invasion of surrounding structures such as muscles, vessels, bones, nerves, and joints.
CT imaging
The anatomic location, size, shape, margin, homogeneity, and density of tumors were assessed on contrast-enhanced CT images (Table 2). We categorized density of the tumor as
“Hypodense”: less than adjacent muscle;
“Isodense”: equal to muscle;
“Hyperdense”: more than adjacent muscle.
Table 2.
MR Imaging Features in 10 Pediatric Low-grade Fibromyxoid Sarcomas
| Pre-Contrast (predominant signal intensity) | |||
|---|---|---|---|
| Sequence | Hypointense N (%) |
Isointense N (%) |
Hyperintense N (%) |
| T1W | 3 (3/10, 30%) | 6 (6/10, 60%) | 1 (1/10, 10%) |
| STIR / T2 W fat sat | 1 (1/10 10%) | 5 (5/10, 50%) | 4(4/10, 40%) |
| Post contrast | |||
| Degree of enhancement | N (%) | ||
| Minimal | 3 (3/10, 30%) | ||
| Moderate | 7 (7/10, 70 %) | ||
| Intense | 0 | ||
| Pattern of Enhancement | N (%) | ||
| Homogenous | 0 | ||
| Heterogenous | 7 (7/10, 70 %) | ||
| Peripheral nodular gyriform | 3 (3/10, 30%) | ||
Areas of calcification and hyperdense hemorrhage within the tumor and the presence and pattern of contrast enhancement were recorded.
Pathological review
In this study, the gross specimen were not available for radiologic-pathologic correlation. All tumors were however reviewed centrally by two expert pediatric pathologists to confirm the histologic subtypes of LGFMS. The gross and microscopic pathology features of LGFMS were obtained from reports issued by local institutions.
Results
Subjects ranged in age from 3 to 18 years with a median of 13 years. Male to female ratio was 2.6:1. All 11 LGFMS were non-metastatic at presentation. There was no evidence for local recurrence or metastasis in our subjects on short-term follow up of 0.5 to 6 years (median 2.7 years). Table 1 summarizes the combined MRI and CT findings. By MR/CT imaging, nine tumors were well circumscribed (>70% tumor surface). LGFMS was located in the lower extremity in 5 patients, the upper extremity in 4, and one each in the tongue muscles and the lung. There was involvement of the pelvic region in two of the three lower extremity tumors. Of the four upper extremity tumors, three were located in the region of the shoulder girdle and one in the upper arm. Two unusual locations were the lung (Fig. 1) and tongue muscles (Fig. 2). On MR/CT imaging, the maximum diameter of tumor varied from 2 to 12 centimeters with a median of 6 cm. In 9 LGFMS, the margin was circumferentially well-defined and one of the tumors was relatively well-defined. Infiltrating margins were seen in one of the tumors involving muscles of the shoulder (Fig. 3).
Figure 1. 12 year old boy with LGFMS arising in the lung.
Axial CT image of the chest with lung window shows a hypodense (compared to muscles on mediastinal window) tumor in the anterior segment of left lower lobe.
Figure 2. 10 year old girl with left tongue mass.
(A) Axial T1 weighted image of the neck shows a predominantly hypointense mass involving the tongue muscles on the left side (arrow). (B) Axial contrast enhanced T1 weighted fat saturation image shows heterogeneous peripheral nodular gyriform enhancement of the mass. (C) Axial contrast enhanced CT image of the face shows mild enhancement of the hypodense mass in the muscles of the tongue on the left side. Note the tiny calcific focus along the medial aspect of the mass (arrow).
Figure 3. 3 year old boy with a left shoulder tumor.
Sagittal contrast enhanced T1 weighted fat saturation of the left shoulder reveals moderate peripheral nodular gyriform enhancement of the tumor with a non-enhancing central component, mostly from fibrosis (hypointense on T2W image, not shown). Notice that the mass is invading the scapula with extension anterior to the scapula (arrow).
MR imaging (10 subjects)
None of the LGFMS appeared uniformly homogenous on T1 and T2 weighted images. Two tumors showed mild inhomogeneity, four showed moderate inhomogeneity, and four had a complex appearance.
On T1-weighted images, all LGFMS had at least some areas with signal intensity less than adjacent muscle and equal to cortical bone. In six tumors (Table 2), the predominant signal intensity was isointense to muscle (Fig. 4). In three, the predominant signal intensity was hypointense to muscles but more than that of the cortical bone (Fig. 5). One of the tumors showed predominant signal intensity greater than muscle but less than that of the subcutaneous fat. Five of the six tumors located in the intramuscular compartment of the extremities showed a “split fat” sign (Fig. 5). None of the tumors showed hemorrhagic components.
Figure 4. 12 year old boy with a right elbow tumor.
(A) Axial T1 weighted image of the right elbow shows a slightly hyperintense mass in the biceps muscle with hypointense areas (arrow) along the lateral aspect of the mass. (B) Axial T2 weighted fat saturation image reveals a solid predominantly T2 hyperintense mass in the biceps muscle with few internal hypointense areas (arrow). (C) Axial contrast enhanced T1 weighted fat saturation image shows moderate heterogeneous enhancement of the tumor with less enhancement of the T2 hypointense area shown in Fig. 4B, consistent with fibrosis.
Figure 5. 15 year old girl with a thigh tumor.
(a) Coronal T1 weighted image of the right femur shows a predominantly hypointense solid tumor involving the right ilio-psoas muscle. Notice the “split fat” sign along the lateral aspect of the mass (arrow). (b) Coronal T2 weighted fat saturation image of the right femur demonstrates that the tumor is predominantly hypointense to the surrounding muscles. Notice that the tumor is abutting the right common femoral vessels. (c) Contrast-enhanced coronal T1 weighted fat saturation image of the right femur shows moderate heterogeneous peripheral nodular enhancement of the tumor. Note that areas that are hypointense on T1 and T2W sequences enhance less than other areas, consistent with fibrosis.
On STIR/T2-weighted fat-saturated images, 8 LGFMS had at least some hypointense areas (Table 1). Eight tumors had areas of fluid signal intensity. Four tumors had predominant signal intensity equal to fluid (Fig. 6). In three tumors, the predominant signal intensity was equal to muscle (Fig. 5). Of the three completely solid tumors, 2 were isointense and one hypointense to muscle on fluid sensitive sequences. Peritumoral edema (Fig. 5) was present in 7 cases. Thick (> 3 mm) internal septations were seen in four tumors.
Figure 6. 12 year old boy with a right groin mass.
(a) Axial T1 weighted image of the pelvis shows a hypointense mass in the subcutaneous plane of the right groin (arrow). (b) On an axial T2 weighted fat saturation image, the mass shows almost entirely fluid signal intensity. Notice the T2 hypointense nodular component along the posterior margin of the mass (arrow). (c) Axial contrast enhanced T1 weighted fat saturation image shows peripheral enhancement with a non-enhancing central component in this complex cystic mass. Notice the nodular enhancement along the posterior margin of the mass (arrow). Synovial sarcoma should be considered in the differential diagnosis of soft tissue tumors with a cystic appearance.
On post-contrast T1 weighted fat-saturated images, all 10 LGFMS revealed mild to moderate inhomogeneous contrast enhancement. Thick enhancing internal septations were seen in four. Areas having T1-and T2 hypointensity and areas with fluid signal on STIR/fat-saturated T2W images showed variable enhancement. In three tumors having a completely solid appearance, peripheral nodular gyriform enhancement was seen (Figs. 2, 3).
Bone involvement of the scapula was seen in association with a tumor arising from the infraspinatus muscle (Fig. 3). Skin involvement was seen in one tumor in the inguinal-scrotal region. Vascular encasement involving small branches of major extremity vessels was seen in two tumors located in the deep muscular compartment. None of the tumors showed joint or obvious nerve encasement.
CT imaging (4 patients)
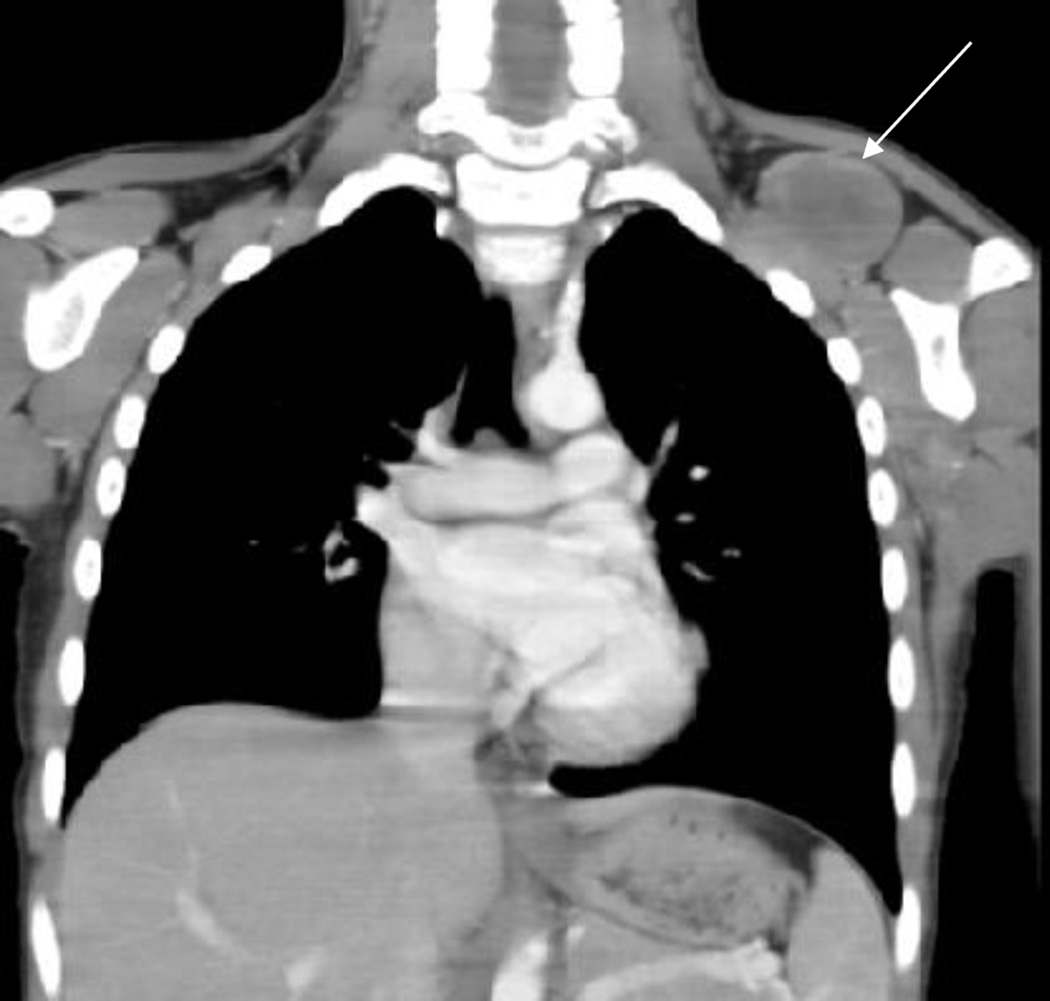
On contrast-enhanced CT imaging, all the 4 LGFMS were hypodense to adjacent muscles. Tumors were located in the subcutaneous plane in two subjects, in the lung parenchyma in one, and in the tongue muscles in one. All 4 tumors showed mild inhomogeneous contrast enhancement (Figs. 2 & 7). The tumor involving the tongue had internal punctate calcific foci (Fig. 2).
Figure 7. 10 year old boy with a left shoulder tumor.
Coronal contrast enhanced CT scan image reveals a slightly hypodense mass in the left supraclavicular region with a thin peripheral rim that is isodense to the surrounding muscles (arrow). Histologic examination shows a solid mass without cystic or necrotic changes.
Pathologic review (11 patients)
Because gross tumor specimens were not available for central pathology review, pathology reports from the local institutions of the excised tumors were reviewed. All tumors were described as well circumscribed, round to oval, and grey-white with a whorled appearance without hemorrhage. The maximum diameter of tumor by report varied from 2 to 12 centimeters with a median of 6 cm. On microscopy, tumors showed two distinct zones -- a paucicellular fibrous zone and a hypervascular myxoid zone with abrupt transition between these zones. Some tumors contained hyalinizing spindle cells with giant rosettes. Fascicular and whorled arrangements were noted in all tumors. In most tumors, mitotic figures were less than 10 per 10 high power fields and cellular atypia was rare. No areas of necrosis or hemorrhage were identified. This finding correlated with the lack of high density hemorrhage on MRI and CT imaging. Cytogenetic studies were performed in 5 tumors, all of which showed the characteristic t(7;16)(q34;p11) translocation. The resection margin was negative in all patients except in one tumor arising from the left shoulder.
Discussion
LGFMS was first described in 1987 and was initially thought to exhibit bland histological features but aggressive clinical behavior. Subsequently many studies revealed that LGFMS usually has a relatively benign course and occurs most commonly in young and middle aged adults. An increasing number of cases have been reported in pediatric patients (1,3).
On histology, these tumors have a low mitotic rate, two distinct zones (myxoid and fibrous), bland regular spindle cells, and a swirling or whorled pattern (2). Myxoid zones are typically hypercellular with prominent vascularity while fibrous zones are hypocellular (1,2). Transition between myxoid and fibrous zones may be gradual or abrupt (1). Hyalinized spindle cell tumor with giant rosettes [HSTGR] is now considered to be a variant of LGFMS that also shows collagen rosettes (1,3,4). Cytogenetic studies show t[7;16] [q34;p11] resulting in a chimeric fusion protein derived from the FUS gene on chromosome 16p11 and the BBF2H7 gene on 17q33 in both LGFMS and HSTGR (1). All LGFMS are positive for Muc-4, an immunohistochemical marker that is very sensitive for the diagnosis of this tumor (5). LGFMS are also positive for vimentin, actins, CD 68, and EMA (epithelial membrane antigen) while negative for CD34, keratins, and S-100 protein (1).
LGFMS are commonly located in the lower extremity, shoulder, trunk, inguinal regions, upper extremity, and vulvovaginal regions (1–3). In our study, the majority of tumors were seen in the lower and upper extremities including the shoulder. Two unusual locations in our study were in the lung parenchyma and tongue musculature. LGFMS is commonly deep and intramuscular in young and middle aged adults. In the pediatric population, a superficial subcutaneous location has been reported to be more common (37%) compared to <10% in large series of including all ages) (1). In our pediatric cohort, deep intramuscular (6/11, 55% of cases) and subcutaneous locations (4/11, 36% of cases) were observed. The reported median size of tumor ranges from 4.2 and 9.4 cm, which is in agreement with a median size of 6 cm seen in our study.
On imaging, LGFMS are solitary and well circumscribed at presentation but they tend to present as multiple infiltrating masses at recurrence (6). All of the tumors in our study were solitary at presentation and the majority of them was well circumscribed and round to oval in shape.
On MRI, LGFMS appears heterogeneous owing to two distinct internal zones: myxoid and fibrous. LGFMS has been reported to show areas of low signal intensity on both T1 and T2W sequences due to the fibrous component (7–11). In our study, the majority of the tumors revealed a heterogeneous appearance on T1- and T2- weighted images. In all tumors, we found internal components that were hypointense to muscle on T1-weighted images. In agreement with prior reports, we found that the majority of T1 hypointense foci (8/10, 80%) were also hypointense on T2-weighted images, likely corresponding to fibrous tissue. On CT imaging, LGFMS have low attenuation components that are hypodense to the skeletal muscle, likely due to the myxoid component and extracellular matrix (6,8,10–11). Though calcification has been reported in these tumors, it is not very common (6,7,11,14) and was seen in only one tumor in our study.
We observed a complex solid-cystic appearance in the majority (9/11, 80%) of tumors in our study. The complex solid-cystic appearance is probably related to the histology of the tumor, in which myxoid zones appear cystic and fibrous zones appear as solid nodular or septated areas. Somewhat similar imaging appearance in the form of intralesional nodules with hyperintense signal had previously been reported in LGFMS (6). A few tumors in our study (3/11, 27%) were entirely solid but no tumor was entirely cystic.
The “split fat sign” on T1 weighted images was seen in 50% (5/10) of our subjects who had an intramuscular tumor. None of the prior studies or case reports has reported this finding in LGFMS. The “split fat sign” (best appreciated on T1W images) represents a rim of fat surrounding a tumor, such as a peripheral nerve sheath tumor, that is located in the intermuscular space. LGFMS are considered to be malignant fibroblastic neoplasms. It is not surprising that LGFMS that occurs in muscles may exhibit this sign.
On fluid sensitive sequences, a distinct gyriform pattern with multiple folded layers of predominantly low signal intensity areas mimicking brain gyri was reported in a prior study (6). We observed a similar gyriform pattern in two tumors that were completely solid. Peritumoral edema was seen in 70% (7/10) of our subjects on T2-weighted images. This imaging feature has not previously been described. In our study, vascular encasement of small branches of major extremity vessels was seen in two subjects, and bone and skin invasion were observed in two different subjects. Though areas of internal hemorrhage have been reported in LGFMS (13), no tumor in our study demonstrated imaging evidence of hemorrhage.
On T1 weighted fat-saturated post-contrast studies, LGFMS have been reported to show heterogeneous contrast enhancement reflecting the two distinct internal zones with varying vascularity (6,12). All tumors in our study showed heterogeneous contrast enhancement. Tumors having a complex solid- cystic appearance showed either enhancing solid components or enhancing thick (>3 mm) internal septations. As on the fluid sensitive sequences, the two tumors that were entirely solid demonstrated a gyriform enhancement pattern on post-contrast T1 weighted fat-saturated images. Areas with hypointense signal on T1-and T2-weighted images showed variable contrast enhancement from minimal to intense. This finding may be a reflection of the degree of fibroblastic activity versus frank fibrosis in these tumors similar to that observed in desmoid tumors (15). Areas with fluid signal intensity on STIR/T2-weighted images showed variable enhancement likely related to the degree of vascularity of the myxoid component of the tumor.
Differential diagnoses for LGFMS of the extremity and trunk in the pediatric population include rhabdomyosarcoma, synovial sarcoma, Ewing sarcoma family tumors, nerve sheath tumors, and solitary fibrous tumor. Apart from some cystic synovial sarcoma, these tumors usually appear solid on imaging and demonstrate heterogeneous contrast enhancement. Rhabdomyosarcoma, myxoid neurofibroma, primitive neuroectodermal tumors, and synovial sarcoma can be distinguished from LGFMS by the absence of fibrous tissue and therefore, lack of T1 and T2 hypointense areas. Solitary fibrous tumors can mimic LGFMS although they lack the T2 bright myxoid component.
Studies with short term follow up have shown that if widely excised, these tumors have relatively low local recurrence (9%) and metastatic (6%) rates. These studies also report that the local recurrence rate of superficial tumors is the same as deep tumors, although metastases occurred less frequently with superficial tumors (1,3). In contrast, prior studies with long term follow up have shown that LGFMS has high local recurrence (68%) and metastatic (41%) rates (2). Because these tumors have an indolent nature, they may recur up to 15 years after completion of therapy with a median time to recurrence of about 3.5 years (2). Lung, pleura, and chest wall are the commonly reported sites of metastasis followed by bone and liver (2). Neither local recurrence nor metastasis was found in our subjects on short term follow up of 0.5 to 6 years (median 2.7 years). Since LGFMS may recur many years after treatment, long term follow up is recommended.
Limitations of our study included a relatively small sample despite a five-year data collection period from all COG institutions, underscoring the rarity of this cancer in children and young adults. Due to the nature of data collection for this study, an exact spatial correlation between imaging and gross pathology appearances of the tumor was not possible. However, local pathology descriptions of the excised tumors provided valuable information regarding tumor composition, which could be correlated to imaging features. Another study limitation is the relatively short-term Follow up of our subjects.
Conclusion
Low grade fibromyxoid sarcoma is a rare soft tissue neoplasm that is increasingly being recognized in pediatric populations. MR imaging features of a complex solid-cystic tumor with fibrotic tissue that appears hypointense to muscle on T1and T2-weighted images and exhibits a variable degree of enhancement, can be very helpful in suggesting this diagnosis before biopsy. Solid-cystic lesions show enhancement of solid components or thick internal septations. Tumors that are predominantly solid may show peripheral nodular gyriform enhancement. Areas with fluid signal intensity on STIR/T2-weighted images may show variable enhancement likely related to the vascularized myxoid component of the tumor. In our study, all tumors underwent complete surgical resection with negative margins (except in one) and without evidence of local recurrence or metastasis on short-term follow up.
Acknowledgments
Supported by CA98543, U10 CA98413
References
- 1.Billings SD, Giblen G, Fanburg-Smith JC. Superficial low-grade fibromyxoid sarcoma (Evans tumor): a clinicopathologic analysis of 19 cases with a unique observation in the pediatric population. Am J Surg Pathol. 2005;29(2):204–210. doi: 10.1097/01.pas.0000146014.22624.8e. [DOI] [PubMed] [Google Scholar]
- 2.Evans HL. Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am J Surg Pathol. 2011;35(10):1450–1462. doi: 10.1097/PAS.0b013e31822b3687. [DOI] [PubMed] [Google Scholar]
- 3.Folpe AL, Lane KL, Paull G, Weiss SW. Low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumor with giant rosettes: a clinicopathologic study of 73 cases supporting their identity and assessing the impact of high-grade areas. Am J Surg Pathol. 2000;24(10):1353–1360. doi: 10.1097/00000478-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Guillou L, Benhattar J, Gengler C, Gallagher G, Ranchere-Vince D, Collin F, et al. Translocation-positive low-grade fibromyxoid sarcoma: clinicopathologic and molecular analysis of a series expanding the morphologic spectrum and suggesting potential relationship to sclerosing epithelioid fibrosarcoma: a study from the French Sarcoma Group. Am J Surg Pathol. 2007;31(9):1387–1402. doi: 10.1097/PAS.0b013e3180321959. [Evaluation Studies Research Support, Non-U S Gov't]. [DOI] [PubMed] [Google Scholar]
- 5.Doyle LA, Moller E, Dal Cin P, Fletcher CD, Mertens F, Hornick JL. MUC4 is a highly sensitive and specific marker for low-grade fibromyxoid sarcoma. Am J Surg Pathol. 2011;35(5):733–741. doi: 10.1097/PAS.0b013e318210c268. [DOI] [PubMed] [Google Scholar]
- 6.Hwang S, Kelliher E, Hameed M. Imaging features of low-grade fibromyxoid sarcoma (Evans tumor) Skeletal Radiol. 2012;41(10):1263–1272. doi: 10.1007/s00256-012-1417-2. [DOI] [PubMed] [Google Scholar]
- 7.Arnaoutoglou C, Lykissas MG, Gelalis ID, Batistatou A, Goussia A, Doukas M, Xenakis TA. Low grade fibromyxoid sarcoma: a case report and review of the literature. J Orthop Surg Res. 2010;5(49):5–49. doi: 10.1186/1749-799X-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SY, Kim MY, Hwang YJ, Han YH, Seo JW, Kim YH, Hur G. Low-grade fibromyxoid sarcoma: CT, sonography, and MR findings in 3 cases. J Thorac Imaging. 2005;20(4):294–297. doi: 10.1097/01.rti.0000171420.81428.16. [Case Reports]. [DOI] [PubMed] [Google Scholar]
- 9.Lee BJ, Park WS, Jin JM, Ha CW, Lee SH. Low grade fibromyxoid sarcoma in thigh. Clin Orthop Surg. 2009;1(4):240–243. doi: 10.4055/cios.2009.1.4.240. [Case Reports]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin J, Wang J, Yu LJ, Ying XL. Low-grade fibromyxoid sarcoma: a clinicopathological analysis of 9 cases. Zhonghua Bing Li Xue Za Zhi. 2009;38(5):302–306. [English Abstract]. [PubMed] [Google Scholar]
- 11.Maeda E, Ohta S, Watadani T, Goto A, Nakajima A, Ohtomo K. Imaging findings of thoracic low-grade fibromyxoid sarcoma: report of three cases. Jpn J Radiol. 2009;27(9):375–380. doi: 10.1007/s11604-009-0351-2. [Case Reports]. [DOI] [PubMed] [Google Scholar]
- 13.Kim SK, Jee WH, Lee AW, Chung YG. Haemorrhagic low-grade fibromyxoid sarcoma: MR findings in two young women. Br J Radiol. 2011;84(2011):e146–e150. doi: 10.1259/bjr/21441528. [Case Reports]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyake M, Tateishi U, Maeda T, Arai Y, Seki K, Hasegawa T, Sugimura K. CT and MRI features of low-grade fibromyxoid sarcoma in the shoulder of a pediatric patient. Radiat Med. 2006;24:511–514. doi: 10.1007/s11604-006-0057-7. [Case Reports]. [DOI] [PubMed] [Google Scholar]
- 14.Torriani M, Etchebehere M, Amstalden EM, Ouellette H. Magnetic resonance imaging of low-grade fibromyxoid sarcoma: Clinics (Sao Paulo) 2006 Jun;61(3):267–270. doi: 10.1590/s1807-59322006000300012. Epub 2006 Jun 30. [DOI] [PubMed] [Google Scholar]
- 15.McCarville M Beth, Hoffer Fredric A, Adelman C Scott, Khoury Joseph D, Li Chenghong, Skapek Stephen X. MRI and biologic behavior of desmoid tumors in children. AJR. 2007;189:633–640. doi: 10.2214/AJR.07.2334. [DOI] [PubMed] [Google Scholar]