Abstract
Background
The prevalence of allergic respiratory disease tends to increase in populations that adopt the so-called Westernized lifestyle. We investigated the association between atopy and several possible lifestyle-related factors in seven Danish population-based studies.
Methods
A total of 20048 persons participated in the seven studies. We used logistic regression to analyse the associations between possible determinants and atopy defined as serum specific IgE or skin prick test positivity against inhalant allergens. Associations were expressed as odds ratios (ORs) with 95% confidence intervals (95% CIs). In addition, individual participant data meta-analyses were performed.
Results
Atopy was significantly associated with younger age (OR per 1 year increase in age: 0.97; 95% CI: 0.97, 0.98); male sex (OR for males versus females: 1.34; 95% CI: 1.24, 1.45), heavy drinking (OR for heavy drinkers versus light drinkers: 1.15; 95% CI: 1.04, 1.27), never smoking (OR for current versus never smokers: 0.73; 95% CI: 0.67, 0.80), and higher educational level (OR for educated versus uneducated: 1.27; 95% CI: 1.15, 1.41). Atopy was not associated with blood pressure, serum total cholesterol, physical activity or body mass except in women only, where we found a positive association (OR for obese vs. normal weight: 1.18; 95% CI: 1.00, 1.39) with ptrend = 0.032.
Conclusions
Of interest for preventive purposes, we found that atopy was associated with some of the reversible lifestyle-related factors that characterize a Westernized lifestyle.
Introduction
The prevalence of allergic respiratory disease such as allergic rhinitis and allergic asthma has increased over recent decades in countries living a Westernized, urbanized, and affluent lifestyle [1,2]. However, the causes of this increase are largely unknown [3]. The rapid rise in the prevalence of allergic respiratory disease is not likely to be explained by changes in genetic factors but rather by changes in environmental factors [4]. Although the environment clearly influences the risk of allergic respiratory disease, there has been increasing attention to lifestyle factors as possible determinants of allergic respiratory disease. For example, there is evidence that obesity is associated with the development of asthma [5–9], but the association with allergic sensitization and the possible mechanisms are not clear [10–12]. Although cholesterol is believed to promote allergic inflammation in rodents [13], few adult studies have addressed this association [4,14]. Likewise, alcohol is a strong immune modulating factor and alcohol consumption has been found to increase serum total IgE levels, but the role of the raised total IgE levels in IgE-mediated allergic reactions and IgE-mediated allergic respiratory disease is not clear [15–20]. Smoking has been suggested to increase the risk of allergic symptoms by increased inflammation in the airways, but it is still unresolved whether smoking confers an increased or a decreased risk of allergy [21].
Many of the previous studies on lifestyle-related factors of allergic respiratory disease have mainly been performed in children or adolescents, have not used objective measures of allergic respiratory disease, or been single-study analyses with limited power to detect associations. In seven Danish population-based studies of adults from different time periods, we investigated the association between several potential lifestyle-related factors of atopy, i.e. serum total cholesterol, systolic blood pressure, smoking habits, alcohol intake, leisure time physical activity, body mass index, age, gender and education, and atopy, defined as specific IgE or skin prick test positivity against inhalant allergens that are both well-accepted objective biomarkers of allergic respiratory disease.
Materials and Methods
Ethics statement
All the included studies were approved by the Ethics Committee of Copenhagen and the Danish Data Protection Agency. We followed the recommendations of the Declaration of Helsinki, and each participant gave informed written consent.
Study populations
We used the seven population based studies: Monica1, Inter99, Health2006, the 1936-cohort, Allergy98, Health2008 and Health2010. Participants were recruited from the Danish Central Personal Register as random samples of the background population living in the Western part of the Copenhagen Region [22]. All studies included comprehensive questionnaire and interview data as well as clinical and biochemical data [22].
The Monica1 study took place from 1982 to 1984. A total of 4,807 persons aged 30, 40, 50 and 60 years old were invited, 3,785 persons participated, and thus the participation rate was 79% [22].
The Health2010 study was conducted between 2010 and 2012 where 3732 persons between 18 and 69 years of age were invited. A total of 1522 persons participated which meant a 40.5% participation rate [23].
A total of 12,934 persons between 30 and 60 years of age were invited to the Inter99 study that took place between 1999 and 2001. The study was a population-based intervention study (CT00289237, ClinicalTrials.gov) that examined the effects of lifestyle intervention on the incidence of cardiovascular disease [24,25]. Data from the baseline examination before intervention was used in the present analyses. A total of 6,784 persons participated, and thus the participation rate was 52.5% [25]. The Health2006 study was conducted between 2006 and 2008. A total of 7,931 persons from 18 to 69 years of age were invited, and 3,471 (participation rate 43.8%) persons were examined [26].
The Health2008 study was conducted between 2008 and 2009 [22,27,28]. A total of 2218 persons 30–60 years of age were invited. Pregnant women, persons with known diabetes, chronic obstructive pulmonary disease, cardiovascular disease, hypertension, a history of blood clots, or unable to participate in physical activities such as climbing stairs were excluded from the study. Thus, a total of 795 participated (participation rate 36%).
The Copenhagen Allergy study that included two groups of persons began in 1990. The first group was randomly selected from the general population, and the other group included persons with allergic respiratory symptoms that were chosen by a screening questionnaire from a random sample of the general population. In the present study, we used data from the follow-up study in 1997–1998 (referred to as ‘Allergy98’). In the Allergy98 study, 1,966 persons aged 15–77 years with Danish nationality were invited, and 1,216 (participation rate 61.9%) participated [29].
The 1936-cohort study took place in 1976–1977, where 1,200 randomly selected persons born in 1936 (40 years of age at the time) were invited to a health examination that focused on cardiovascular risk. A total of 1,052 persons were examined, and the participation rate was 87.7% [30,31].
A total of 20048 persons participated in the seven studies. We excluded 2654 persons that had no data on specific IgE measurements or skin prick test and 249 persons that had participated in one of the former studies. Thus, we included a total of 17145 persons from the seven studies (Monica1: 3481, 1936-cohort: 989, Allergy98: 1172, Inter99: 5961, Health2006: 3246, Health2008: 792, Health2010: 1504). Please also see the S1 Fig and S1 Table.
Covariates
The questionnaires provided data on the covariates education (no education beyond basic [basic education includes primary and lower secondary education for nine or ten years], education including students); physical activity during leisure time (sedentary, light, or moderate/vigorous); alcohol consumption (abstinent: 0, light drinkers >0–7, moderate drinkers >7–14, or heavy drinkers >14 drinks per week); and smoking habits (never smokers, former smokers, current smokers). Year of birth was divided into decades (nineteen twenties, nineteen thirties, nineteen fifties, nineteen sixties or nineteen seventies and above) except for the last group that contained persons born in the nineteen seventies, nineteen eighties, and nineteen nineties.
Height and weight were measured, and body mass index (BMI) was calculated as weight divided by height squared, expressed in kg/m2 and classified as (underweight: <18.5, normal weight: 18.5-<25, overweight: 25-<30, and obese: ≥30 kg/m2). Serum total cholesterol in mmol/l and systolic blood pressures in mmHg were used as continuous variables.
The number of missing values was: education (Nmissing = 298); physical activity during leisure time (Nmissing = 205); smoking habits (Nmissing = 332); alcohol consumption (Nmissing = 735); BMI (Nmissing = 7); total cholesterol (Nmissing = 13); and systolic blood pressure (Nmissing = 38) giving a total of 1317 persons (7.7%) with a missing value in at least one of the covariates. S2 Table shows the baseline characteristics of the participants with no missing variables.
Atopy
Atopy was defined by determination of serum specific IgE or skin prick test positivity to inhalant allergens as described in previous studies [32–35]. In the 1936-cohort and the Monica1 study, serum specific IgE positivity was tested by the ADVIA Centaur Allergy Screen assay (Bayer HealthCare Diagnostics division, Tarrytown, N.Y., USA) [36]. This is a multi-allergen assay to detect specific serum IgE antibodies to 19 different common inhalant allergens. Atopy was defined as a positive result according to the manufacturer’s instructions.
In the Health2006, Health2008 and Allergy98 studies, we used the ADVIA Centaur sIgE assay (Bayer Corporation, New York, NY) to test serum specific IgE to mite (Dermatophagoides [D.] pteronyssinus), grass, cat, and birch (In the Allergy98 study in addition dog and mugwort) [37]. In the Inter99 study, serum samples were analyzed for specific IgE to mite (D. pteronyssinus), grass, cat, and birch by the IMMULITE 2000 Allergy Immunoassay System [38]. In the Allergy98, the Inter99, the Health2006, and the Health2008 study, the specific IgE analysis was positive if the measurement was ≥0.35 kU/l. Specific IgE positivity, atopy, was defined as one or more positive tests for specific IgE against the tested allergens.
In the Health2010 study, skin prick testing (SPT) was performed by the Soluprick SQ® (ALK Abelló, Hørsholm, Denmark) system. The procedure was performed using lancets and a standard panel of aeroallergens comprising birch, grass (Phleum pratense) mugwort, horse, cat, dog, two house dust mites (Dermatophagoides pteronyssinis and D. farinae), and two moulds (Cladosporium herbarum and Alternaria alternata). A negative control and a positive control (10 mg/mL histamine) were also included. The mean wheal diameter was calculated as the average of the widest diameter and the perpendicular bisector. A positive SPT was defined as a mean wheal diameter ≥3 mm. Atopy was defined as a positive reaction to at least one of ten allergens.
Statistical analyses
The analyses were performed with SAS, version 9.4 (SAS Institute Inc. Cary, NC USA). P-values were two-sided and p-values <0.05 defined as statistically significant. Table 1 shows the baseline characteristics of the participants according to study population and is expressed as mean (standard deviation, SD) or % (number).
Table 1. Characteristics of the study populations.
| Mean (SD) or % (n) | |||||||
|---|---|---|---|---|---|---|---|
| 1936-cohort | Monica1 | Allergy98 | Inter99 | Health2006 | Health2008 | Health2010 | |
| Age (years) | 40.4 (0.4) | 45.0 (11.1) | 40.0 (15.1) | 46.1 (7.9) | 49.0 (13.1) | 46.8 (8.2) | 48.8 (13.9) |
| Systolic BP (mmHg) | 120.7 (14.0) | 123.6 (16.9) | 128.9 (17.9) | 130.2 (17.4) | 130.3 (17.9) | 121.6 (15.2) | 129.0 (18.2) |
| S-cholesterol (mmol/l) | 6.3 (1.2) | 6.1 (1.2) | 5.8 (1.3) | 5.5 (1.1) | 5.3 (1.1) | 5.3 (1.0) | 5.3 (1.0) |
| Gender | |||||||
| Male | 46.7 (462) | 50.6 (1761) | 45.7 (535) | 49.1 (2930) | 45.2 (1467) | 43.6 (345) | 44.1 (664) |
| Female | 53.3 (527) | 49.4 (1720) | 54.3 (637) | 50.9 (3031) | 54.8 (1779) | 56.4 (447) | 55.9 (840) |
| Alcohol (drinks/week) | |||||||
| 0 | 20.3 (201) | 14.5 (504) | 19.2 (225) | 9.7 (550) | 6.6 (194) | 7.0 (52) | 10.0 (139) |
| >0–7 | 43.1 (426) | 47.8 (1664) | 48.2 (564) | 45.1 (2562) | 48.2 (1422) | 55.1 (411) | 50.0 (698) |
| >7–14 | 17.2 (170) | 18.3 (635) | 19.4 (227) | 21.7 (1235) | 22.7 (670) | 21.3 (159) | 20.9 (291) |
| >14 | 19.4 (192) | 19.4 (675) | 13.2 (154) | 23.5 (1337) | 22.5 (662) | 16.6 (124) | 19.1 (267) |
| Education | |||||||
| Basic | 28.5 (282) | 29.9 (1041) | 28.8 (336) | 16.8 (962) | 13.8 (440) | 8.4 (66) | 15.4 (229) |
| Beyond basic | 71.5 (706) | 70.1 (2440) | 71.2 (832) | 83.2 (4777) | 86.2 (2756) | 91.6 (717) | 84.6 (1263) |
| BMI (kg/m2) | |||||||
| <18.5 | 3.0 (30) | 2.1 (73) | 1.3 (15) | 1.0 (60) | 1.7 (56) | 1.0 (8) | 0.6 (10) |
| 18.5-<25 | 65.2 (645) | 58.0 (2018) | 49.4 (579) | 42.1 (2507) | 46.6 (1511) | 48.4 (383) | 45.4 (683) |
| 25-<30 | 26.0 (257) | 30.8 (1071) | 33.4 (392) | 39.7 (2367) | 35.5 (1151) | 34.7 (275) | 38.0 (571) |
| ≥30 | 5.8 (57) | 9.1 (318) | 15.9 (186) | 17.2 (1023) | 16.2 (526) | 15.9 (126) | 16.0 (240) |
| Physical activity | |||||||
| Sedentary | 34.7 (343) | 28.3 (985) | 26.0 (303) | 21.6 (1257) | 18.5 (595) | 14.8 (117) | 18.0 (268) |
| Light | 51.0 (505) | 51.4 (1787) | 50.3 (587) | 61.5 (3581) | 60.4 (1938) | 58.2 (460) | 57.2 (851) |
| Moderate/vigorous | 14.3 (141) | 20.3 (705) | 23.7 (277) | 16.9 (981) | 21.1 (676) | 27.0 (214) | 24.8 (369) |
| Smoking habits | |||||||
| Current smokers | 54.0 (534) | 53.8 (1874) | 39.9 (463) | 36.8 (2093) | 22.8 (733) | 18.3 (145) | 17.8 (266) |
| Former smokers | 13.2 (131) | 16.1 (561) | 17.8 (207) | 26.5 (1505) | 32.2 (1035) | 33.4 (264) | 35.5 (529) |
| Never smokers | 32.8 (324) | 30.1 (1046) | 42.3 (492) | 36.7 (2087) | 45.0 (1446) | 48.3 (382) | 46.7 (696) |
| Atopy | |||||||
| Non-atopics | 85.1 (842) | 82.9 (2887) | 62.7 (735) | 65.5 (3903) | 76.7 (2488) | 72.7 (576) | 70.3 (1057) |
| Atopics* | 14.9 (147) | 17.1 (594) | 37.3 (437) | 34.5 (2058) | 23.3 (758) | 27.3 (216) | 29.7 (447) |
Abbreviations: BMI, body mass index; BP, blood pressure; SD, standard deviation.
* Serum specific IgE or skin prick test positivity to inhalant allergens.
Multivariate logistic regression analyses were used to model the cross-sectional associations between different covariates and atopy. In model 1, we adjusted for gender, age, and study population (with an indicator of the study population that the participant belonged to). In model 2, we further adjusted for birth year (except when using age as exposure), education, physical activity, smoking habits, alcohol intake, BMI, systolic pressure and serum total cholesterol. We used complete case analysis, i.e. persons with missing values in one or more of the covariates were excluded from the regression analyses.
In additional analyses, we only included the six studies that defined atopy according to serum specific IgE positivity. We also stratified by gender the analyses regarding BMI and atopy.
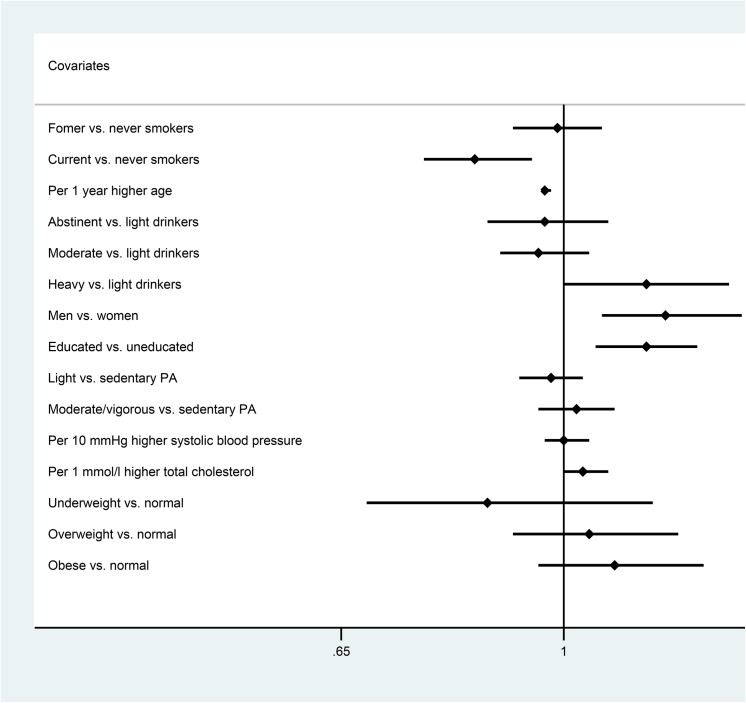
We used Stata, version 12.1 (StataCorp LP, College Station, Texas, USA), to perform meta-analyses of the fully adjusted study specific estimates obtained from logistic regression models for each study separately. This is an alternative to the analyses of the pooled studies, mainly to account for the higher correlation within studies than between studies and to assess the heterogeneity across the studies. We combined the OR estimates with the inverse variance method in random rather than the fixed effects models because of heterogeneity across studies for some of the analyses, detected by the I2-test. An overview of the results from meta-analyses is shown in Fig 1.
Fig 1. Overview of the results of meta-analyses of the study-specific estimates of the associations of atopy with possible lifestyle-related factors.
Abbreviations: OR, odds ratio; CI, confidence interval; vs., versus.
The specific meta-analyses are shown in S2–S10 Fig. Due to the narrow age span in the 1936-cohort study, we excluded it from the meta-analysis of age and atopy (S5 Fig).
Results
Participants’ birth years were distributed over the following decades (nineteen twenties: 5.1% (n = 874), nineteen thirties: 13.8% (n = 2368), nineteen forties: 24.6% (n = 4219), nineteen fifties: 29.3% (n = 5023), nineteen sixties: 18.1% (n = 3096); or nineteen seventies and above: 9.1% (n = 1565).
The mean age of the study participants varied between approximately 40 years (allergy98) to approximately 50 years (Health2006) (Table 1). In all but one study, Monica1, more females participated. The number of uneducated, sedentary participants and serum cholesterol levels were highest in the old studies and lower in the younger ones as opposed to BMI and the number of never smokers and atopics that were lowest in the old studies and somewhat higher in the youngest studies. Systolic blood pressure and alcohol intake showed no consistent time trends across the studies.
In the adjusted analyses of the merged study data, atopy was significantly associated with young age, male sex, high intake of alcohol, never smoking, and education beyond basic school, but not with systolic blood pressure or serum cholesterol (Table 2).
Table 2. The associations of atopy with possible factors of atopy (n = 15828).
| OR (95% CI) of atopy compared to reference group/per increase | ||
|---|---|---|
| Model 1* | Model 2** | |
| Age (years) | ||
| Per 1 year older | 0.98 (0.97, 0.98) | 0.97 (0.97, 0.98) |
| P-value | <0.0001 | <0.0001 |
| Systolic blood pressure (mmHg) | ||
| Per 10 mmHg higher systolic blood pressure | 1.02 (0.99, 1.04) | 1.00 (0.97, 1.02) |
| P-value | P = 0.224 | 0.881 |
| Serum cholesterol (mmol/l) | ||
| Per 1 mmol/l higher s-cholesterol | 1.03 (1.00, 1.07) | 1.03 (1.00, 1.07) |
| P-value | 0.092 | 0.077 |
| Gender | ||
| Male | 1.41 (1.31, 1.51) | 1.34 (1.24, 1.45) |
| Female | 1 (reference) | 1 (reference) |
| P-value | <0.0001 | <0.0001 |
| BMI (kg/m2) | ||
| <18.5 | 0.85 (0.61, 1.18) | 0.89 (0.64, 1.23) |
| 18.5-<25 | 1 (reference) | 1 (reference) |
| 25-<30 | 1.06 (0.98, 1.15) | 1.05 (0.97, 1.15) |
| ≥30 | 1.07 (0.96, 1.19) | 1.06 (0.94, 1.19) |
| P-value*** | 0.085 | 0.176 |
| Physical activity | ||
| Sedentary | 1 (reference) | 1 (reference) |
| Light | 1.01 (0.92, 1.10) | 0.98 (0.89, 1.07) |
| Moderate/vigorous | 1.06 (0.95, 1.18) | 1.02 (0.91, 1.14) |
| P-value*** | 0.318 | 0.790 |
| Alcohol (drinks/week) | ||
| 0 | 0.96 (0.85, 1.09) | 0.99 (0.87, 1.12) |
| >0–7 | 1 (reference) | 1 (reference) |
| >7–14 | 1.00 (0.90, 1.10) | 1.00 (0.91, 1.11) |
| >14 | 1.10 (1.00, 1.22) | 1.15 (1.04, 1.27) |
| P-value*** | 0.050 | 0.017 |
| Smoking habits | ||
| Current smokers | 0.73 (0.67, 0.80), p<0.0001 | 0.73 (0.67, 0.80), p<0.0001 |
| Former smokers | 0.82 (0.75, 0.90), p = 0.296 | 0.81 (0.74, 0.89), p = 0.194 |
| Never smokers | 1 (reference) | 1 (reference) |
| Education | ||
| Basic | 1 (reference) | 1 (reference) |
| Beyond basic | 1.31 (1.19, 1.44) | 1.27 (1.15, 1.41) |
| P-value | <0.0001 | <0.0001 |
* Adjusted for gender, age, and study population.
** Further adjusted for birth year (except for age), education, physical activity, smoking habits, alcohol intake, BMI, systolic blood pressure and serum total cholesterol.
*** P-value for trend.
Abbreviations: OR, odds ratio; BMI, body mass index; CI, confidence interval.
The additional analyses including only the six studies using serum specific IgE positivity for the definition of atopy (the Health2010 study was excluded) showed similar results (data not shown). The meta-analyses of the study-specific estimates were quite similar to results of the pooled data analyses which shows that differences between the studies did not seriously bias our results (Fig 1 and S2–S10 Figs).
BMI was not associated with atopy in men only (OR for underweight vs. normal weight: 0.83; 95% CI: 0.40, 1.70; OR for overweight vs. normal weight: OR = 1.03; 95% CI: 0.92, 1.16; and OR for obese vs. normal weight: 0.96; 95% CI: 0.81, 1.13). The p-value for trend among men was ptrend = 0.891. However, BMI was significantly associated with atopy in women (OR for underweight vs. normal weight: OR = 0.91; 95% CI: 0.63, 1.32; OR for overweight vs. normal weight: 1.07; 95% CI: 0.95, 1.22; and OR for obese vs. normal weight: 1.18; 95% CI: 1.00, 1.39). The p-value for trend among women was ptrend = 0.032.
Discussion
In analyses including more than 20,000 persons, we found that atopy was significantly associated with younger age, male sex, heavy drinking, not smoking, and educational level, but not with blood pressure, serum cholesterol, physical activity or body mass index. The large sample size allowed us to estimate the independent effects of these factors with a reasonably high precision. Data come from a pool of seven large population-based studies conducted on Danish adults since the early 1980's up to recent years, and the use of objective factors of atopy and standardized methods in general enabled the exploration of disease prevalence and risk factors over time [1,39,40].
The observed positive trend between time and prevalence of atopy is well-known and in line with several previous reports using parts of the data used in the present analyses [1,39,40]. The increasing trends of atopy support questionnaire/interview surveys on symptoms/diagnoses performed in Denmark in this period. The associations of atopy with male sex [18,41], higher education [41], and young age [18,31,42] are in line with several previous studies [42]. A possible mechanism underlying the association with age could be that the immune response in general decreases with increasing age rendering older persons less susceptible to allergic reactions and novel sensitisation. An alternative explanation could be that younger generations may have been exposed to novel environmental risk factors in early life that have changed their susceptibility to allergy. This so-called cohort effect is believed to have mainly affected generations born from the 1960s and onwards [40].
Although smoking is a known risk factor for asthma, its association with development of allergic sensitization is controversial. In line with the observed lower prevalence of atopy among current smokers compared to never smokers in the present study, Linneberg et al found that smoking was associated with a lower prevalence [18] and incidence [43] of IgE-mediated sensitization to inhalant allergens. Wüthrich et al also found smoking to be negatively associated with atopy in the previously mentioned cross-sectional Swiss study of 8344 persons [42]. On the other hand, Gergen et al found no association between smoking and atopy [44] and—although not entirely comparable to our results—, a recent meta-analysis by Saulyte et al. found no associations between active smoking and allergic rhinitis [21]. One possible explanation for a negative association between smoking and atopy may be that smokers who develop allergies tend to quit smoking, i.e., an example of reverse causation, although this is inconsistent with studies reporting that the risk of atopy among former smokers is intermediate of that of current and never smokers [18]. The association may also reflect an immunosuppressive effect of smoking [43]. We found statistically significant heterogeneity between studies in the analyses of current smoking and odds ratio of atopy (S4 Fig). Considering the individual study results, the 1936 cohort seems to be the cause of heterogeneity, since it is the only study with a positive association with atopy. Therefore, we consider this finding incidental.
The effect of alcohol consumption on sensitisation is also not clear. In 2003, Linneberg et al found that alcohol consumption was positively associated with serum total IgE but not with IgE sensitisation [19], and in 2010, Linneberg et al found that alcohol consumption was associated with prevalent but not incident aeroallergen sensitisation [31]. Also, Assing et al found no association between alcohol consumption and skin prick test positivity among 1668 Danish students [15]. The observed positive association between alcohol intake and atopy in the present study is, however, in line with several other studies [16,18,20]. The possible mechanisms by which alcohol consumption could affect allergic sensitization are not fully understood but may include a direct effect on the B-cells or the alcohol-induced increased permeability in the gut lumen [17]. There is also evidence that high intake of alcohol increases IgE sensitization to cross-reactive carbohydrate determinants that may interfere with allergy testing [31,45].
Previous studies examining the association between obesity and atopy in adults have shown conflicting results [9]. There are both studies supporting a positive association [46–48] and studies against such an association [49–58]. The positive association in the present study between BMI and atopy among women in particular, is in line with several studies that include mainly children or adolescents [56–58]. The mechanisms by which obesity might be related to atopy—and possibly in women only—are not clear but include a possible influence of sex hormones on the development and expression of atopy and atopic disorders [59–61] or the fact that for a given BMI, women have a higher percentage of body fat than men [9]. However, given the fact that overweight and obesity are in increase, it is important to address any possible effect of overweight and obesity in the pathogenesis of atopy in future studies.
In addition, theoretically both serum lipids and blood pressure could mediate a possible effect of obesity on atopy since obesity has a detrimental effect on both, and both are related to some form of inflammation [62]. However, we found no association between either systolic blood pressure or serum total cholesterol and atopy. The lack of association between serum total cholesterol and atopy is somewhat in line with a nested case-control study by Schäfer et al who found no association between serum total cholesterol and allergic sensitization in adjusted analyses [4]. However, Fessler et al found that the odds ratio of atopy defined as serum specific IgE positivity against allergens was OR = 1.17 (95% CI: 1.00, 1.38) per two standard deviation increase in total cholesterol which is comparable to ORs of serum cholesterol for myocardial infarction [14]. The possible underlying mechanism may be an effect of serum lipids on pro-atopic Th2 immunity and allergic inflammation [13,14].
The strengths of our study include the large population-based samples and the inclusion of multiple studies. Also a strength, is the use of objective factors of atopy, i.e. serum specific IgE positivity and skin prick test positivity against inhalant allergens, which may be more reliable than self-reported diagnoses and symptoms. Although different methods were employed for assessment of atopy, our previous comparison of e.g. the ADVIA Centaur Allergy Screen assay (used in the 1936-cohort and the Monica1 studies) against skin prick test reactivity (used in the Health2010 study) showed good agreement in our background population [36]. We have also compared the ADVIA Centaur sIgE assay (used in the Health2006, Health2008 and Allergy98 studies) against skin prick test positivity showing good agreement [37]. In general, previous studies have also shown good agreement in the different ways of measuring serum specific IgE against inhalant allergens [63,64].
One limitation is that the questionnaires used for collection of data on symptoms and diagnoses of diseases in the various studies differed markedly over time period and some studies lacked questions on allergic respiratory disease. Hence, we could not perform meaningful analyses for these outcomes across populations.
In conclusion, in this large cross-sectional study we confirmed that atopy, objectively assessed by IgE or skin prick test sensitization to inhalant allergens, is associated with sex and age and in addition with several lifestyle-related factors. Although environment is clearly important in the aetiology allergic respiratory disease, lifestyle factors could also contribute to the allergy epidemic.
Supporting Information
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Study participants were drawn as a random samples of the general population living in a defined area of the Western part of Copenhagen.
(DOCX)
(DOCX)
Data Availability
Data cannot be made publicly available for ethical and legal reasons. Public availability may compromise participant privacy, and this would not comply with Danish legislation. Requests for data should be addressed to Professor Allan Linneberg who will provide the data access in accordance with the Danish Data Protection Agency.
Funding Statement
Tea Skaaby was supported by a grant from the Lundbeck Foundation (Grant number R165-2013-15410). Betina Heinsbæk Thuesen was supported by a grant from the Lundbeck Foundation (Grant number R108-A10225). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Linneberg A, Gislum M, Johansen N, Husemoen LL, Jorgensen T. Temporal trends of aeroallergen sensitization over twenty-five years. Clin Exp Allergy 2007. August;37(8):1137–42. [DOI] [PubMed] [Google Scholar]
- 2. Linneberg A. Are we getting enough allergens? Int Arch Allergy Immunol 2008;147(2):93–100. 10.1159/000135695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ring J, Kramer U, Schafer T, Behrendt H. Why are allergies increasing? Curr Opin Immunol 2001. December;13(6):701–8. [DOI] [PubMed] [Google Scholar]
- 4. Schafer T, Ruhdorfer S, Weigl L, Wessner D, Heinrich J, Doring A, et al. Intake of unsaturated fatty acids and HDL cholesterol levels are associated with manifestations of atopy in adults. Clin Exp Allergy 2003. October;33(10):1360–7. [DOI] [PubMed] [Google Scholar]
- 5. van GR, van der Ent CK, Rovers MM, Kimpen JL, van Essen-Zandvliet LE, de MG. Excessive body weight is associated with additional loss of quality of life in children with asthma. J Allergy Clin Immunol 2007. March;119(3):591–6. [DOI] [PubMed] [Google Scholar]
- 6. Visness CM, London SJ, Daniels JL, Kaufman JS, Yeatts KB, Siega-Riz AM, et al. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999–2006. J Asthma 2010. September;47(7):822–9. 10.3109/02770903.2010.489388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Arch Dis Child 2006. April;91(4):334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boulet LP. Obesity and atopy. Clin Exp Allergy 2014. October 16. [DOI] [PubMed] [Google Scholar]
- 10. Chen Y, Rennie D, Cormier Y, Dosman J. Atopy, obesity, and asthma in adults: the Humboldt study. J Agromedicine 2009;14(2):222–7. 10.1080/10599240902724051 [DOI] [PubMed] [Google Scholar]
- 11. Chen Y, Rennie D, Cormier Y, Dosman J. Association between obesity and atopy in adults. Int Arch Allergy Immunol 2010;153(4):372–7. 10.1159/000316348 [DOI] [PubMed] [Google Scholar]
- 12. Hersoug LG, Linneberg A. The link between the epidemics of obesity and allergic diseases: does obesity induce decreased immune tolerance? Allergy 2007. October;62(10):1205–13. [DOI] [PubMed] [Google Scholar]
- 13. Yeh YF, Huang SL. Enhancing effect of dietary cholesterol and inhibitory effect of pravastatin on allergic pulmonary inflammation. J Biomed Sci 2004. September;11(5):599–606. [DOI] [PubMed] [Google Scholar]
- 14. Fessler MB, Jaramillo R, Crockett PW, Zeldin DC. Relationship of serum cholesterol levels to atopy in the US population. Allergy 2010. July;65(7):859–64. 10.1111/j.1398-9995.2009.02287.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Assing K, Bodtger U, Linneberg A, Malling HJ, Poulsen LK. Association between alcohol consumption and skin prick test reactivity to aeroallergens. Ann Allergy Asthma Immunol 2007. January;98(1):70–4. [DOI] [PubMed] [Google Scholar]
- 16. Gonzalez-Quintela A, Gude F, Boquete O, Rey J, Meijide LM, Suarez F, et al. Association of alcohol consumption with total serum immunoglobulin E levels and allergic sensitization in an adult population-based survey. Clin Exp Allergy 2003. February;33(2):199–205. [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez-Quintela A, Vidal C, Gude F. Alcohol, IgE and allergy. Addict Biol 2004. September;9(3–4):195–204. [DOI] [PubMed] [Google Scholar]
- 18. Linneberg A, Nielsen NH, Madsen F, Frolund L, Dirksen A, Jorgensen T. Factors related to allergic sensitization to aeroallergens in a cross-sectional study in adults: The Copenhagen Allergy Study. Clin Exp Allergy 2001. September;31(9):1409–17. [DOI] [PubMed] [Google Scholar]
- 19. Linneberg A, Petersen J, Nielsen NH, Madsen F, Frolund L, Dirksen A, et al. The relationship of alcohol consumption to total immunoglobulin E and the development of immunoglobulin E sensitization: the Copenhagen Allergy Study. Clin Exp Allergy 2003. February;33(2):192–8. [DOI] [PubMed] [Google Scholar]
- 20. Vidal C, Armisen M, Dominguez-Santalla MJ, Gude F, Lojo S, Gonzalez-Quintela A. Influence of alcohol consumption on serum immunoglobulin E levels in atopic and nonatopic adults. Alcohol Clin Exp Res 2002. January;26(1):59–64. [PubMed] [Google Scholar]
- 21. Saulyte J, Regueira C, Montes-Martinez A, Khudyakov P, Takkouche B. Active or passive exposure to tobacco smoking and allergic rhinitis, allergic dermatitis, and food allergy in adults and children: a systematic review and meta-analysis. PLoS Med 2014. March;11(3):e1001611 10.1371/journal.pmed.1001611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osler M, Linneberg A, Glumer C, Jorgensen T. The cohorts at the Research Centre for Prevention and Health, formerly 'The Glostrup Population Studies'. Int J Epidemiol 2011. June;40(3):602–10. 10.1093/ije/dyq041 [DOI] [PubMed] [Google Scholar]
- 23. Aadahl M, Linneberg A, Moller TC, Rosenorn S, Dunstan DW, Witte DR, et al. Motivational counseling to reduce sitting time: a community-based randomized controlled trial in adults. Am J Prev Med 2014. November;47(5):576–86. 10.1016/j.amepre.2014.06.020 [DOI] [PubMed] [Google Scholar]
- 24. Skaaby T, Husemoen LL, Thuesen BH, Pisinger C, Hannemann A, Jorgensen T, et al. Longitudinal associations between lifestyle and vitamin D: A general population study with repeated vitamin D measurements. Endocrine 2015. May 30. [DOI] [PubMed] [Google Scholar]
- 25. Jorgensen T, Borch-Johnsen K, Thomsen TF, Ibsen H, Glumer C, Pisinger C. A randomized non-pharmacological intervention study for prevention of ischaemic heart disease: baseline results Inter99. Eur J Cardiovasc Prev Rehabil 2003. October;10(5):377–86. [DOI] [PubMed] [Google Scholar]
- 26. Berg ND, Husemoen LL, Thuesen BH, Hersoug LG, Elberling J, Thyssen JP, et al. Interaction between filaggrin null mutations and tobacco smoking in relation to asthma. J Allergy Clin Immunol 2012. February;129(2):374–80, 380. 10.1016/j.jaci.2011.08.045 [DOI] [PubMed] [Google Scholar]
- 27. Aadahl M, Zacho M, Linneberg A, Thuesen BH, Jorgensen T. Comparison of the Danish step test and the watt-max test for estimation of maximal oxygen uptake: the Health2008 study. Eur J Prev Cardiol 2013. December;20(6):1088–94. 10.1177/2047487312462825 [DOI] [PubMed] [Google Scholar]
- 28. Byberg S, Hansen AL, Christensen DL, Vistisen D, Aadahl M, Linneberg A, et al. Sleep duration and sleep quality are associated differently with alterations of glucose homeostasis. Diabet Med 2012. September;29(9):e354–e360. 10.1111/j.1464-5491.2012.03711.x [DOI] [PubMed] [Google Scholar]
- 29. Husemoen LL, Fenger M, Friedrich N, Tolstrup JS, Beenfeldt FS, Linneberg A. The association of ADH and ALDH gene variants with alcohol drinking habits and cardiovascular disease risk factors. Alcohol Clin Exp Res 2008. November;32(11):1984–91. 10.1111/j.1530-0277.2008.00780.x [DOI] [PubMed] [Google Scholar]
- 30. Drivsholm T, Eplov LF, Davidsen M, Jorgensen T, Ibsen H, Hollnagel H, et al. Representativeness in population-based studies: a detailed description of non-response in a Danish cohort study. Scand J Public Health 2006;34(6):623–31. [DOI] [PubMed] [Google Scholar]
- 31. Linneberg A, Friedrich N, Husemoen LL, Thuesen B, Gonzalez-Quintela A, Vidal C, et al. Incidence and remission of specific IgE aeroallergen sensitization from age of 40 to 60 years, and association with alcohol consumption. Int Arch Allergy Immunol 2010;151(2):142–8. 10.1159/000236004 [DOI] [PubMed] [Google Scholar]
- 32. Skaaby T, Husemoen LL, Thuesen BH, Hammer-Helmich L, Linneberg A. Atopy and cause-specific mortality. Clin Exp Allergy 2014. November;44(11):1361–70. 10.1111/cea.12408 [DOI] [PubMed] [Google Scholar]
- 33. Skaaby T, Husemoen LL, Roswall N, Thuesen BH, Linneberg A. Atopy and development of cancer: A population-based prospective study. JACI: In practice 2014. [DOI] [PubMed] [Google Scholar]
- 34. Skaaby T, Husemoen LL, Thuesen BH, Jeppesen J, Linneberg A. The association of atopy with incidence of ischemic heart disease, stroke, and diabetes. Endocrine 2014. June 12. [DOI] [PubMed] [Google Scholar]
- 35. Skaaby T, Husemoen LL, Thuesen BH, Fenger RV, Linneberg A. Specific IgE positivity against inhalant allergens and development of autoimmune disease. Autoimmunity 2015. August;48(5):282–8. 10.3109/08916934.2014.1003640 [DOI] [PubMed] [Google Scholar]
- 36. Linneberg A, Husemoen LL, Nielsen NH, Madsen F, Frolund L, Johansen N. Screening for allergic respiratory disease in the general population with the ADVIA Centaur Allergy Screen Assay. Allergy 2006. March;61(3):344–8. [DOI] [PubMed] [Google Scholar]
- 37. Petersen AB, Gudmann P, Milvang-Gronager P, Morkeberg R, Bogestrand S, Linneberg A, et al. Performance evaluation of a specific IgE assay developed for the ADVIA centaur immunoassay system. Clin Biochem 2004. October;37(10):882–92. [DOI] [PubMed] [Google Scholar]
- 38. Thuesen BH, Husemoen LL, Ovesen L, Jorgensen T, Fenger M, Gilderson G, et al. Atopy, asthma, and lung function in relation to folate and vitamin B(12) in adults. Allergy 2010. November;65(11):1446–54. 10.1111/j.1398-9995.2010.02378.x [DOI] [PubMed] [Google Scholar]
- 39. Linneberg A, Nielsen NH, Madsen F, Frolund L, Dirksen A, Jorgensen T. Secular trends of allergic asthma in Danish adults. The Copenhagen Allergy Study. Respir Med 2001. April;95(4):258–64. [DOI] [PubMed] [Google Scholar]
- 40. Linneberg A, Nielsen NH, Madsen F, Frolund L, Dirksen A, Jorgensen T. Is the increase in allergic respiratory disease caused by a cohort effect? Clin Exp Allergy 2002. December;32(12):1702–5. [DOI] [PubMed] [Google Scholar]
- 41. Ferraz E, Garcia CA, Bettiol H, Caldeira RD, Cardoso VC, Arruda LK, et al. Atopy risk factors at birth and in adulthood. J Pediatr (Rio J) 2011. July;87(4):336–42. [DOI] [PubMed] [Google Scholar]
- 42. Wuthrich B, Schindler C, Medici TC, Zellweger JP, Leuenberger P. IgE levels, atopy markers and hay fever in relation to age, sex and smoking status in a normal adult Swiss population. SAPALDIA (Swiss Study on Air Pollution and Lung Diseases in Adults) Team. Int Arch Allergy Immunol 1996. December;111(4):396–402. [DOI] [PubMed] [Google Scholar]
- 43. Linneberg A, Nielsen NH, Madsen F, Frolund L, Dirksen A, Jorgensen T. Smoking and the development of allergic sensitization to aeroallergens in adults: a prospective population-based study. The Copenhagen Allergy Study. Allergy 2001. April;56(4):328–32. [DOI] [PubMed] [Google Scholar]
- 44. Gergen PJ, Turkeltaub PC. The association of allergen skin test reactivity and respiratory disease among whites in the US population. Data from the Second National Health and Nutrition Examination Survey, 1976 to 1980. Arch Intern Med 1991. March;151(3):487–92. [PubMed] [Google Scholar]
- 45. Gonzalez-Quintela A, Garrido M, Gude F, Campos J, Linneberg A, Lojo S, et al. Sensitization to cross-reactive carbohydrate determinants in relation to alcohol consumption. Clin Exp Allergy 2008. January;38(1):152–60. [DOI] [PubMed] [Google Scholar]
- 46. Chen Y, Rennie D, Cormier Y, Dosman J. Association between obesity and atopy in adults. Int Arch Allergy Immunol 2010;153(4):372–7. 10.1159/000316348 [DOI] [PubMed] [Google Scholar]
- 47. Vieira VJ, Ronan AM, Windt MR, Tagliaferro AR. Elevated atopy in healthy obese women. Am J Clin Nutr 2005. September;82(3):504–9. [DOI] [PubMed] [Google Scholar]
- 48. Xu B, Pekkanen J, Laitinen J, Jarvelin MR. Body build from birth to adulthood and risk of asthma. Eur J Public Health 2002. September;12(3):166–70. [DOI] [PubMed] [Google Scholar]
- 49. Bruno A, Pace E, Cibella F, Chanez P. Body mass index and comorbidities in adult severe asthmatics. Biomed Res Int 2014;2014:607192 10.1155/2014/607192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Flexeder C, Bruske I, Magnussen H, Heinrich J. Association between obesity and atopy in adults? Int Arch Allergy Immunol 2011;156(1):117–8. 10.1159/000322296 [DOI] [PubMed] [Google Scholar]
- 51. van Veen IH, Ten BA, Sterk PJ, Rabe KF, Bel EH. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy 2008. May;63(5):570–4. 10.1111/j.1398-9995.2007.01597.x [DOI] [PubMed] [Google Scholar]
- 52. Santamaria F, Montella S, De SS, Sperli F, Barbarano F, Spadaro R, et al. Asthma, atopy, and airway inflammation in obese children. J Allergy Clin Immunol 2007. October;120(4):965–7. [DOI] [PubMed] [Google Scholar]
- 53. Jarvis D, Chinn S, Potts J, Burney P. Association of body mass index with respiratory symptoms and atopy: results from the European Community Respiratory Health Survey. Clin Exp Allergy 2002. June;32(6):831–7. [DOI] [PubMed] [Google Scholar]
- 54. Schachter LM, Salome CM, Peat JK, Woolcock AJ. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax 2001. January;56(1):4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Silverberg JI, Silverberg NB, Lee-Wong M. Association between atopic dermatitis and obesity in adulthood. Br J Dermatol 2012. March;166(3):498–504. 10.1111/j.1365-2133.2011.10694.x [DOI] [PubMed] [Google Scholar]
- 56. Hancox RJ, Milne BJ, Poulton R, Taylor DR, Greene JM, McLachlan CR, et al. Sex differences in the relation between body mass index and asthma and atopy in a birth cohort. Am J Respir Crit Care Med 2005. March 1;171(5):440–5. [DOI] [PubMed] [Google Scholar]
- 57. Schachter LM, Peat JK, Salome CM. Asthma and atopy in overweight children. Thorax 2003. December;58(12):1031–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med 2001. May;163(6):1344–9. [DOI] [PubMed] [Google Scholar]
- 59. Chen W, Mempel M, Schober W, Behrendt H, Ring J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy 2008. November;63(11):1418–27. 10.1111/j.1398-9995.2008.01880.x [DOI] [PubMed] [Google Scholar]
- 60. Salam MT, Wenten M, Gilliland FD. Endogenous and exogenous sex steroid hormones and asthma and wheeze in young women. J Allergy Clin Immunol 2006. May;117(5):1001–7. [DOI] [PubMed] [Google Scholar]
- 61. Bergeron C, Boulet LP, Hamid Q. Obesity, allergy and immunology. J Allergy Clin Immunol 2005. May;115(5):1102–4. [DOI] [PubMed] [Google Scholar]
- 62. Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999. January 14;340(2):115–26. [DOI] [PubMed] [Google Scholar]
- 63. Jahn-Schmid B, Harwanegg C, Hiller R, Bohle B, Ebner C, Scheiner O, et al. Allergen microarray: comparison of microarray using recombinant allergens with conventional diagnostic methods to detect allergen-specific serum immunoglobulin E. Clin Exp Allergy 2003. October;33(10):1443–9. [DOI] [PubMed] [Google Scholar]
- 64. Lee YW, Sohn JH, Lee JH, Hong CS, Park JW. Allergen-specific IgE measurement with the IMMULITE 2000 system: intermethod comparison of detection performance for allergen-specific IgE antibodies from Korean allergic patients. Clin Chim Acta 2009. March;401(1–2):25–32. 10.1016/j.cca.2008.10.034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Abbreviations: a98, Allergy98; h06, Health2006; h08, Health2008; h10, Health2010; i99, Inter99; k36, 1936-cohort; m1, Monica1; OR, odds ratio; CI, confidence interval.
(TIF)
Study participants were drawn as a random samples of the general population living in a defined area of the Western part of Copenhagen.
(DOCX)
(DOCX)
Data Availability Statement
Data cannot be made publicly available for ethical and legal reasons. Public availability may compromise participant privacy, and this would not comply with Danish legislation. Requests for data should be addressed to Professor Allan Linneberg who will provide the data access in accordance with the Danish Data Protection Agency.