Abstract
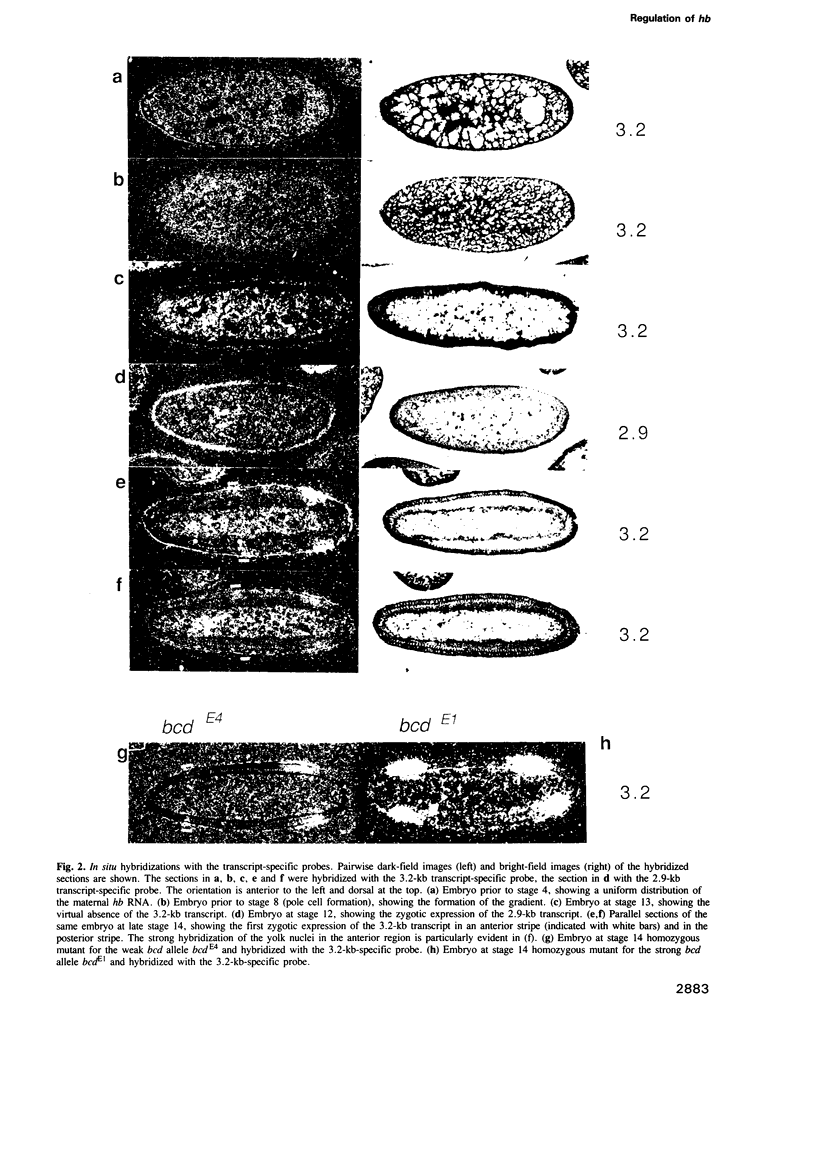
The Drosophila gap gene hunchback (hb) is required for the establishment of the anterior segment pattern of the embryo, and also for a small region of the posterior segment pattern. The hb gene encodes two transcripts from two promoters which show a differential regulation, although they code for the same protein product. The 3.2-kb transcript is expressed during oogenesis and forms an anterior-posterior gradient during the early stages of development. The first zygotic expression of hb during cleavage stages 11-12 is due to the 2.9-kb transcript. Its expression is under the control of the anterior pattern organizer gene bicoid (bcd) and it appears to be necessary and sufficient for the anterior segmentation. The 3.2-kb transcript is expressed again at syncytial blastoderm stage in the anterior yolk nuclei, as well as in an anterior stripe which is posteriorly adjacent to the domain of the 2.9-kb transcript, and as a posterior stripe. Using hb-promoter/lacZ fusion gene constructs in combination with germ line transformation, we have delimited a regulatory region for the 2.9-kb transcript to approximately 300 bp upstream of the site of transcription initiation and show that this region is sufficient to confer the full regulation by bcd.
Full text
PDF

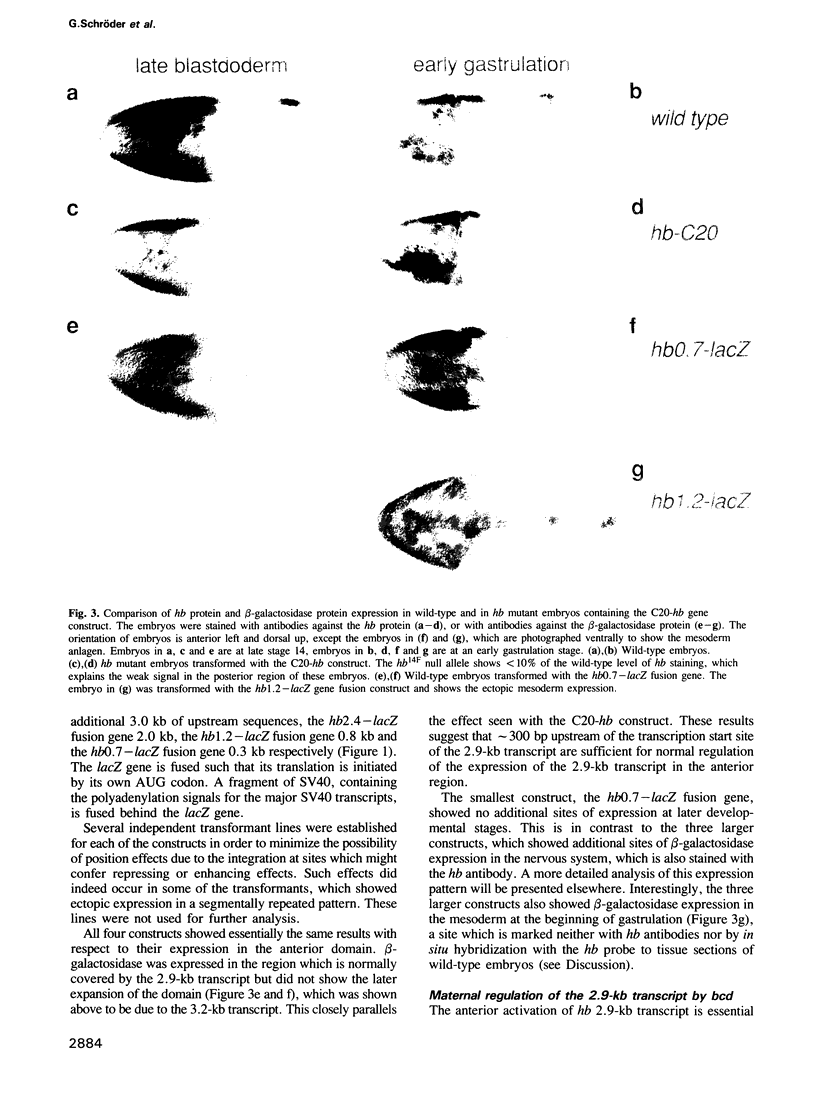
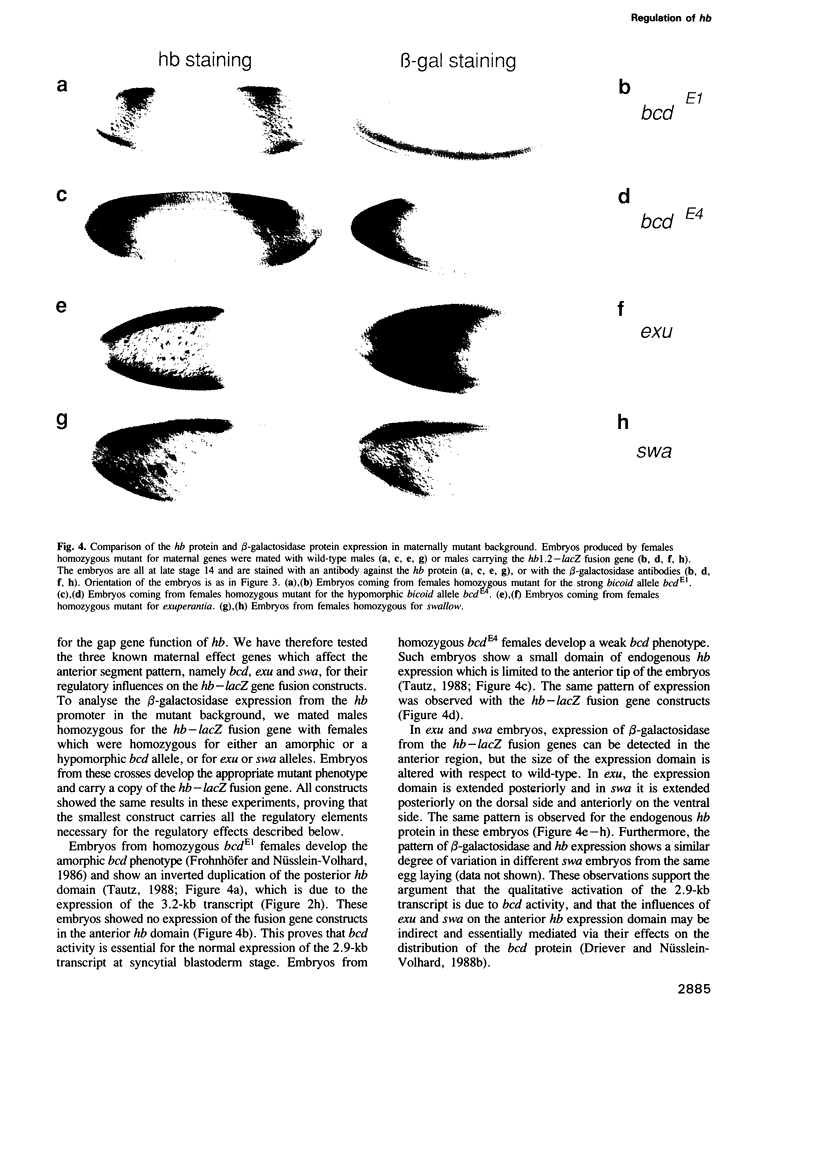


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987 Sep;101(1):1–22. [PubMed] [Google Scholar]
- Bender M., Turner F. R., Kaufman T. C. A development genetic analysis of the gene regulator of postbithorax in Drosophila melanogaster. Dev Biol. 1987 Feb;119(2):418–432. doi: 10.1016/0012-1606(87)90046-7. [DOI] [PubMed] [Google Scholar]
- Foe V. E., Alberts B. M. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci. 1983 May;61:31–70. doi: 10.1242/jcs.61.1.31. [DOI] [PubMed] [Google Scholar]
- Frigerio G., Burri M., Bopp D., Baumgartner S., Noll M. Structure of the segmentation gene paired and the Drosophila PRD gene set as part of a gene network. Cell. 1986 Dec 5;47(5):735–746. doi: 10.1016/0092-8674(86)90516-7. [DOI] [PubMed] [Google Scholar]
- Gaul U., Jäckle H. Pole region-dependent repression of the Drosophila gap gene Krüppel by maternal gene products. Cell. 1987 Nov 20;51(4):549–555. doi: 10.1016/0092-8674(87)90124-3. [DOI] [PubMed] [Google Scholar]
- Gaul U., Seifert E., Schuh R., Jäckle H. Analysis of Krüppel protein distribution during early Drosophila development reveals posttranscriptional regulation. Cell. 1987 Aug 14;50(4):639–647. doi: 10.1016/0092-8674(87)90037-7. [DOI] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Harding K., Levine M. Gap genes define the limits of antennapedia and bithorax gene expression during early development in Drosophila. EMBO J. 1988 Jan;7(1):205–214. doi: 10.1002/j.1460-2075.1988.tb02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi Y., Kuroiwa A., Gehring W. J. Control elements of the Drosophila segmentation gene fushi tarazu. Cell. 1985 Dec;43(3 Pt 2):603–613. doi: 10.1016/0092-8674(85)90232-6. [DOI] [PubMed] [Google Scholar]
- Knipple D. C., Seifert E., Rosenberg U. B., Preiss A., Jäckle H. Spatial and temporal patterns of Krüppel gene expression in early Drosophila embryos. Nature. 1985 Sep 5;317(6032):40–44. doi: 10.1038/317040a0. [DOI] [PubMed] [Google Scholar]
- Lehmann R., Nüsslein-Volhard C. hunchback, a gene required for segmentation of an anterior and posterior region of the Drosophila embryo. Dev Biol. 1987 Feb;119(2):402–417. doi: 10.1016/0012-1606(87)90045-5. [DOI] [PubMed] [Google Scholar]
- Muriel W. J., Cole J., Lehmann A. R. Molecular analysis of ouabain-resistant mutants of the mouse lymphoma cell line L5178Y. Mutagenesis. 1987 Sep;2(5):383–389. doi: 10.1093/mutage/2.5.383. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Frohnhöfer H. G., Lehmann R. Determination of anteroposterior polarity in Drosophila. Science. 1987 Dec 18;238(4834):1675–1681. doi: 10.1126/science.3686007. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983 Sep 24;11(18):6341–6351. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson E. C., Mahowald A. P. Isolation of Drosophila clones encoding maternally restricted RNAs. Dev Biol. 1987 Nov;124(1):1–8. doi: 10.1016/0012-1606(87)90453-2. [DOI] [PubMed] [Google Scholar]
- Tautz D. Regulation of the Drosophila segmentation gene hunchback by two maternal morphogenetic centres. Nature. 1988 Mar 17;332(6161):281–284. doi: 10.1038/332281a0. [DOI] [PubMed] [Google Scholar]