Abstract
Lichens produce various unique chemicals that can be used for pharmaceutical purposes. To screen for novel lichen secondary metabolites showing inhibitory activity against lung cancer cell motility, we tested acetone extracts of 13 lichen samples collected in Chile. Physciosporin, isolated from Pseudocyphellaria coriacea (Hook f. & Taylor) D.J. Galloway & P. James, was identified as an effective compound and showed significant inhibitory activity in migration and invasion assays against human lung cancer cells. Physciosporin treatment reduced both protein and mRNA levels of N-cadherin with concomitant decreases in the levels of epithelial-mesenchymal transition markers such as snail and twist. Physciosporin also suppressed KITENIN (KAI1 C-terminal interacting tetraspanin)-mediated AP-1 activity in both the absence and presence of epidermal growth factor stimulation. Quantitative real-time PCR analysis showed that the expression of the metastasis suppressor gene, KAI1, was increased while that of the metastasis enhancer gene, KITENIN, was dramatically decreased by physciosporin. Particularly, the activity of 3’-untranslated region of KITENIN was decreased by physciosporin. Moreover, Cdc42 and Rac1 activities were decreased by physciosporin. These results demonstrated that the lichen secondary metabolite, physciosporin, inhibits lung cancer cell motility through novel mechanisms of action.
Introduction
Lung cancer is the most common cancer and the leading cause of cancer-related death in humans worldwide [1, 2]. There were about 1.8 million new cases of lung cancer diagnosed in 2012, accounting for 12.9% of the total cases of cancer [1, 2]. Due to the lack of efficient treatment for advanced disease, the prognosis of lung cancer is still poor, with less than 15% surviving 5 years after diagnosis [3]. When lung cancer is diagnosed at presentation with symptoms, it carries an extremely poor prognosis, with an overall 5-year survival of 16% in the USA and less than 10% in the UK [4]. In lung cancer, metastasis is the leading cause of death, and unraveling the mechanism of tumor progression and metastasis is thus of great importance [5]. In the initial steps of local invasion, the signaling pathways that control the cytoskeletal dynamics of tumor cells, the turnover of cell–matrix and the cell–cell junctions were activated [6]. Therefore, development of new chemical agents that inhibit cancer cell migration and invasion by targeting the above signal is required in treatment of advanced cancers.
Lichens produce diverse secondary metabolites that show a variety of biological activities including anti-cancer activity [7]. To date, nearly a thousand secondary metabolites of lichens have been discovered and some of these unique lichen metabolites are effective against various in vitro cancer models [8]. However, although lichens are a source for screening for anti-cancer active compounds, only a small number of compounds have been tested [9]. Therefore, the current study examined the inhibitory activity of 13 Chilean lichen species against migration and invasion ability of human lung cancer cells and further investigated the possible molecular mechanisms underlying their anti-metastatic activity to identify potential novel anti-metastasis agents.
Materials and Methods
Preparation of lichen extracts
Thalli of the lichens were collected from Chile in January of 2009 and 2012 during field trips in the National Park of Torres Del Paine, Patagonia, organized by Dr. Pereira at Talca University, Talca, Chile. The permit to collect lichen specimens from location was issued by the Administration of the National Forestry Corporation (CONAF) of Punta Arenas and the Administration of the National Park Torres del Paine, Magallanes region and Chilean Antarctic, which is part of the National System of Protected Wild Areas of the State of Chile. The field studies did not involve any endangered or protected species. The duplicates were deposited at the Korean Lichen and Allied Bioresource Center (KOLABIC) in the Korean Lichen Research Institute (KoLRI), Sunchon National University, Korea.
Thin Layer Chromatography (TLC) analysis of lichen material
Lichen thalli were soaked in 1 mL acetone for 5 minutes in 1.5 mL EP tubes, and the concentrated solution was spotted on Silica gel 60 F254 pre-coated plates (Merck Millipore, Darmstadt, Germany) using a microcap several times. Solvent A [Toluene: Dioxin: Acetic acid 180: 45: 5 (v/v/v)], described in Culberson’s improved standardized method [10], was used in this study. The TLC plate spotted with samples was loaded in a twin trough chamber pre-saturated with solvent system A and was removed from the chamber when the solvent front reached 14cm from the starting baseline. The results were visualized by examination and marking in daylight (for pigments) and under UV at 254 and 350 nm. Following this, the plates were sprayed with 10% aqueous sulfuric acid and heated at 110°C to ensure full visualization. After charring, the specific colors of substances, both in daylight and under UV (350nm), were noted.
Two lichen species Lethariella cladonioides (Nyl.) Krog (Atranorin) and Usnea longissimi (usnic acid) were used as the standard control. Based on the standardized method [10, 11], relative Rf value was determined to help identify each spot [12].
Separation and identification of physciosporin
Pseudocyphellaria coriacea (CL090002) extract was separated by TLC as described above. The spot identified as physciosporin was scratched off and eluted with acetone. The supernatant was dried and weighed after centrifugation. High-performance liquid chromatography (HPLC) assay was performed to confirm single peak within the purified sample, and, then, structure of physciosporin was confirmed by nuclear magnetic resonance (NMR) analysis (Figs A and B in S1 File).
Cell culture
The human lung cancer cells including A549, H1650 and H1975 were used in this study. Cells were cultured in RPMI 1640 culture medium supplemented with 10% fetal bovine serum, 1% Penicillin-Streptomycin solution under a humidified 5% CO2 atmosphere at 37°C in an incubator.
Wound healing assay
A549 cells were plated at a density of 2.5~3 × 105 cells/well on 6-well tissue culture plates (Corning, New York, USA) and grown overnight to confluence. Monolayer cells were scratched with a sterile pipette tip to create a wound. The cells were then washed twice with serum-free RPMI 1640 to remove floating cells and incubated in RPMI1640 culture medium supplemented with 2% FBS with 5 μg/mL of acetone extract of P. coriacea or physciosporin. Photographs of cells were taken at 0, 24, 48, and 72 h after wounding to measure the width of the wound. For each sample, an average of five wound assays was taken to determine the average rate of migration. Experiments were repeated at least three times.
Invasion assay
Invasion assays were performed in Boyden chambers (Corning, New York, USA). The polycarbonate filters (8 μm pore size, Corning) pre-coated with 1% gelatin were used for invasion assays. Cells (2×106) in 120 μL of medium (RPMI 1640 containing 0.2% BSA dissolved in PBS) with or without 5 μg/mL crude lichen extracts or physciosporin were seeded in the upper chamber. Then 400 μL medium with 10 μg/mL fibronectin was added to the lower chamber to serve as a chemotactic agent. After 24 h incubation, the cells in the upper chamber were fixed with Diff Quik kit (Sysmex). Then the cells attached in the upper chamber were mechanically removed by cotton swab. The cells adhering to the under-side of the filter were stained and counted under upright microscope (5 fields/chamber). Each invasion assay was repeated in three independent experiments.
Western blotting
Cells treated with 5 μg/mL of acetone extract of P. coriacea or physciosporin for 24 h were washed twice with ice-cold PBS and lysed in lysis buffer [13]. The antibodies used were purchased from Cell Signaling Technology (N-cadherin, α-tubulin) and BD Biosciences (E-cadherin). Antibodies were detected with horseradish peroxidase-conjugated secondary antibody (Thermo) using Immobilon Western Chemiluminescent HRP Substrate Kit (MILLIPORE) and luminescence imaging (Image Quant LAS 4000 mini). Bands were measured by Multi-Gauge 3.0 and their relative density calculated based on the density of the α-tubulin bands in each sample. Values were expressed as arbitrary densitometric units corresponding to signal intensity.
Quantitative RT-PCR
The quantitative RT-PCR (qRT-PCR) was performed as previously described [14]. Briefly, total RNA was isolated from human lung cancer cells by using RNAiso Plus (TaKaRa, Otsu, Shiga Shiga 520–2193, Japan) according to the manufacturer’s instructions. Total RNA from each group of treated cells was converted to cDNA using a M-MLV reverse Transcriptase kit (Invitrogen, Carlsbad, USA) and SYBR green (Enzynomics, Seoul, Korea). The primers used for real-time PCR were Snail (forward) 5'-tcccgggcaatttaacaatg-3' and (reverse) 5'-tgggagacacatcggtcga-3'; Twist (forward) 5'-cgggagtccgcagtctta-3' and (reverse) 5'-tgaatcttgctcagcttgtc-3'; N-cadherin (forward) 5'-ctcctatgagtggaacaggaacg-3' and (reverse) 5'-ttggatcaatgtcataatcaagtgctgta-3'; KAI1 (forward) 5'-gctcatgggcttgggct-3' and (reverse) 5'-gagctcagtcacgatgccgc-3'; KITENIN (forward) 5’-cggaataaagacggcagagg-3’ and (reverse) 5’-tgctccgaggtgcctgtgat-3’; GAPDH (forward) 5’-atcaccatcttccaggagcga-3’ and (reverse) 5’-agttgtcatggatgaccttggc-3’. qRT-PCR reactions and analyses were performed using CFX (Bio-Rad, Hercules, USA).
Reporter assay
For AP-1 reporter assay, HEK293T cells were transfected with KITENIN expression plasmid and AP1 reporter plasmid. After 12 h transfection, cells were treated with 5 μg/mL of acetone extracts of P. coriacea or physciosporin for 48h with or without EGF and then analyzed using a Dual-Luciferase® reporter assay system (Promega, Madison, WI, USA). The Renilla luciferase reporter plasmid (pRL-TK) was used as an internal control for the transfection efficiency. The activity for TOPFLASH reporter, NF-κb and STAT reporter were also tested in our study.
For KITENIN promoter reporter assay, human KITENIN promoter (-2344 to + 536) was amplified by PCR from genomic DNA of HEK293T cells using primers designed from the genomic sequence of the KITENIN locus (1p13.1). The PCR products were digested with KpnI/BglII and cloned into the pGL3basic plasmid (Promega, Madison, WI, USA) upstream of the luciferase reporter gene. Construction was confirmed by sequencing. The KITENIN 3’-untranlated region (3’-UTR) assay was performed as previously described [15]. The experiments were performed in triplicate, and at least three results from independent experiments were included in an analysis. Fold changes were calculated using values normalized to Renilla luciferase activity.
Affinity-precipitation of cellular GTPases
The cellular Rac1 and Cdc42 activities were determined using GST-RBD/PBD as previously described [16]. Briefly, the cells were lysed in lysis buffer. The lysates were incubated with GST- RBD/PBD beads at RT for 1 h. The beads were then washed four times with washing buffer. The bound Rac1 and Cdc42 proteins were detected by immunoblotting using a monoclonal antibody against Rac1 (MILLIPORE 05–389) and Cdc42 (SANTA CRUZ SC-87). The relative activity of each GTPase was determined by quantifying each band of GTP-bound GTPase and the total amount of GTPase using Multi-Gauge 3.0, and the values of the GTP-bound bands were normalized to that of the total amount. All results were determined using three different exposures from at least three independent experiments.
Results
Acetone extracts of lichens collected in Chile inhibit A549 cell motility
Invasion and migration play a crucial role in the metastasis of cancer cells. To find an inhibitory substance from lichen secondary metabolites, wound healing assays were performed in A549 human lung cancer cells as an initial screening for acetone extracts of 13 Chilean lichens (Table 1) [17–20]. As shown in Fig 1A, Rhizoplaca melanophthalma, Hypotrachyna sinuosa, Pseudocyphellaria coriacea, Pseudocyphellaria glabra and Nephroma sp. inhibited the migration of A549 cells at a concentration of 5 μg/mL. This concentration was used as no cytotoxicity was observed at this concentration (data not shown). The distances between the edges of the wounds for cells treated with the five lichen extracts were wider than those for DMSO-treated cells (Fig 1B).
Table 1. Thirteen Chile lichen species used in this study.
| Collection No. | Family | Species name | Known lichen substances | Reference |
|---|---|---|---|---|
| CL090001 | Lobariaceae | Pseudocyphellaria glabra | 7β-acetoxyhopan-22-ol hopane-15α,22-diol, stictic and constictic acid | [17] |
| CL090002 | Lobariaceae | Pseudocyphellaria coriacea | 7β-acetoxyhopan-22-ol hopane-7β,22-diol, 7β-acetoxyhopan-22-ol | [17] |
| CL090003 | Nephromataceae | Nephroma sp. | Zeorin | [18] |
| CL090006 | Physciaceae | Physcia sp. | Atranorin, zeorin | [18] |
| CL120051 | Parmeliaceae | Flavoparmelia caperata | Usnic,ptorocetraric,caperatic acid and atranorin | [18] |
| CL120055 | Parmeliaceae | Hypotrachyna sinuosa | Usnic acid salazinic acid | [18] |
| CL120113 | Lecanoraceae | Rhizoplaca melanophthalma | Usnic acid | [18] |
| CL120124 | Lecanoraceae | Rhizoplaca melanophthalma | Usnic acid | [18] |
| CL120181 | Lobariaceae | Pseudocyphellaria argyracea | 7β-acetoxyhopan-22-ol hopane-7β,22-diol, 7β-acetoxyhopan-22-ol,methyl gyrophorate and gyrophoric acid | [17] |
| CL120206 | Lobariaceae | Pseudocyphellaria verrucosa | Gyrophoric acid | [19] |
| CL120330 | Parmeliaceae | Xanthoparmelia sp. | Usnic, salazinic,norstic,stictic,,barbatic and diffractaic acid | [18] |
| CL120340 | Parmeliaceae | Protousnea sp. | Sekikaic and divaricatic acid | [20] |
| CL120371 | Parmeliaceae | Xanthoparmelia sp. | Usnic, salazinic,norstic,stictic,,barbatic and diffractaic acid | [18] |
Fig 1. Acetone extracts of lichens collected in Chile inhibited A549 cell motility.
(A) Quantitative analysis of migration assays of A549 cells treated with 5 μg/mL of acetone extracts of Pseudocyphellaria glabra, Pseudocyphellaria coriacea, Nephroma sp., Physcia sp., Flavoparmelia caperata, Hypotrachyna sinuosa, Rhizoplaca melanophthalma, Rhizoplaca melanophthalma, Pseudocyphellaria argyracea, Pseudocyphellaria verrucosa, Xanthoparmelia sp., Protousnea sp. and Xanthoparmelia sp.. (B) Representative images of migration assays of A549 cells treated with the extracts of R. melanophthalma, H. sinuosa, P. coriacea, P. glabra and Nephroma sp. (C-D) Invasion assays of A549 cells treated with 5 μg/mL of acetone extracts of R. melanophthalma, H. sinuosa, P. coriacea, P. glabra and Nephroma sp. (C) and quantitative analysis of invaded cell numbers in each treatment (D). Quantitative data were obtained from three independent experiments, n = 3. Data represent mean ± S.E.M. (standard error of the mean). ***p<0.001 compared to DMSO-treated A549 cells.
To further check whether the above lichen extracts had inhibitory activity against invasion of A549 cells, invasion assays were performed using gelatin-coated two-well chambers. As shown in Fig 1C, cells treated with the acetone extracts of the five lichens showed fewer numbers of invaded cells when compared to DMSO-treated control. Quantitative analysis of the data revealed that the differences were significant (Fig 1D). These results indicate that acetone extracts of R. melanophthalma, H. sinuosa, P. coriacea, P. glabra and Nephroma sp. exhibit inhibitory activity against A549 cell motility.
Physciosporin was identified as an active lichen secondary metabolite from P. coriacea in the inhibition of lung cancer cell motility
To investigate the main compound of these five candidate lichens, five lichen acetone extracts were individually analyzed by TLC (Fig 2A). Based on the relative Rf values [12], R. melanophthalma, H. sinuosa, and Nephroma sp. share the same major component, usnic acid (spot a, Fig 2A), while P. glabra and P. coriacea have distinct main compounds (spot c, Fig 2A). As usnic acid is the most extensively studied lichen metabolite for anti-cancer activity, we chose ‘spot c’ for further study. ‘Spot c’ was distinctively observed within P. glabra and P. coriacea among the other Pseudocyphellaria genus that possesses tenuiorin (spot d) and other unknown metabolites as the main compound (left panel, Fig 2B). As ‘spot c’ in P. glabra and P. coriacea shared an identical TLC Rf value with physciosporin in P. granulata and was pale or dark grey in daylight and became black and denser under UV (left and right panel, Fig 2B), we identified ‘spot c’ as physciosporin [21–23]. P. faveolata and P. valdiviana also possess physciosporin as their main compound (right panel, Fig 2B). The physciosporin used in this study was isolated from P. coriacea as the amounts of other physciosporin containing lichens were limited. Purity and structure of physciosporin was confirmed by HPLC and NMR analysis (Fig 2C; S1 File).
Fig 2. Physciosporin was identified as an active lichen secondary metabolite from P. coriacea in the inhibition of lung cancer cell motility.
(A-B) Thin Layer Chromatography (TLC) analysis using (Toluene: Dioxin: Acetic acid = 180: 45: 5, v/v/v) solvent system for lichen extracts having inhibitory activity in A549 cell motility (A), the lichens in Pseudocyphellaria genus (B). ‘a’ denotes location of spot for usnic acid; ‘b’, for atranorin; ‘c’, for physciosporin; ‘d’, for tenuiorin. Lichen species L. cladonioides and U. longissimi were used as standard control for atranorin and usnic acid, respectively. (C) Chemical structure of physciosporin. (D-E) Migration assay of A549 cells treated with 5 μg/mL of acetone extract of P. coriacea or 5 μg/mL physciosporin (D), and quantitative analysis of wound length (E). (F–G) Invasion assays of A549 cells treated with 5 μg/mL of acetone extract of P. coriacea or physciosporin (F), and quantitative analysis of invaded cell numbers in each treatment (G). Quantitative data were obtained from three independent experiments, n = 3. Data represent mean ± S.E.M. (standard error of the mean). ***p<0.001 compared to DMSO-treated A549 cells.
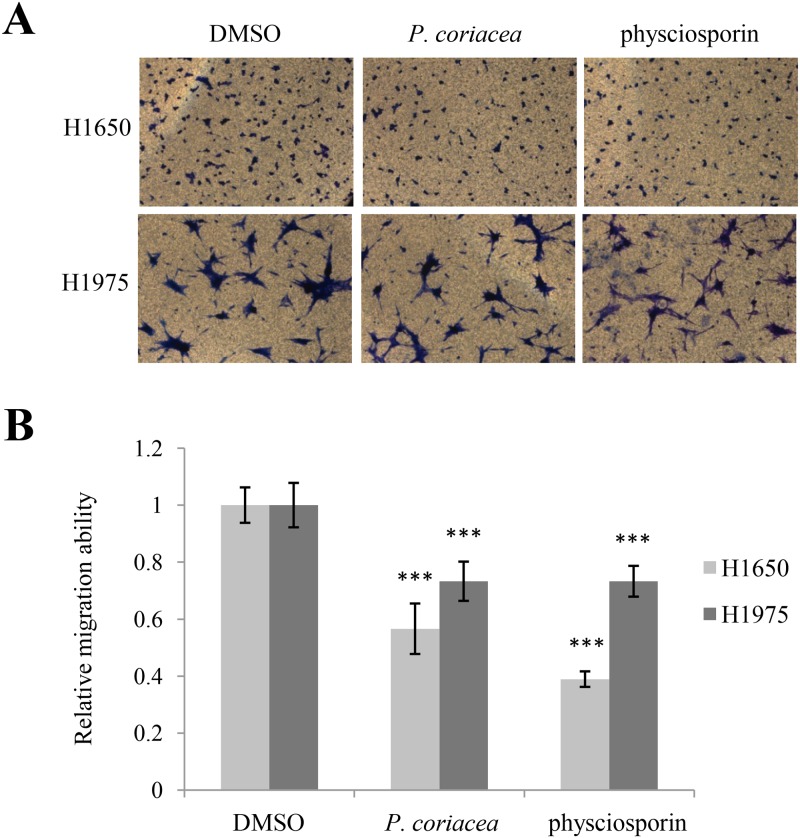
In order to check whether physciosporin is the active compound inhibiting A549 cell motility, physciosporin was tested in wound healing assays and invasion assays. Compared with the acetone extract of P. coriacea, physciosporin showed similar inhibitory activity against migration and invasion of A549 cells (Fig 2D–2G). In particular, physciosporin inhibited A549 cell invasion in a dose-dependent manner (Fig 2F and 2G). Interestingly, the inhibition of the purified compound was higher than that of crude extracts in the invasion assay but slightly lower in the migration assay (Fig 2E and 2G). Inhibitory activity of acetone extract of P. coriaea and physciosporin on lung cancer cell motility was also observed in H1650, H1975 cells (Fig 3). Cell viability was not altered at the given concentration of physciosporin (data not shown). Above all, these results demonstrated that physciosporin is an active lichen secondary metabolite from P. coriacea in the inhibition of lung cancer cell motility.
Fig 3. Acetone extract of P. coriacea and physciosporin inhibited lung cancer cell motility.
(A-B) Invasion assays of H1650 and H1975 lung cancer cells treated with 5 μg/mL of acetone extract of P. coriacea or 5 μg/mL physciosporin (A), and quantitative analysis of invaded cell number in each treatment (B). Quantitative data were obtained from three independent experiments, n = 3. Data represent mean ± S.E.M. (standard error of the mean). ***p<0.001 compared to DMSO-treated A549 cells.
Acetone extract of P. coriacea and physciosporin decreased the level of epithelial-mesenchymal transition marker
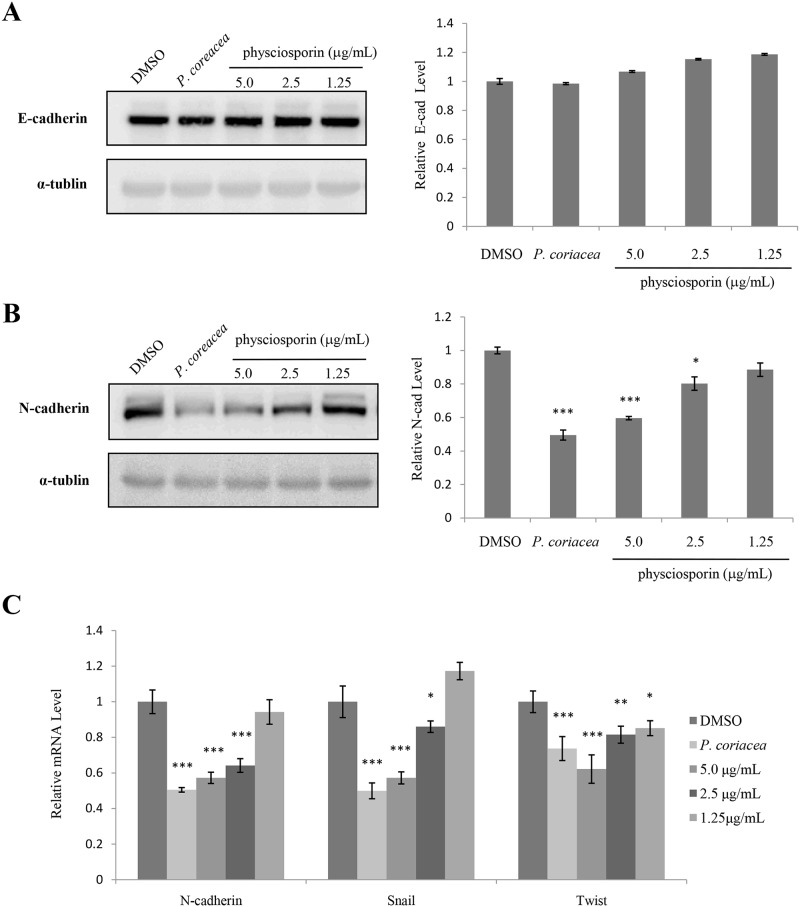
To investigate the mechanism of action for the inhibitory activity of physciosporin against lung cancer cell motility, the effects of acetone extract of P. coriacea and physciosporin were examined in several signaling pathways. First, changes in the levels of epithelial-mesenchymal (EMT) marker were measured in treated cells (Fig 4). In our study, decreases in the expression of N-cadherin were prominent in A549 cells treated with acetone extract of P. coriacea or physciosporin at both the protein and mRNA levels (Fig 4B and 4C), while those of E-cadherin remained marginal (Fig 4A and data not shown for E-cadherin mRNA). In addition, the expressions of Snail and Twist were dose-dependently decreased by acetone extract of P. coriacea or physciosporin treatment (Fig 4C). Taken together, these results suggest that acetone extract of P. coriacea and physciosporin have inhibitory activity against lung cancer cell motility in part through inhibition of EMT.
Fig 4. Acetone extract of P. coriacea and physciosporin decreased the level of epithelial-mesenchymal transition marker.
(A-B) Western blot analysis of E-cadherin (A) and N-cadherin (B) in A549 cells treated with 5 μg/mL of acetone extracts of P. coriacea or physciosporin. (C) Quantitative analysis of the mRNA levels of N-cadherin, Snail and Twist in A549 cells treated with 5 μg/mL of acetone extracts of P. coriacea or physciosporin. Quantitative data were obtained from at least two independent experiments. Data represent mean ± S.E.M. (standard error of the mean). *p<0.05; **p<0.01; ***p<0.001 compared to DMSO-treated A549 cells.
Acetone extract of P. coriacea and physciosporin suppressed KITENIN-mediated AP-1 activity and affected the expression of KAI1 and KITENIN
Next, changes in transcriptional activities of β-catenin/LEF-1 (TOPFLASH), AP-1 (AP-1-luc), NF-κB (NF-κB-luc) and STAT (STAT-luc) were measured in treated cells. In a preliminary test, only AP-1 activity was decreased by acetone extract of P. coriacea or physciosporin treatment in a dose-dependent manner in the absence of co-activator (data not shown). In our previous study, we showed that KITENIN (KAI1 C-terminal interacting tetraspanin) promotes the tumorigenic potential of cancers, and epidermal growth factor (EGF) stimulates KITENIN-mediated AP-1 activation [24]. To further test the effects of acetone extract of P. coriacea or physciosporin on KITENIN-mediated AP-1 activity, AP-1 reporter assays were performed in cells co-transfected with KITENIN. As shown in Fig 5A, KITENIN-mediated AP-1 activity was decreased by acetone extract of P. coriacea or physciosporin treatment in a dose-dependent manner, both with and without EGF stimulation. In order to investigate how acetone extract of P. coriacea and physciosporin suppressed KITENIN-mediated AP1 activity, we tested KITENIN levels in treated cells. KITENIN mRNA levels were decreased by the treatment (Fig 5B). The metastatic suppressor gene, KAI1, is often down-regulated in metastatic tumor cells [25, 26]. As KITENIN has an inverse relationship with KAI1 or other metastasis suppressor genes [27], we tested the mRNA level of KAI1 in treated cells and found a similar inverse relationship between KITENIN and KAI1 (Fig 5C). Taken together, these results suggest that acetone extract of P. coriacea and physciosporin inhibit A549 cell motility in part through the suppression of KITENIN-mediated AP-1 activity by regulating KITENIN and KAI1 expressions. To check how mRNA level of KITENIN can be changed by the treatment, luciferase assays for KITENIN promoter and 3’-UTR were performed. As a result, the activity of KITENIN 3’-UTR was dramatically diminished by the treatment (Fig 5E) while that of KITENIN promoter remained constant (Fig 5D).
Fig 5. Acetone extract of P. coriacea and physciosporin suppressed KITENIN-mediated AP-1 activity and affected the expression of KAI1 and KITENIN.
(A) AP-1 luciferase assay of HEK293T cells transfected with KITENIN and treated with 5 μg/mL of acetone extract of P. coriacea or physciosporin in the absence or presence of epidermal growth factor stimulation. (B-C) Quantitative analysis of the mRNA level of KITENIN (B) and KAI1 (C) in A549 cells treated with 5 μg/mL of acetone extract of P. coriacea or physciosporin. (D-E) KITENIN promoter (D) and 3’-UTR (E) luciferase assays of HEK293T cells treated with 5 μg/mL of acetone extract of P. coriacea or physciosporin. Quantitative data were obtained from at least three independent experiments. Data represent mean ± S.E.M. (standard error of the mean). *p<0.05; **p<0.01; ***p<0.001 compared to DMSO-treated cells.
Acetone extract of P. coriacea and physciosporin reduced RhoGTPase activity
Next, changes in the activities of RhoGTPases were measured in treated cells (Fig 6). To investigate the effects of acetone extract of P. coriacea and physciosporin on the activities of Cdc42 and Rac1, GST pull-down assays were performed using GST-PBD (p21-binding domain). The results showed that the activities of Cdc42 and Rac1 were decreased by 20% and 33%, respectively, in the presence of physciosporin (Fig 6A and 6B). RhoA activity was also decreased by acetone extract of P. coriacea and physciosporin (data not shown). Taken together, these results suggest that acetone extract of P. coriacea and physciosporin inhibit A549 cell motility in part through the reduction of the RhoGTPase activity. The results demonstrate that the lichen secondary metabolite, physciosporin, inhibits cancer cell motility with specific mechanisms of action.
Fig 6. Acetone extract of P. coriacea and physciosporin reduced RhoGTPase activity.
(A-B) The levels of GTP-bound Cdc42 (A) and Rac1 (B) were measured in A549 cells treated with 5 μg/mL of acetone extract of P. coriacea or physciosporin. GTP-Cdc42 and -Rac1 were measured using GST-PBD. The total amounts of Cdc42 and Rac1 were shown to measure relative activity. Quantitative data were obtained from three independent experiments, n = 3. Data represent the mean ± S.E.M. (standard error of the mean). ***p<0.001 compared to DMSO-treated A549 cells.
Discussion
A lot of unique chemicals have been isolated from lichen used for pharmaceutical purpose. In this study, an effective chemical compound physciosporin has been isolated from Chile lichen Pseudocyphellaria coriacea for their inhibitory activity against lung cancer cell motility. Physciosporin, a chlorinated depsidone, was first isolated in 1977 from the lichen Pseudocyphellaria physciospora and identified as methyl 2-chloro-4-formyl-3,8-dihydroxy-l,6,9-trimethyl-11-oxo-11H-dibenzo [b,e] [l,4] dioxepin-7-carboxylate (methyl 5-chlorovirensate) [22], but their biological activity has not been reported to date. In this study, we found the following: 1) the acetone extract of P. coriacea and its subcomponent, physciosporin, inhibit motility of lung cancer cells; 2) the extract of P. coriacea and physciosporin suppress EMT; 3) the extract of P. coriacea and physciosporin decrease KITENIN-mediated AP-1 activity and affect the expression of KAI1 and KITENIN; and 4) the extract of P. coriacea and physciosporin reduce RhoGTPase activity.
EMT, an essential phenotypic conversion during embryonic development, tissue remodeling and wound healing, plays an indispensable role in tumor invasion and metastasis [28–30]. Increases in N-cadherin expression and decreases in E-cadherin level (called “cadherin switching”) are crucial in EMT and arise during cancer progression [31]. Especially, the level of N-cadherin seems to represent the mesenchymal phenotype of the cells and a group of transcription factors including Snail, Slug, ZEB and Twist has a crucial role in the control of EMT [32]. Snail, a zinc-finger transcription factor first identified in Drosophila, is a key EMT regulator [33]. Twist also has an important role in cancer metastasis. For example, inhibition of Twist expression by short-interfering RNAs affects metastatic vigor [34], and the regulation of Twist-1 may contribute to resistance to paclitaxel and antimicrotubule drugs in cancer patients [35]. Our result showed that the levels of N-cadherin, Snail, and Twist were decreased by acetone extract of P. coriacea and physciosporin treatment while those of E-cadherin remained marginal (Fig 4). Of note is that, in some case, changes in E-cadherin level are not essential for EMT [36] or metastatic activity of tumor [37].
KITENIN is a metastasis-enhancing gene. Recently, we identified that KITENIN/ErbB4-Dvl2-c-Jun axis is an unconventional EGFR-independent downstream signal of EGF and mediates the invasiveness and tumorigenesis of cancer cells [24]. In our results, KITENIN-mediated AP-1 activity was decreased by the extract of P. coriacea and physciosporin. Especially, the expression of KITENIN was decreased by the treatment and, for this, 3’-UTR activity of KITENIN was dramatically diminished (Fig 5). Few KITENIN-targeting microRNAs suppressing the migration and invasion of the cells via modulating KITENIN expression were reported and miR-27a, miR-30b, and miR-124 are among the examples [15]. Currently, we do not know whether physciosporin likely acts as microRNAs to suppress 3’-UTR activity of KITENIN. However, as unconventional KITENIN/ErbB4-mediated downstream signal of EGF plays one of the molecular basis for conferring resistance to anti-EGFR agents, the extract of P. coriacea and physciosporin may have potential beneficial activity in overcoming the limited clinical efficacy of anti-EGFR therapy.
Rho GTPases, members of the Ras superfamily of small GTPases such as Rac1, Cdc42, and RhoA, are adhesion and growth factor–activated molecular switches that play important roles in tumor development and progression [38]. Cdc42 is responsible for the fibroblastic morphology that leads to pulmonary metastasis of the cells via a mesenchymal mode of migration [39], and Rac1 drives the mesenchymal mode of migration [40]. Rac and Cdc42 also play a role in cell–cell adhesion in epithelial cells in addition to their effects on the actin cytoskeleton and motility. Our result showed that the activities of Rho GTPases were decreased by acetone extract of P. coriacea and physciosporin treatment (Fig 6). All of above results showed that physciosporin has anti-metastatic activity with molecular mechanisms of action, and further study is required to evaluate the potential anticancer activity of physiosporin in various in vivo cancer models.
Supporting Information
(PDF)
Abbreviations
- DMSO
dimethylsulfoxide
- EGF
Epidermal Growth Factor
- HPLC
high-performance liquid chromatography
- KITENIN
KAI1 C-terminal interacting tetraspanin
- NMR
nuclear magnetic resonance
- PBD
p21 binding domain
- TLC
Thin Layer Chromatography
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the National Research Foundation of Korea (NRF-2013R1A1A2004677, MRC-2011-0030132) and by the Korea National Research Resource Center Program (NRF-2014M3A9B8002115). This study also received support from a research grant funded by the Sunchon Research Center for Natural Medicine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(1):9–29. Epub 2014/01/09. 10.3322/caac.21208 . [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer Journal international du cancer. 2015;136(5):E359–86. Epub 2014/09/16. 10.1002/ijc.29210 . [DOI] [PubMed] [Google Scholar]
- 3. Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA: a cancer journal for clinicians. 2000;50(1):7–33. Epub 2000/03/29. . [DOI] [PubMed] [Google Scholar]
- 4. Lung cancer: a global scourge. Lancet. 2013;382(9893):659 Epub 2013/08/27. 10.1016/S0140-6736(13)61759-6 . [DOI] [PubMed] [Google Scholar]
- 5. Reck M, Heigener DF, Mok T, Soria J-C, Rabe KF. Management of non-small-cell lung cancer: recent developments. The Lancet. 2013;382(9893):709–19. [DOI] [PubMed] [Google Scholar]
- 6. Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nature Reviews Cancer. 2003;3(5):362–74. [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto Y, Hara K, Kawakami H, Komine M. Lichen Substances and Their Biological Activities Recent Advances in Lichenology: Springer; 2015. p. 181–99. [Google Scholar]
- 8. Kim H, Kim KK, Hur JS. Anticancer activity of lichen metabolites and their mechanisms at the molecular level In: Upreti DK, Divakar PK, Shukla V, Bajpai R, editors. Modern methods and approaches in lichen systematics and culture techniques. 2: Springer; India; 2015. p. 201–8. [Google Scholar]
- 9. Stanojković T. Investigations of Lichen Secondary Metabolites with Potential Anticancer Activity Lichen Secondary Metabolites: Springer; 2015. p. 127–46. [Google Scholar]
- 10. Culberson CF. Improved conditions and new data for the identification of lichen products by a standardized thin-layer chromatographic method. Journal of chromatography. 1972;72(1):113–25. Epub 1972/10/05. . [DOI] [PubMed] [Google Scholar]
- 11. White F, James PW. A new guide to microchemical techniques for the identification of lichen substances: British Lichen Society; 1985. [Google Scholar]
- 12. Orange A, James PW, White F. Microchemical methods for the identification of lichens: Twayne Publishers; 2001. [Google Scholar]
- 13. Kim H, Ki H, Park HS, Kim K. Presenilin-1 D257A and D385A mutants fail to cleave Notch in their endoproteolyzed forms, but only presenilin-1 D385A mutant can restore its gamma-secretase activity with the compensatory overexpression of normal C-terminal fragment. The Journal of biological chemistry. 2005;280(23):22462–72. Epub 2005/03/31. . [DOI] [PubMed] [Google Scholar]
- 14. Nguyen TT, Yoon S, Yang Y, Lee HB, Oh S, Jeong MH, et al. Lichen secondary metabolites in Flavocetraria cucullata exhibit anti-cancer effects on human cancer cells through the induction of apoptosis and suppression of tumorigenic potentials. PloS one. 2014;9(10):e111575 Epub 2014/11/02. 10.1371/journal.pone.0111575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park SY, Kim H, Yoon S, Bae JA, Choi SY, Jung YD, et al. KITENIN-targeting microRNA-124 suppresses colorectal cancer cell motility and tumorigenesis. Molecular therapy: the journal of the American Society of Gene Therapy. 2014;22(9):1653–64. Epub 2014/06/10. 10.1038/mt.2014.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim H, Han J-R, Park J, Oh M, James SE, Chang S, et al. δ-Catenin-induced Dendritic Morphogenesis AN ESSENTIAL ROLE OF p190RhoGEF INTERACTION THROUGH AKT1-MEDIATED PHOSPHORYLATION. Journal of Biological Chemistry. 2008;283(2):977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galloway DJ. Flora of New Zealand: Lichens, including lichen-forming and lichenicolous fungi, Vol 1 and 2: Manaaki Whenua Press, Landcare Research; 2007. [Google Scholar]
- 18. Brodo IM, Sharnoff SD, Sharnoff S. Lichens of north America: Yale University Press; 2001. [Google Scholar]
- 19. Galloway D. Nomenclatural notes on Pseudocyphellaria IV: some South American taxa. The Lichenologist. 1989;21(01):88–9. [Google Scholar]
- 20. Kinoshista K, Matsubara H, Koyama K, Takahashi K, Yoshimura I, Yamamoto Y, et al. New phenolics from Protousnea species. Hattori Shokubutsu Kenkyujo Hokoku. 1994;75:359–64. [Google Scholar]
- 21. Wilkins AL, James P. The chemistry of Pseudocyphellaria impressa S. Lat. in New Zealand. The Lichenologist. 1979;11(03):271–81. [Google Scholar]
- 22. Maass W, McInnes A, Smith D, Taylor A. Lichen substances. X. Physciosporin, a new chlorinated depsidone. Canadian Journal of Chemistry. 1977;55(15):2839–44. [Google Scholar]
- 23. McCarthy PM. Flora of Australia Volume 58A: Lichens 3: CSIRO Publishing; 2001. [Google Scholar]
- 24. Bae JA, Yoon S, Park S-Y, Lee JH, Hwang J-E, Kim H, et al. An Unconventional KITENIN/ErbB4-Mediated Downstream Signal of EGF Upregulates c-Jun and the Invasiveness of Colorectal Cancer Cells. Clinical Cancer Research. 2014;20(15):4115–28. 10.1158/1078-0432.CCR-13-2863 [DOI] [PubMed] [Google Scholar]
- 25. Dong JT, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT, et al. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. 1995;268(5212):884–6. Epub 1995/05/12. . [DOI] [PubMed] [Google Scholar]
- 26. Ueda T, Ichikawa T, Tamaru J, Mikata A, Akakura K, Akimoto S, et al. Expression of the KAI1 protein in benign prostatic hyperplasia and prostate cancer. The American journal of pathology. 1996;149(5):1435–40. Epub 1996/11/01. [PMC free article] [PubMed] [Google Scholar]
- 27. Lee JH, Park SR, Chay K-O, Seo Y-W, Kook H, Ahn KY, et al. KAI1 COOH-terminal interacting tetraspanin (KITENIN), a member of the tetraspanin family, interacts with KAI1, a tumor metastasis suppressor, and enhances metastasis of cancer. Cancer research. 2004;64(12):4235–43. [DOI] [PubMed] [Google Scholar]
- 28. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 29. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119(6):1420 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu Y, Zhou BP. Snail: more than EMT. Cell adhesion & migration. 2010;4(2):199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. Journal of cell science. 2008;121(6):727–35. [DOI] [PubMed] [Google Scholar]
- 32. Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nature Reviews Cancer. 2007;7(6):415–28. [DOI] [PubMed] [Google Scholar]
- 33. Nieto MA. The snail superfamily of zinc-finger transcription factors. Nature reviews Molecular cell biology. 2002;3(3):155–66. [DOI] [PubMed] [Google Scholar]
- 34. Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–39. Epub 2004/06/24. 10.1016/j.cell.2004.06.006 . [DOI] [PubMed] [Google Scholar]
- 35. Wang X, Ling MT, Guan X-Y, Tsao SW, Cheung HW, Lee DT, et al. Identification of a novel function of TWIST, a bHLH protein, in the development of acquired taxol resistance in human cancer cells. Oncogene. 2004;23(2):474–82. [DOI] [PubMed] [Google Scholar]
- 36. Nilsson GM, Akhtar N, Kannius-Janson M, Baeckstrom D. Loss of E-cadherin expression is not a prerequisite for c-erbB2-induced epithelial-mesenchymal transition. International journal of oncology. 2014;45(1):82–94. Epub 2014/05/09. 10.3892/ijo.2014.2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu X, Huang H, Remmers N, Hollingsworth MA. Loss of E-cadherin and epithelial to mesenchymal transition is not required for cell motility in tissues or for metastasis. Tissue barriers. 2014;2(4):e969112 Epub 2015/01/23. 10.4161/21688362.2014.969112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prudnikova TY, Rawat SJ, Chernoff J. Molecular Pathways: Targeting the Kinase Effectors of RHO-Family GTPases. Clinical Cancer Research. 2015;21(1):24–9. 10.1158/1078-0432.CCR-14-0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yui Y, Itoh K, Yoshioka K, Naka N, Watanabe M, Hiraumi Y, et al. Mesenchymal mode of migration participates in pulmonary metastasis of mouse osteosarcoma LM8. Clinical & experimental metastasis. 2010;27(8):619–30. [DOI] [PubMed] [Google Scholar]
- 40. Murali A, Rajalingam K. Small Rho GTPases in the control of cell shape and mobility. Cellular and Molecular Life Sciences. 2014;71(9):1703–21. 10.1007/s00018-013-1519-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.