Abstract
Background
Whether the occurrence of posterior atrophy (PA) and medial temporal lobe atrophy (MTA) was correlated with cognitive and non-cognitive symptoms in Alzheimer’s disease (AD) and mild cognitive impairment (MCI) patients are unclear.
Methods
Patients with probable AD and MCI from a medical center outpatient clinic received attention, memory, language, executive function evaluation and Mini-Mental Status Examination (MMSE). The severity of dementia was rated by the Clinical Dementia Rating (CDR) Sum of Box (CDR-SB). The neuropsychiatric inventory (NPI) subscale of agitation/aggression and mood symptoms was also applied. Magnetic resonance imaging (MRI) was scored visually for the MTA, PA and white matter hyperintensity (WMH) scores.
Results
We recruited 129 AD and 31 MCI (mean age 78.8 years, 48% female) patients. MMSE scores, memory, language and executive function were all significantly decreased in individuals with AD than those with MCI (p < 0.01). MTA and PA scores reflected significant atrophy in AD compared to MCI; however, the WMH scores did not differ. The MTA scores were significantly correlated with the frontal, parieto-occipital and global WMH scores (p < 0.01) while the PA scores showed a correlation with the parieto-occipital and temporal WMH scores (p < 0.01). After adjusting for age, education, APOE4 gene and diagnostic group covariates, the MTA scores showed a significant association with MMSE and CDR-SB, while the right side PA scores were significantly associated with NPI-agitation/aggression subscales (p < 0.01).
Conclusion
Regional atrophy is related to different symptoms in patients with AD or MCI. PA score is useful as a complementary measure for non-cognitive symptom.
Introduction
Alzheimer’s disease (AD) is the most common form of dementia worldwide. Recently, a nationwide survey in Taiwan showed the age-adjusted prevalence of 8.04% for all type of dementia [1]. In AD, the earliest prominent cognitive symptoms can be characterized as either amnestic or non-amnestic dysfunction [2]. This variable presentation, which reflects the heterogeneity of AD, may be related to the initial dysfunction of the different brain regions as well as additional factors such as genetic or neuropathological findings [3–5]. In addition, 75–90% of AD patients develop “non-cognitive” behavior and psychological symptoms of dementia (BPSD), which are classified into various clusters using the Neuropsychiatric Inventory (NPI) [6–8]. These non-cognitive symptoms have been linked to the dysfunction of different brain structures such as limbic system or cerebral white matter [9–12]. To study the relationships between behavior symptoms and regional brain dysfunction, non-invasive neuroimaging tools, such as brain magnetic resonance imaging (MRI) are extremely helpful.
In structural brain MRI, many automatic computational measurements could be derived, such as regional brain volume, cortical thickness and surface area [13–15]. These measurements allow for an objective evaluation of structural integrity while also permitting the assessment of the entire brain. To gain a precise quantification of brain volume using automatic computational measurements, sophisticated and time-consuming post-imaging analyses are necessary, which may also require specific scanning protocols. In contrast, the visual rating of MR scans can yield sufficient regional anatomical information for both clinical diagnosis and research, with the advantage of speed, flexibility and clinical applicability. Thus, visual rating methods may serve as an appealing alternative to computational methods [16, 17]
Recently-developed visual rating system have been applied to the evaluation of medial temporal lobe atrophy (MTA) and posterior atrophy (PA); these analyses showed that regional atrophy is associated with impairment in specific cognitive domains in AD, providing support for the utility of these method in the diagnostic evaluation of AD [18–21]. These rating scales also had good inter and intra-rater reliability [19, 22]. However, it is unknown whether similar associations may exist between neuropsychiatric, or BPSD symptoms and MTA and PA scores. In the current work, we have endeavored to address this question by examining the relationships between these visual rating scores and clinical symptoms in patients diagnosed with AD as well as those with mild cognitive impairment (MCI).
Methods
Participants
Subjects were recruited from the outpatient clinic of Taipei Veterans General Hospital between August 2012 and July 2014. As part of the diagnostic procedure, all patients received a standardized assessment that included dementia history, neuropsychological assessment, laboratory tests and MRI scans. Diagnoses were made during a multidisciplinary consensus meeting. The diagnosis of probable AD followed the clinical criteria for probable AD described by the National Institute on Aging–Alzheimer’s Association [2]. The diagnosis of MCI was made according to the revised 2004 consensus criteria [23]. The cut-off value for a diagnosis of MCI was set to 1.5 SD below the age-adjusted norm for the logical memory test of the Wechsler Memory Scale III (WMS-III)[24]. The logical memory test component of the WMS-III assesses verbal memory by asking the subject to recall two stories immediately following an oral presentation (Part I), and again after a 30-min delay (Part II)[25]. The disease duration was defined as the period between the initial onset of symptom reported by caregiver and the performance of this study. Genomic DNA was isolated from whole blood using the Gentra Puregene kit according to the manufacturer’s protocols (Qiagen, Hilden, Germany). The presence of the ε2, ε3, and ε4 alleles of the APOE gene were determined by assessing the sequences at two SNPs (rs429358 and rs7412) [26, 27]. The APOE4 gene carrier was defined as one of alleles contain ε4 gene.
Neuropsychological assessment
Cognitive function was assessed using standardized tests that included several domains. The Mini-Mental Status Examination (MMSE) was used to assess global cognition. To evaluate the severity of dementia, the Clinical Dementia Rating (CDR) scores and sum of box (CDR-SB) tests were given with the caregiver input [28–30]. To assess memory, we used the delay recall of 12-items memory test [31, 32]. To examine the language function, we used both a modified Boston Naming Test [33]. A category verbal fluency test was used to evaluate both verbal ability and executive control ability. In the verbal fluency test, the subject named as many fruits as possible within 1 minute [34]. A forward and backward digit span test was used to assess attention and working memory domain [35]. Finally, the Neuropsychiatric Inventory (NPI) questionnaire was administered to assess the frequency and severity of BPSD [36–38]. Two NPI subscale scores were used in this study, namely, NPI-agitation and NPI-mood symptoms subscales [8]. The NPI-agitation/aggression subscale score included agitation/aggression, dis-inhibition, irritability/lability and aberrant motor behavior items from NPI. The NPI-mood subscale score included depression, anxiety and irritability/lability items from NPI. Each subscale score was calculated by the sum of the items’ severity multiplied by its duration.
MRI and image analysis
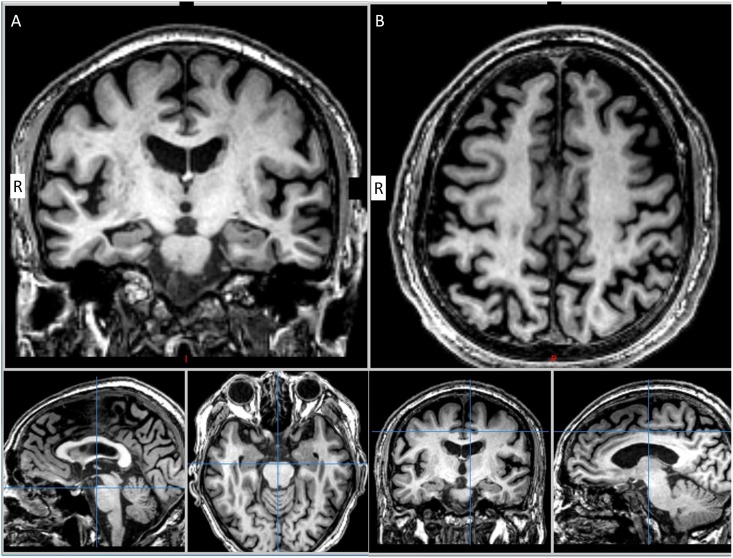
All participants received whole-brain MRI scans (GE, 3T DISCOVERY 750) in the clinical assessment. First, trans-axial T2-weighted scans (TR/TE = 1130/80 ms, NEX = 2, voxel size 0.55 x 0.55 x 10 mm3), 3D fluid-attenuated inversion Recovery (FLAIR) images (TR/TE = 6000/126 ms, inversion time 1861 ms, NEX = 1, voxel size 0.56 x 0.56 x 1 mm3), and high-resolution sagittal T1-weighted images (TR/TE = 9.1/3.7 ms, NEX = 1, voxel size 0.5 x 0.5 x 1.0 mm3) were acquired. The image analysis included a visual rating of MTA and PA on the T1-weighted images. T1-weighted images were viewed in the coronal plane, and MTA scores for the left and right hemispheres were given. MTA was rated on a 5-point scale (0 point, absent; 1 point, minimal; 2 points, mild; 3 points, moderate; and 4 points, severe) based on the height of the hippocampal formation and the width of the choroid fissure and the temporal horn [20]. PA was rated on a 4-point scale (0 point, absent; 1 point, mild sulcal widening and mild atrophy; 2 points, substantial widening and substantial atrophy; and 3 points, end-stage atrophy) based on the posterior cingulate and parieto-occipital sulcus and sulci of the parietal lobes and precuneus [19]. Fig 1 demonstrates the moderate atrophy of MTA and PA scores. The mean scores for MTA and PA from both hemispheres were calculated for statistic analysis. White matter hyperintensity (WMH) was evaluated using a scale of age-related white matter change (ARWMC) scale on both axial T2-weighted image and 3D-FLAIR images [39]. The ARWMC scale rates WMH on a 4-points scale (0 point, no lesions; 1 point, focal lesions; 2 points, beginning confluence of lesions; and 3 points, diffuse involvement of the entire region, with or without involvement of U fibers). A total of five locations (frontal, parieto-occipital, temporal, basal ganglia and infratentorium) were assessed and the sum of both left and right hemisphere ARWMC scores in each location were used for further analysis. All the visual rating scores were evaluated by one of the author (Dr. Hsu). To confirm the consistency of the visual rating scores between investigators, the first 24 AD cases were selected to have both the MTA and PA score evaluated by both Dr. Hsu and Prof. Philip Scheltens [20]. The Ethics Committee and Institutional Review Board of Taipei Veterans General Hospital approved the study. The written informed consent was provided by the participants and their next of kin or legally authorized representatives.
Fig 1. Illustrating the moderate atrophy of MTA and PA scores.
(A). the right side MTA score is 2 and the left side MTA score is 1. (B). the both side of PA score is 2. R: right side.
Statistic analysis
All statistic analyses were performed using SPSS (version 21.0). Independent two sample t-tests and X2 tests were conducted to compare age, gender, neuropsychological tests results, MTA, PA and ARWMC scores between AD and MCI patients. The kappa test was used to assess the inter-rater reliability in visual rating scores. Correlation analysis using Pearson correlation coefficient values was conducted to study the relationship between visual rating scales and age, education, MMSE, CDR-SB and NPI subscales. To assess the relationships between the visual rating scores, cognitive functions and neuropsychiatric symptoms, we used regression analysis with MTA and PA as independent variables, and MMSE, CDR-SB and NPI subscale scores as dependent variables. Age, education, APOE4 gene data and diagnostic group were entered as covariates. Statistic significance was defined as a p-value < 0.01.
Results
A total 160 patients (probable AD, n = 129; amnestic MCI, n = 31) were recruited during the study period. The average age (mean + standard deviation) was 79.6 + 7.8 years for the AD and 75.4 + 8.6 years for the MCI patients (p = 0.02). Female patients represented 50.4% of the AD and 38.7% of the MCI patients (Table 1). The average disease duration was significant longer in AD group (AD vs. MCI: 41.3 + 48.8 months vs. 26.3 + 21.3 months, p < 0.01). Gender distribution, years of education, and body mass index (BMI) did not differ between the AD and MCI groups. The mean MMSE, delayed recall of 12-item memory test, category verbal fluency and modified Boston naming test were significant lower in the AD than in the MCI groups (p < 0.01). In measurements of non-cognitive symptoms, the AD group displayed significantly higher average subscale scores for NPI-agitation/aggression symptoms and NPI-mood symptoms than did the MCI group. There was also a trend towards a higher proportion of ApoE4 carriers in the AD compared to the MCI group (p < 0.02).
Table 1. Demographic and clinical data of AD and MCI patients.
| AD (n = 129) | MCI (n = 31) | P value* | |
|---|---|---|---|
| Female patients | 65 (50.4%) | 12 (38.7%) | 0.25 |
| Age | 79.6 (7.8) | 75.4 (8.6) | 0.02 |
| Education years | 9.9 (4.4) | 11.1 (4.7) | 0.19 |
| Disease duration (months) | 41.3 (48.8) | 26.3 (21.3) | 0.01 |
| BMI | 23.7 (3.3) | 24.3 (3.9) | 0.41 |
| MMSE | 18.9 (5.5) | 25.5 (3.3) | < 0.001 |
| Delayed recall of 12-item Memory test | 1.3 (2.0) | 4.8 (2.7) | < 0.001 |
| Forward digit span | 8.7 (2.9) | 9.7 (2.4) | 0.04 |
| Backward digit span | 4.2 (1.9) | 5.2 (2.3) | 0.03 |
| Category verbal fluency | 6.7 (2.9) | 9.1 (2.5) | < 0.001 |
| Modified Boston naming test | 11.5 (2.8) | 13.3 (1.9) | < 0.001 |
| NPI-agitation/aggression subscale | 7.0 (10.9) | 1.2 (2.8) | < 0.001 |
| NPI-mood subscale | 5.4 (8.9) | 1.6 (4.3) | 0.001 |
| CDR | < 0.001 | ||
| 0.5 | 12 (9.3%) | 31 (100%) | |
| 1 | 89 (68.9%) | ||
| 2 | 25 (19.4%) | ||
| 3 | 3 (2.3%) | ||
| ApoE4 carrier | 44 (34.1%) | 4 (12.9%) | 0.02 |
Values are means with standard deviations unless otherwise indicated. Values in boldface indicate statistically significant differences between groups.
AD, Alzheimer’s disease; MCI, mild cognitive impairment; BMI, body mass index; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; CDR, clinical dementia rating;
* Independent two sample t-tests or Chi-square tests.
MRI rating
We observed a moderate agreement between the rater and the original author reflected by the inter-rater reliability of the visual rating MTA and PA scores. The Kappa coefficient agreement varies from 0.51 and 0.51 for both hemisphere scores of MTA (p < 0.001 on both side) and 0.57 and 0.43 for both hemisphere scores of PA scores (p < 0.001 on the right side and p = 0.001 on the left side). There was a significantly higher MTA and PA scores for the AD vs. MCI patients. However, for the WMH measurements, the total and the regional ARWMC scores did not differ significantly between the AD and MCI group (Table 2). The MTA and PA scores within each hemisphere did not show a significant correlation (Pearson correlation coefficient: 0.17, p = 0.03 for the right side and 0.13, p = 0.12 for the left side). In terms of anatomical association, we observed significant correlations between the MTA score and the frontal, parieto-occipital and total WMH scores were found in both the AD and the MCI group (Pearson correlation coefficient: 0.36, 0.28 and 0.31 respectively, all p < 0.01). The PA scores also showed a significant association with the parieto-occipital and temporal WMH scores (Pearson correlation coefficient: 0.21 and 0.21, p < 0.01) (Table 3).
Table 2. Brain MRI visual rating scores of AD and MCI patients.
| AD (n = 129) | MCI (n = 31) | P value* | |
|---|---|---|---|
| MTA | |||
| Right | 1.9 (0.9) | 1.4 (0.9) | 0.01 |
| Left | 2.0 (0.9) | 1.5 (0.9) | 0.006 |
| Mean | 1.9 (0.8) | 1.4 (0.9) | 0.006 |
| PA | |||
| Right | 1.5 (0.6) | 1.1 (0.7) | 0.002 |
| Left | 1.5 (0.7) | 0.9 (0.6) | < 0.001 |
| Mean | 1.5 (0.6) | 1.0 (0.6) | < 0.001 |
| WMH | |||
| Frontal | 2.7 (2.1) | 2.5 (2.0) | 0.70 |
| Right | 1.3 (1.0) | 1.3 (1.0) | 0.74 |
| Left | 1.4 (1.0) | 1.3 (1.1) | 0.67 |
| Parieto-occipital | 2.6 (2.2) | 2.4 (2.1) | 0.60 |
| Right | 1.4 (1.1) | 1.2 (1.1) | 0.51 |
| Left | 1.3 (1.1) | 1.2 (1.1) | 0.70 |
| Temporal | 1.0 (1.7) | 0.9 (1.6) | 0.65 |
| Right | 0.5 (0.9) | 0.4 (0.8) | 0.46 |
| Left | 0.5 (0.9) | 0.5 (0.9) | 0.87 |
| Basal ganglion | 0.1 (0.6) | 0.1 (0.4) | 0.51 |
| Right | 0.1 (0.3) | 0.0 (0.2) | 0.33 |
| Left | 0.1 (0.3) | 0.0 (0.2) | 0.53 |
| Infratentorium | 0.0 (0.2) | 0.0 (0.0) | 0.18 |
| Right | 0.0 (0.2) | 0.0 (0.0) | 0.08 |
| Left | 0.0 (0.1) | 0.0 (0.0) | 0.32 |
| Total | 6.5 (5.5) | 5.9 (5.1) | 0.53 |
Values are means with standard deviations. Values in boldface indicate statistically significant differences between groups.
MRI, magnetic resonance imaging; AD, Alzheimer’s disease; MCI, mild cognitive impairment; MTA, medial temporal atrophy; PA, posterior atrophy; WMH, white matter hyperintensity;
* Independent two sample t-test tests.
Table 3. Correlation analysis of brain MRI visual rating scores in AD and MCI patients.
| MTA | PA | FWMH | POWMH | TWMH | TOMWH | |
|---|---|---|---|---|---|---|
| MTA | - | 0.16 | 0.36* | 0.28* | 0.18 | 0.31* |
| PA | - | 0.10 | 0.21* | 0.21* | 0.17 | |
| FWMH | - | 0.78* | 0.60* | 0.90* | ||
| POWMH | - | 0.65* | 0.92* | |||
| TWMH | - | 0.82* | ||||
| TOWMH | - |
Values are Pearson correlation coefficients.
MRI, magnetic resonance imaging; AD, Alzheimer’s disease; MCI, mild cognitive impairment; MTA, medial temporal atrophy; PA, posterior atrophy; FWMH, frontal white matter hyperintensity; POWMH, parieto-occipital white matter hyperintensity; TWMH, temporal white matter hyperintensity; TOWMH, total white matter hyperintensity.
*p < 0.01.
We then explored the relationship between the visual rating scores and the clinical parameters that were assessed. MTA scores showed a significant positive association with age and CDR-SB for both the AD and MCI groups. Additionally, MTA scores showed a significant negative correlation with the MMSE scores in both the AD and MCI patients. Only the age and the NPI-agitation/aggression subscale scores were significantly correlated with PA (Table 4).
Table 4. Correlation analysis of clinical data and brain MRI visual rating scores in AD and MCI patients.
| Age | Education years | MMSE | CDR-SB | NPI-agitation /aggression | NPI-mood | |
|---|---|---|---|---|---|---|
| MTA | 0.31* | 0.19 | -0.30* | 0.34* | 0.18 | 0.09 |
| PA | 0.40* | 0.06 | -0.09 | 0.15 | 0.26* | 0.16 |
| FWMH | 0.27* | 0.04 | -0.14 | 0.10 | 0.14 | 0.13 |
| POWMH | 0.32* | 0.07 | -0.00 | 0.03 | 0.17 | 0.19 |
| TWMH | 0.19 | 0.16 | -0.02 | 0.07 | 0.22* | 0.23 |
| TOWMH | 0.28* | 0.08 | -0.06 | 0.08 | 0.20 | 0.22* |
Values are Pearson correlation coefficients.
MRI, magnetic resonance imaging; AD, Alzheimer’s disease; MCI, mild cognitive impairment; MTA, medial temporal atrophy; PA, posterior atrophy; FWMH, frontal white matter hyperintensity; POWMH, parieto-occipital white matter hyperintensity; TWMH, temporal white matter hyperintensity; TOWMH, total white matter hyperintensity; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; CDR-SB, clinical dementia rating sum of boxes.
*p < 0.01.
To further evaluate the relationship between the visual rating scores and the clinical measurements, we performed a multivariate regression analysis. Following adjustments for age, education, APOE4 gene and diagnostic group, the MMSE was still significantly correlated with MTA scores. We also observed a significant association of the CDR-SB score with MTA. PA scores, in contrast, did not exhibit any significant correlation with MMSE and CDR-SB scores following adjustments for age and education covariates (p = 0.56 and P = 0.28). The PA, but not the MTA, score show a trend of association with the NPI-agitation/aggression subscale values following adjustment for age (p = 0.03 for PA vs. P = 0.18 for MTA). We further separated the right and left hemisphere PA scores to perform the regression study. In this regression analysis, the right hemisphere PA scores showed a higher R-square value than did the left hemisphere scores (R-square = 0.36, p = 0.003 (right) vs. R-square = 0.29, p = 0.19 (left); Table 5).
Table 5. Summary of regression analyses for variables predicting the MMSE, CDR-SB scores and subscale of NPI-agitation scores in AD and MCI patients.
| Variables entered | R | R2 | R2 Change | F Change | df |
|---|---|---|---|---|---|
| MMSE as dependent variable | |||||
| Block 1. Age, education years, APOE4 carrier and diagnostic group | 0.49 | 0.24 | 0.24 | 11.97* | 155 |
| Block 2. MTA* | 0.55 | 0.30 | 0.07 | 14.93* | 154 |
| CDR-SB as dependent variable | |||||
| Block 1. Age, education years, APOE4 carrier and diagnostic group | 0.52 | 0.27 | 0.27 | 14.01* | 155 |
| Block 2. MTA* | 0.57 | 0.32 | 0.05 | 12.27* | 154 |
| NPI-agitation/aggression as dependent variable | |||||
| Block 1. Age, education years, APOE4 carrier and diagnostic group | 0.28 | 0.08 | 0.08 | 3.25 | 155 |
| Block 2. PA (right side)* | 0.36 | 0.13 | 0.05 | 8.79* | 154 |
MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; CDR-SB, clinical dementia rating sum of boxes; AD, Alzheimer’s dementia; MCI, mild cognitive impairment; MTA, medial temporal atrophy visual rating score.
*p < 0.01
Discussion
In this study, we used a method of simple visual rating to evaluate the association between morphological brain features and clinical symptoms. We show that MTA scores are correlated with performance on the MMSE and CDR-SB scores, measures of cognition, in AD and MCI patients, whereas PA scores were associated with scores on the NPI-agitation/aggression subscale. These findings provide support for the idea that regional atrophy in different brain structure may be intimately related to cognitive and non-cognitive symptoms in AD and MCI. Moreover, we provide the first evidence that the degree of PA score correlates with non-cognitive symptoms, as measured by the NPI subscales. Our findings further show that the application of visual rating scores (both MTA and PA) is useful for the evaluation of AD and MCI patients in clinical studies.
The MTA score had been in use since 1992, and it is one of the widely-used visual rating systems used in clinical and research field [20, 40]. Evidence for MTA has previously been used to support the diagnosis of AD [20, 41]. In studies of cognition, the MTA score has been shown to correlate with both memory and executive function [42–44]. In previous studies, the degree of MTA has also been applied to predict the conversion from MCI to AD [45, 46]. The MTA score may be influenced by the age and WMH but it appears to be independent of the PA score, at least in AD patients [19, 47, 48]. Consistent with previous findings, our current study found that MTA scores were correlated with age (Pearson correlation coefficient = 0.31, p < 0.01) but not with PA scores (Pearson correlation coefficient = 0.16, p = 0.04). In addition, MTA scores showed a significant correlation with both global and regional WMH scores (both frontal and parieto-occipital), in line with previous literatures [47–49].
The PA score is a recently developed visual rating scale that has been shown to be able to discriminated AD from dementia with Lewy bodies and frontotemporal lobar degeneration [19]. Furthermore, PA ratings can distinguish between younger control subjects and early-onset AD, but not between older control subjects and late-onset AD [21]. AD patients with higher PA scores have been shown to have worse performance on tasks of visuospatial and executive function [18]. In the current work, we observed that PA scores showed a significant correlation with posterior regional WMH (parieto-occipital and temporal regions) in AD and MCI patients. This anatomical association may reflect concomitant regional gray matter and white matter changes in the brain. We did not find any significant association between PA scores and digit backward and category verbal fluency test in both AD and MCI patients (p = 0.18 and p = 0.33). In addition, only a trend was observed for a correlation between the PA scores and the digit backward in AD group (p = 0.02).
In tests of non-cognitive symptoms, we found that the mean PA scores had a trend of association with scores on the NPI-agitation/aggression subscale (p = 0.03). More specifically, we found that PA scores in the right hemisphere, but not the left, were significantly associated with the NPI-agitation/aggression subscale. In addition to the PA, we observed that the temporal WMH scores is significant correlated with scores of the NPI-agitation/aggression subscale. Symptoms of agitation/aggression have previously been shown to be associated with brain structure such as lateral frontal, parietal, temporal and anterior cingulate gyrus in AD or MCI patients [12, 50–53]. Another study showed that right fronto-temporal dysfunction may be linked to aggression behavior in dementia patients [54]. In a functional MRI study, Kumari et al found that bilateral frontal and right inferior parietal lobe activity was associated with aggression behavior in schizophrenia patients [55, 56]. From previous studies, the right hemisphere rather than left hemisphere may have stronger association with aggression behavior [54, 56]. Our results demonstrate that both right PA and temporal WMH scores have a significant association with ratings on the NPI-agitation/aggression subscale in both AD and MCI patients, which has not been reported before.
Limitation
There had some limitations to the current work. First, we have no postmortem data from the individuals that comprise our dataset. Therefore, the possibility of misdiagnosis cannot be ruled out. Nevertheless, we used the standardized workup procedure and all patients fulfilled the criteria of probable AD and MCI. These standardized measures may help to reduce the potential likelihood of the misclassification of patients. Secondary, only two atrophy measures were used in the current study and the PA score was measured from a large aggregate of regions, therefore the finer localization was not possible. Both MTA and PA are the areas that are more commonly affected in AD than MCI. The kappa values of interrater reliability of MTA and PA in the current study are around 0.5, which is in moderate agreement. It might influence the study findings. We included the APOE4 gene data and diagnostic group as covariates in multivariate regression analysis to decrease the confounding effect. Third, the irritability/lability from NPI items were included in both NPI-agitation and NPI-mood symptoms subscales according our prior study. The multivariate regression analysis between irritability scores and PA scores is non-significant. It needs further study to confirm this finding. Additionally, we did not perform an extensive cognitive examination that included assessments of executive, visuospatial and praxis function. The category verbal fluency is more related to measure the semantic memory function then executive function [57]. The lack of such data may restrict the interpretation of our study as to the relationship between the PA score and more subtle aspects of cognition function. A great strength of the current study, we believe, is the inclusion of the NPI assessment to explore non-cognitive symptoms in MCI and AD patients, and how these may relate to aspects of brain structure.
Conclusion
Our results showed that, in MCI and AD patients, data gained by the visual rating of MTA and PA scores are not a reduplication data; rather they provide complementary information relevant to both cognitive and non-cognitive symptoms. In addition, we find that the visual rating of PA scores provides additional relevance to symptoms of agitation. Further study, with a large patient sample, combined with a more detailed battery of neuropsychological tests, will be necessary to confirm these findings.
Acknowledgments
We thank Professor Phillips Scheltens’s help in visual rating score. Academia Sinica of Taiwan (Taiwan biobank: biosiganture study in Alzheimer disease) supports this study.
Data Availability
All relevant data are within the paper.
Funding Statement
Ministry of Science and Technology of Taiwan (NSC 101-2314-B-075-037-MY2, 102-2321-B-010-030, 103-2314-B-075-063-), Taipei Veterans General Hospital (V103E9-006, V103E3-005, V103C-083), Ministry of Science and Technology support for the Centre for Dynamical Biomarkers and Translational Medicine, National Central University, Taiwan (NSC 102-2911-I-008-001), Brain Research Center, National Yang-Ming University and a grant from Ministry of Education, Aim for the Top University Plan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sun Y, Lee HJ, Yang SC, Chen TF, Lin KN, et al. (2014) A nationwide survey of mild cognitive impairment and dementia, including very mild dementia, in Taiwan. PloS one 9: e100303 10.1371/journal.pone.0100303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., et al. (2011) The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia: the journal of the Alzheimer's Association 7: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galton CJ, Patterson K, Xuereb JH and Hodges JR (2000) Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain: a journal of neurology 123 Pt 3: 484–498. [DOI] [PubMed] [Google Scholar]
- 4. Koedam EL, Lauffer V, van der Vlies AE, van der Flier WM, Scheltens P, et al. (2010) Early-versus late-onset Alzheimer's disease: more than age alone. Journal of Alzheimer's disease: JAD 19: 1401–1408. 10.3233/JAD-2010-1337 [DOI] [PubMed] [Google Scholar]
- 5. Dickerson BC, Wolk DA and Alzheimer's Disease Neuroimaging I (2011) Dysexecutive versus amnesic phenotypes of very mild Alzheimer's disease are associated with distinct clinical, genetic and cortical thinning characteristics. Journal of neurology, neurosurgery, and psychiatry 82: 45–51. 10.1136/jnnp.2009.199505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hart DJ, Craig D, Compton SA, Critchlow S, Kerrigan BM, et al. (2003) A retrospective study of the behavioural and psychological symptoms of mid and late phase Alzheimer's disease. International journal of geriatric psychiatry 18: 1037–1042. [DOI] [PubMed] [Google Scholar]
- 7. Fuh JL, Wang SJ and Cummings JL (2005) Neuropsychiatric profiles in patients with Alzheimer's disease and vascular dementia. Journal of neurology, neurosurgery, and psychiatry 76: 1337–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trzepacz PT, Saykin A, Yu P, Bhamditipati P, Sun J, et al. (2013) Subscale validation of the neuropsychiatric inventory questionnaire: comparison of Alzheimer's disease neuroimaging initiative and national Alzheimer's coordinating center cohorts. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry 21: 607–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kandiah N, Chander R, Zhang A and Yee CC (2014) Cerebral white matter disease is independently associated with BPSD in Alzheimer's disease. Journal of the neurological sciences 337: 162–166. 10.1016/j.jns.2013.11.042 [DOI] [PubMed] [Google Scholar]
- 10. Berlow YA, Wells WM, Ellison JM, Sung YH, Renshaw PF, et al. (2010) Neuropsychiatric correlates of white matter hyperintensities in Alzheimer's disease. International journal of geriatric psychiatry 25: 780–788. 10.1002/gps.2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bruen PD, McGeown WJ, Shanks MF and Venneri A (2008) Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer's disease. Brain: a journal of neurology 131: 2455–2463. [DOI] [PubMed] [Google Scholar]
- 12. Tsai CF, Hung CW, Lirng JF, Wang SJ and Fuh JL (2013) Differences in brain metabolism associated with agitation and depression in Alzheimer's disease. East Asian archives of psychiatry: official journal of the Hong Kong College of Psychiatrists = Dong Ya jing shen ke xue zhi: Xianggang jing shen ke yi xue yuan qi kan 23: 86–90. [PubMed] [Google Scholar]
- 13. Feczko E, Augustinack JC, Fischl B and Dickerson BC (2009) An MRI-based method for measuring volume, thickness and surface area of entorhinal, perirhinal, and posterior parahippocampal cortex. Neurobiology of aging 30: 420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fischl B and Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America 97: 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Good CD, Scahill RI, Fox NC, Ashburner J, Friston KJ, et al. (2002) Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. NeuroImage 17: 29–46. [DOI] [PubMed] [Google Scholar]
- 16. Varon D, Barker W, Loewenstein D, Greig M, Bohorquez A, et al. (2014) Visual rating and volumetric measurement of medial temporal atrophy in the Alzheimer's Disease Neuroimaging Initiative (ADNI) cohort: baseline diagnosis and the prediction of MCI outcome. International journal of geriatric psychiatry. [DOI] [PubMed] [Google Scholar]
- 17. Davies RR, Scahill VL, Graham A, Williams GB, Graham KS, et al. (2009) Development of an MRI rating scale for multiple brain regions: comparison with volumetrics and with voxel-based morphometry. Neuroradiology 51: 491–503. 10.1007/s00234-009-0521-z [DOI] [PubMed] [Google Scholar]
- 18. Smits LL, Tijms BM, Benedictus MR, Koedam EL, Koene T, et al. (2013) Regional atrophy is associated with impairment in distinct cognitive domains in Alzheimer's disease. Alzheimer's & dementia: the journal of the Alzheimer's Association. [DOI] [PubMed] [Google Scholar]
- 19. Koedam EL, Lehmann M, van der Flier WM, Scheltens P, Pijnenburg YA, et al. (2011) Visual assessment of posterior atrophy development of a MRI rating scale. European radiology 21: 2618–2625. 10.1007/s00330-011-2205-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, et al. (1992) Atrophy of medial temporal lobes on MRI in "probable" Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. Journal of neurology, neurosurgery, and psychiatry 55: 967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lehmann M, Koedam EL, Barnes J, Bartlett JW, Ryan NS, et al. (2012) Posterior cerebral atrophy in the absence of medial temporal lobe atrophy in pathologically-confirmed Alzheimer's disease. Neurobiology of aging 33: 627 e621–627 e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cavallin L, Loken K, Engedal K, Oksengard AR, Wahlund LO, et al. (2012) Overtime reliability of medial temporal lobe atrophy rating in a clinical setting. Acta radiologica 53: 318–323. 10.1258/ar.2012.110552 [DOI] [PubMed] [Google Scholar]
- 23. Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, et al. (2004) Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of internal medicine 256: 240–246. [DOI] [PubMed] [Google Scholar]
- 24. Wechsler D (1998) Wechsler Memory Scale-III. San Antonio, Texas: The Psychological Corporation. [Google Scholar]
- 25. Härting C, Neufeld MH, Calabrese P, Deisinger K and J. K (2000) WMS-R manual. Bern: Hans Huber Verlag. [Google Scholar]
- 26. Chen CS, Ouyang P, Yeh YC, Lai CL, Liu CK, et al. (2012) Apolipoprotein E polymorphism and behavioral and psychological symptoms of dementia in patients with Alzheimer disease. Alzheimer disease and associated disorders 26: 135–139. 10.1097/WAD.0b013e31821f5787 [DOI] [PubMed] [Google Scholar]
- 27. Liao YC, Lee WJ, Hwang JP, Wang YF, Tsai CF, et al. (2014) ABCA7 gene and the risk of Alzheimer's disease in Han Chinese in Taiwan. Neurobiology of aging 35: 2423 e2427,–2423 e2413. [DOI] [PubMed] [Google Scholar]
- 28. Folstein MF, Folstein SE and McHugh PR (1975) "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 29. Hughes CP, Berg L, Danziger WL, Coben LA and Martin RL (1982) A new clinical scale for the staging of dementia. The British journal of psychiatry: the journal of mental science 140: 566–572. [DOI] [PubMed] [Google Scholar]
- 30. Burke WJ, Miller JP, Rubin EH, Morris JC, Coben LA, et al. (1988) Reliability of the Washington University Clinical Dementia Rating. Archives of neurology 45: 31–32. [DOI] [PubMed] [Google Scholar]
- 31. Vakil E and Blachstein H (1993) Rey Auditory-Verbal Learning Test: structure analysis. Journal of clinical psychology 49: 883–890. [DOI] [PubMed] [Google Scholar]
- 32. Vanderploeg RD, Schinka JA, Jones T, Small BJ, Graves AB, et al. (2000) Elderly norms for the Hopkins Verbal Learning Test-Revised. The Clinical neuropsychologist 14: 318–324. [DOI] [PubMed] [Google Scholar]
- 33. Mack WJ, Freed DM, Williams BW and Henderson VW (1992) Boston Naming Test: shortened versions for use in Alzheimer's disease. Journal of gerontology 47: P154–158. [DOI] [PubMed] [Google Scholar]
- 34. Barr A and Brandt J (1996) Word-list generation deficits in dementia. Journal of clinical and experimental neuropsychology 18: 810–822. [DOI] [PubMed] [Google Scholar]
- 35. Wechsler D (1997) Wechsler Adult Intelligence Scale-Third Edition. San Antonio, TX: The Pshchological Corporation. [Google Scholar]
- 36. Fuh JL, Liu CK, Mega MS, Wang SJ and Cummings JL (2001) Behavioral disorders and caregivers' reaction in Taiwanese patients with Alzheimer's disease. International psychogeriatrics / IPA 13: 121–128. [DOI] [PubMed] [Google Scholar]
- 37. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, et al. (1994) The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 44: 2308–2314. [DOI] [PubMed] [Google Scholar]
- 38. Cummings JL (1997) The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 48: S10–16. [DOI] [PubMed] [Google Scholar]
- 39. Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, et al. (2001) A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke; a journal of cerebral circulation 32: 1318–1322. [DOI] [PubMed] [Google Scholar]
- 40. Wattjes MP (2011) Structural MRI. International psychogeriatrics / IPA 23 Suppl 2: S13–24. 10.1017/S1041610211000913 [DOI] [PubMed] [Google Scholar]
- 41. Bouwman FH, Verwey NA, Klein M, Kok A, Blankenstein MA, et al. (2010) New research criteria for the diagnosis of Alzheimer's disease applied in a memory clinic population. Dementia and geriatric cognitive disorders 30: 1–7. [DOI] [PubMed] [Google Scholar]
- 42. Oosterman JM, Oosterveld S, Rikkert MG, Claassen JA and Kessels RP (2012) Medial temporal lobe atrophy relates to executive dysfunction in Alzheimer's disease. International psychogeriatrics / IPA 24: 1474–1482. 10.1017/S1041610212000506 [DOI] [PubMed] [Google Scholar]
- 43. Launer LJ, Scheltens P, Lindeboom J, Barkhof F, Weinstein HC, et al. (1995) Medial temporal lobe atrophy in an open population of very old persons: cognitive, brain atrophy, and sociomedical correlates. Neurology 45: 747–752. [DOI] [PubMed] [Google Scholar]
- 44. Shim YS, Youn YC, Na DL, Kim SY, Cheong HK, et al. (2011) Effects of medial temporal atrophy and white matter hyperintensities on the cognitive functions in patients with Alzheimer's disease. European neurology 66: 75–82. 10.1159/000329277 [DOI] [PubMed] [Google Scholar]
- 45. Lehmann M, Koedam EL, Barnes J, Bartlett JW, Barkhof F, et al. (2013) Visual ratings of atrophy in MCI: prediction of conversion and relationship with CSF biomarkers. Neurobiology of aging 34: 73–82. 10.1016/j.neurobiolaging.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 46. Prins ND, van der Flier WM, Brashear HR, Knol DL, van de Pol LA, et al. (2013) Predictors of progression from mild cognitive impairment to dementia in the placebo-arm of a clinical trial population. Journal of Alzheimer's disease: JAD 36: 79–85. 10.3233/JAD-122233 [DOI] [PubMed] [Google Scholar]
- 47. Pereira JB, Cavallin L, Spulber G, Aguilar C, Mecocci P, et al. (2014) Influence of age, disease onset and ApoE4 on visual medial temporal lobe atrophy cut-offs. Journal of internal medicine 275: 317–330. 10.1111/joim.12148 [DOI] [PubMed] [Google Scholar]
- 48. Jang JW, Kim S, Na HY, Ahn S, Lee SJ, et al. (2013) Effect of white matter hyperintensity on medial temporal lobe atrophy in Alzheimer's disease. European neurology 69: 229–235. 10.1159/000345999 [DOI] [PubMed] [Google Scholar]
- 49. Appel J, Potter E, Bhatia N, Shen Q, Zhao W, et al. (2009) Association of white matter hyperintensity measurements on brain MR imaging with cognitive status, medial temporal atrophy, and cardiovascular risk factors. AJNR American journal of neuroradiology 30: 1870–1876. 10.3174/ajnr.A1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rafii MS, Taylor CS, Kim HT, Desikan RS, Fleisher AS, et al. (2014) Neuropsychiatric symptoms and regional neocortical atrophy in mild cognitive impairment and Alzheimer's disease. American journal of Alzheimer's disease and other dementias 29: 159–165. 10.1177/1533317513507373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Trzepacz PT, Yu P, Bhamidipati PK, Willis B, Forrester T, et al. (2013) Frontolimbic atrophy is associated with agitation and aggression in mild cognitive impairment and Alzheimer's disease. Alzheimer's & dementia: the journal of the Alzheimer's Association 9: S95–S104 e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hirono N, Mega MS, Dinov ID, Mishkin F and Cummings JL (2000) Left frontotemporal hypoperfusion is associated with aggression in patients with dementia. Archives of neurology 57: 861–866. [DOI] [PubMed] [Google Scholar]
- 53. Lanctot KL, Herrmann N, Nadkarni NK, Leibovitch FS, Caldwell CB, et al. (2004) Medial temporal hypoperfusion and aggression in Alzheimer disease. Archives of neurology 61: 1731–1737. [DOI] [PubMed] [Google Scholar]
- 54. Mychack P, Kramer JH, Boone KB and Miller BL (2001) The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology 56: S11–15. [DOI] [PubMed] [Google Scholar]
- 55. Kumari V, Aasen I, Taylor P, Ffytche DH, Das M, et al. (2006) Neural dysfunction and violence in schizophrenia: an fMRI investigation. Schizophrenia research 84: 144–164. [DOI] [PubMed] [Google Scholar]
- 56. Soyka M (2011) Neurobiology of aggression and violence in schizophrenia. Schizophrenia bulletin 37: 913–920. 10.1093/schbul/sbr103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Neill E, Gurvich C and Rossell SL (2014) Category fluency in schizophrenia research: is it an executive or semantic measure? Cognitive neuropsychiatry 19: 81–95. 10.1080/13546805.2013.807233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.