SUMMARY
Regulation of neuronal excitability and cardiac excitation-contraction coupling requires proper localization of L-type Ca2+ channels. We show that the actin-binding protein α-actinin binds to the C-terminal surface targeting motif of α11.2, the central pore-forming CaV1.2 subunit, to foster its surface expression. Disruption of α-actinin function by dominant negative or shRNA constructs reduces CaV1.2 surface localization in HEK293 and neuronal cultures, and dendritic spine localization in neurons. We demonstrate that calmodulin displaces α-actinin from their shared binding site on α11.2 upon Ca2+ influx through L-type channels but not through NMDAR, thereby triggering loss of CaV1.2 from spines. Coexpression of a Ca2+-binding deficient calmodulin mutant does not affect basal CaV1.2 surface expression, but inhibits its internalization upon Ca2+ influx. We conclude that α-actinin stabilizes CaV1.2 at the plasma membrane, and that its displacement by Ca2+-calmodulin induces Ca2+-induced endocytosis of CaV1.2, thus providing an important negative feedback mechanism for Ca2+ influx.
INTRODUCTION
Voltage-dependent Ca2+ channels constitute a major source of Ca2+ influx into cells. They consist of a pore-forming α1 subunit and auxiliary α2δ, β, and γ subunits. Four genes encode α1 subunits (α11.1–1.4) that give rise to the dihydropyridine-sensitive L-type Ca2+ channels CaV1.1–1.4. The auxiliary subunits facilitate release from the endoplasmic reticulum (ER) by inhibiting ubiquitination and proteasomal degradation and influence electrophysiological properties of Ca2+ channels such as activation and inactivation (Altier et al., 2011; Dai et al., 2009; Dolphin, 2009; Waithe et al., 2011). CaV1.2 is the main L-type channel in brain (Hell et al., 1993b; Sinnegger-Brauns et al., 2004) and heart (Seisenberger et al., 2000). CaV1.2 is important for numerous neuronal functions, including synaptic plasticity (Moosmang et al., 2005) (Qian and Hell, unpublished), learning and memory (Moosmang et al., 2005), and gene expression (Dolmetsch et al., 2001; Graef et al., 1999). CaV1.2 is enriched at postsynaptic sites (Davare et al., 2001; Hell et al., 1996; Obermair et al., 2004; Tippens et al., 2008) (see also (Higley and Sabatini, 2008; Hoogland and Saggau, 2004)). Precise subcellular localization of CaV1.2 is important for generating Ca2+ nanodomains that control excitability (Berkefeld et al., 2006; Liu et al., 2004; Marrion and Tavalin, 1998) and long-term potentiation (Grover and Teyler, 1990). Auxiliary subunits promote surface expression of CaV1.2, but the molecular mechanisms that determine the precise subcellular localization of CaV1.2 are unknown.
Dihydropyridine binding to L-type channels in the plasma membrane of intact GH4C1 cells decreases upon K+-induced depolarization in a dose- and time-dependent manner but reappears within minutes upon return to basal [K+] (Liu et al., 1994). Similar activity-dependent internalization of CaV1.2 occurs in cultured cortical neurons (Green et al., 2007). Disruption of F-actin decreases L-type currents in neurons (Johnson and Byerly, 1993), cardiomyocytes (Dzhura et al., 2002; Lader et al., 1999), and vascular smooth muscle cells (Nakamura et al., 2000). The activity of other ion channels, such as the NMDAR, also decreases upon actin depolymerization (Rosenmund and Westbrook, 1993). The actin-binding protein α-actinin, which links filamentous actin to integral membrane proteins, binds directly to NMDAR and disruption of this interaction reduces NMDAR channel activity (Krupp et al., 1999; Wyszynski et al., 1997; Zhang et al., 1998). We now report that α-actinin binds to residues 1584 to ~1670 in the proximal C-terminus of rabbit α11.2. Earlier independent work found that deletion of residues 1623–1666 impairs surface expression of CaV1.2 (Gao et al., 2000) (see also (Kepplinger et al., 2000)). This region includes the IQ segment (residues 1644–1666), which mediates Ca2+-dependent inactivation (CDI) by binding to Ca2+-CaM (Fallon et al., 2005; Kim et al., 2004; Peterson et al., 1999; Van Petegem et al., 2005; Zhou et al., 1997; Zuhlke et al., 1999). Recent evidence suggests that overexpression of CaM can support trafficking of CaV1.2 to the cell surface in heterologous expression systems, perhaps by helping to mask an ER retention signal within the quaternary structure of CaV1.2 for forward trafficking (Ravindran et al., 2008; Wang et al., 2007). However, it is unclear whether endogenous CaM promotes CaV1.2 surface expression in neurons. Most importantly, it is unknown how CaV1.2 is retained at the cell surface and targeted to specific locations in the plasma membrane where CaV1.2 accumulation is required, such as postsynaptic sites.
We show that α-actinin binds to the membrane targeting and CaM-binding region in the α11.2 C-terminus and fosters CaV1.2 surface expression in HEK293 cells and neurons. CaM competes with α-actinin for α11.2 binding in the presence but not absence of Ca2+. Ca2+ influx triggers displacement of α-actinin from CaV1.2 by Ca2+/CaM, which results in loss of CaV1.2 from the cell surface and dendritic spines if lasting long enough for endocytosis to occur.
RESULTS
CaV1.2 Interacts with α-Actinin in Brain
Previous work (Lu et al., 2007) suggests that α-actinin and CaV1.2 interact, which was evaluated by co-immunoprecipitation (coIP) and immunofluorescence microscopy (IF). The antibodies CNC1 and FP1 against α11.2 specifically recognize the full length form of α11.2 (~240 kDa apparent MR) and a C-terminally truncated form (~200 kDa) generated by proteolytic processing (Davare et al., 2001; Hell et al., 1996; Hell et al., 1993a; Hell et al., 1993b). Their specificity for immunofluorescence staining was confirmed by comparing untransfected HEK293 cells with those transfected with either α11.2 or α11.3 (Figure S1A). Because these antibodies are highly selective for α11.2 and directed against the same region of α11.2, they were used interchangeably throughout the study. The isoform selectivity of the two widely used monoclonal antibodies BM-75.2 and EA-53 against α-actinin was similarly defined (Figure S1B,C). Four different genes encode four highly homologous α-actinin isoforms. BM-75.2 recognizes α-actinin-1 through -4 equally well by immunoblotting (IB) but α-actinin-1 selectively by immunofluorescence (IF). EA-53 interacts preferentially with α-actinin-2 by IB but with α-actinin-2 through -4, though not -1, by IF. BM-75.2 was thus primarily used, and supplemented with EA-53 for IF experiments to detect all isoforms.
CoIP of α-actinin and CaV1.2 was observed from solubilized rat forebrain (Figure 1A bottom, lane 3; Figure 8A, lanes 1–6). This CoIP was specific as it was absent in control IgG antibody samples (Figure 1A, lane 2; Fig. 8A, lanes 7 and 8). The two main size forms of α11.2 often appear as multiple partially resolved bands due to splice variations (Figure 1A top). IF labeling of rat brain sections showed extensive colocalization of CaV1.2 puncta and α-actinin puncta in cortical neurons (Figure 1B). Both α11.2 and α-actinin were detected within somata of pyramidal neurons and their dendritic arbors. Colocalization between α11.2 and α-actinin was also observed in the dendrites of neurons in primary hippocampal cultures (see Figure S4). High magnification clearly illustrates overlapping punctate staining for α11.2 and α-actinin (Figure 1C). Many of these puncta are likely synapses, as both CaV1.2 (Davare et al., 2001; Hell et al., 1996; Obermair et al., 2004; Tippens et al., 2008) and α-actinin (Allison et al., 1998; Wyszynski et al., 1998; Wyszynski et al., 1997) are mostly clustered at postsynaptic sites.
Figure 1. α-Actinin Interacts and Colocalizes with CaV1.2 in Neurons.
(A) CoIP of α-actinin with Cav1.2 from Triton X-100 solubilized rat forebrain.
(B) Confocal images from murine cortical slices showing colocalization between α11.2 and α-actinin within the soma (arrows) and neurites (arrowheads) of cortical neurons. Bottom left panels show a magnified view of the area indicated in the top row panels.
(C) Confocal images from the CA1 region of the hippocampus in rat brain slices.
Scale bars: B: 20 μm; C: 10 μm. See Figure S1 for antibody specificity.
Figure 8. CaM Displaces α-Actinin from CaV1.2 upon Ca2+ Influx.
Forebrain slices were pre-incubated for 5 min with vehicle or 20 μM isradipine, 10 μM MK801, 10 μM DCKA, 1 μM TTX, 10 μM NBQX, 10 μM W7, 20 μM TFP, or 30 μM calmidazolium (CMZ) if indicated, treated with vehicle (control, CT), 10 μM ionomycin, 20 μM FPL64176, or 100 μM glutamate for indicate time periods, and extracted with 1% Triton X-100 before IP of CaV1.2 and IB for α11.2 and α-actinin.
(A) Ionomycin reduced coIP of α-actinin with CaV1.2.
(B) FPL and glutamate reduced coIP; the glutamate effect was prevented by the L-type channel blocker isradipine.
(C) The effect of 5 min glutamate treatment was not prevented by the two NMDAR inhibitors MK801 and DCKA.
(D) The effect of 5 min FPL treatment was prevented by reducing network activity with TTX or NBQX.
(E) The FPL effect was complete after 1 min.
(F) The effect of 5 min FPL treatment was prevented by three different CaM inhibitors.
(A–F) Bars are mean±SEM from n samples in ≥ 3 experiments (*p<0.05, **p<0.01, ***p<0.001).
α-Actinin Binds to the CaM Binding and Surface Targeting Region of α11.2
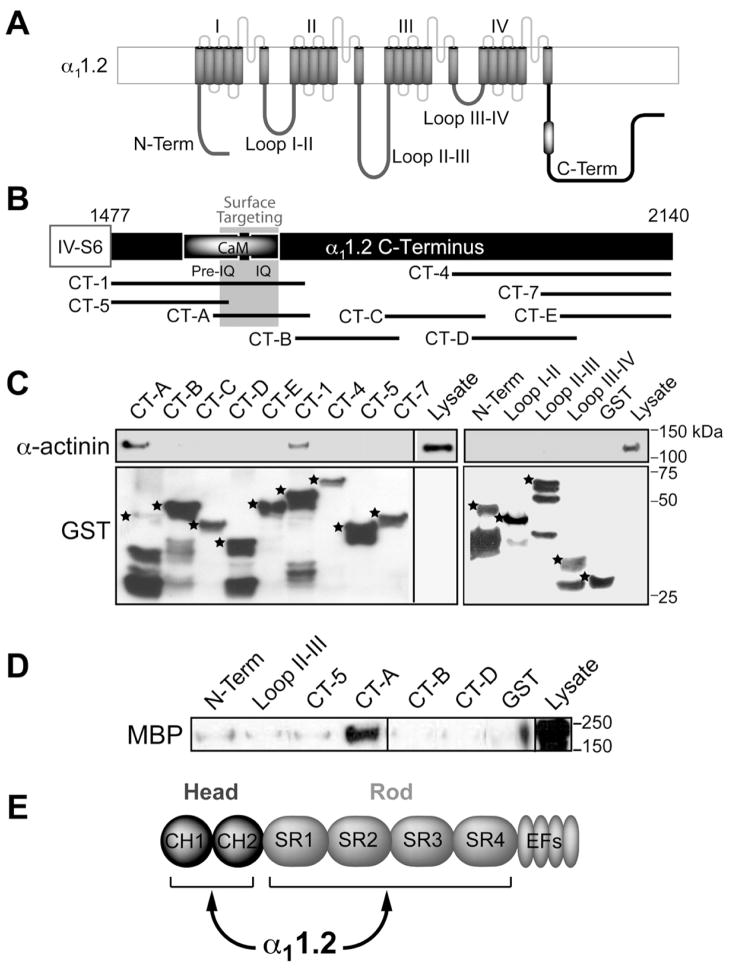
To confirm the α11.2 - CaV1.2 interaction and to obtain potential leads for its physiological functions we determined the α-actinin binding site on α11.2 by pull down experiments. Intracellular N- and C-termini and loops between the 4 homologous membrane domains (Figure 2A) were expressed in E. coli as GST-fusion proteins and immobilized on glutathione-Sepharose. The C-terminus of α11.2 was divided into several overlapping fragments (Figure 2B). The overlapping α11.2 C-terminal fragments CT-A and CT-1, but no other fusion protein, pulled down α-actinin from brain homogenates (Figure 2C, top). Probing of the lower portion of the blots with anti-GST antibody indicates that comparable amounts of the various fusion proteins were present in the different pull down samples (Figure 2C, bottom). The cDNA in our GST-CT-A plasmid covers residues 1584–1707 with GST being placed at the N-terminus of this fragment. C-terminal truncation of 40 residues of GST-CT-A as suggested by GST probing (Figure 2C, bottom) would retain residues 1584–1667 including residues 1623–1666, which are critical for proper trafficking of CaV1.2 to the cell surface (Gao et al., 2000). Some of the α11.2-derived GST-fusion proteins were also incubated with purified maltose binding protein (MBP) - α-actinin-1 expressed in E. coli (Figure 2D). Again, CT-A, but none of the other GST fusion proteins, recruited α-actinin indicating a direct interaction. Further extensive binding studies showed that α-actinin can bind independently with 3 different sites to residues 1588–1609, 1614–1635, and 1644–1670, with the latter being of very high affinity (apparent Kd: 3.5±0.5 nM) and sensitive to displacement by Ca2+/CaM (Malik, Shea, and Hell, unpublished data).
Figure 2. α-Actinin-1 Interacts with the CaM Binding and Surface Targeting Region of the α11.2 C-terminus.
(A) Diagram of α11.2 depicts intracellular N- and C-termini and four homologous domains, which form the channel pore and are connected by intracellular loops. Black and white oval: Pre-IQ + IQ region.
(B) Diagram of the α11.2 C-terminus depicts the Pre-IQ + IQ CaM binding region, the surface targeting region (residues 1626–1666 (Gao et al., 2000)), and the overlapping C-terminal GST fusion proteins used.
(C) IB of α-actinin pull-down from rat forebrain homogenates by indicated GST fusion proteins. Top, probing for α-actinin binding to GST fusion proteins. α-actinin bound to CT-1 [corresponding to rabbit α11.2 residues 1507–1733 (NFDYL…GGLFG)] and to CT-A [corresponding to rat α11.2 residues 1584–1707 (ELRAI…FGNHV); the latter are homologous to rabbit residues 1614–1737; the change in numbering is because the rat sequence lacks 30 residues at the very N-terminus compared to rabbit]. Bottom, probing for total GST fusion protein present within each pull-down reaction. While there are comparable amounts of expected full length forms for most fusion proteins (*), the C-terminal half of CT-A is largely removed identifying the N-terminal half as α-actinin binding site. In addition, the 20 residues at the N-terminus, which would overlap with the C-terminal 20 residues of CT-A, (SAASEDDIFRRAGGLFGNHV) do not apparently bind α-actinin.
(D) IB of pull-down of purified MBP-α-actinin-1 by immobilized α11.2 GST fusion proteins using an anti-MBP antibody. CT-A directly interacts with α-actinin.
(E) Schematic illustrates head and rod domain of α-actinin.
See also Table S1 for details on subcloning.
Functional Interaction of α-Actinin and CaV1.2 in HEK293 Cells
α11.21623–1666 also binds CaM, which mediates CDI of CaV1.2. Similarly, CaM and α-actinin bind to overlapping sites on the NMDAR and mediate its Ca2+-dependent desensitization (Krupp et al., 1999; Leonard et al., 2002; Merrill et al., 2007; Wyszynski et al., 1997; Zhang et al., 1998). We therefore tested whether α-actinin controls CaV1.2 functions. The α-actinin head consists of two calponin homology (CH) domains followed by a rod domain that consists of four spectrin repeats (SR) (Fig. 2E). The head as well as the rod domain can act in a dominant negative fashion (Pavalko and Burridge, 1991; Schnizler et al., 2009; Zhang and Gunst, 2006). Furthermore, the CH and SR domains can independently interact with α11.21623–1666 (Malik, Shea, and Hell, unpublished data). Accordingly, the Head and Rod domain have the potential to exert a dominant negative effect by disrupting the interaction between α11.21623–1666 and endogenous α-actinin.
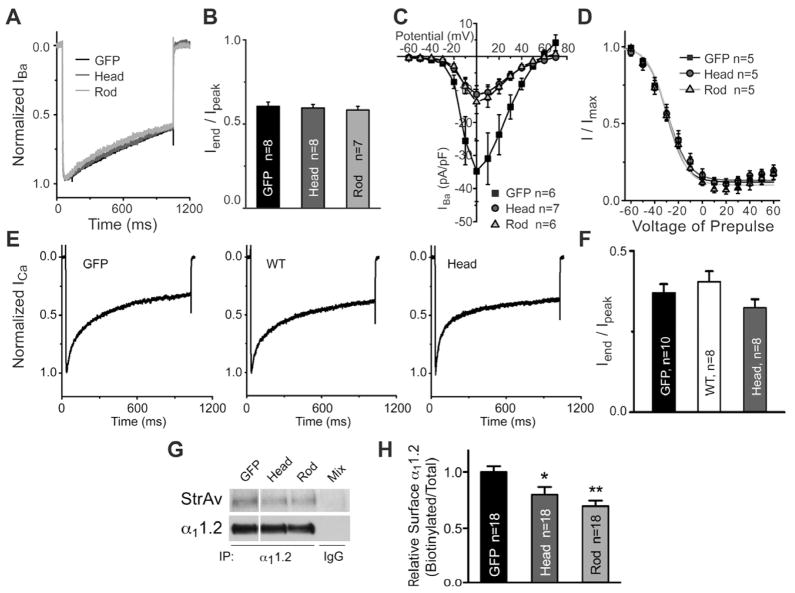
Contrary to our initial hypothesis, coexpression of the Head or Rod domain of α-actinin-1, which was tagged with EGFP, did not alter activation or inactivation of either Ba2+ or Ca2+ currents through CaV1.2 (Figure 3A,B,E,F). Current-voltage relationships and steady-state inactivation were also unaffected (Figure 3C,D). However, the density of Ba2+ current through Cav1.2 was significantly reduced by both constructs (Figure 3C). Because such a reduction in current density could be due to a decrease in surface localization of Cav1.2, we quantified this parameter by surface biotinylation. HEK293 cells were cotransfected with CaV1.2 containing an HA-tagged variant of α11.2 (Obermair et al., 2004) and EGFP-head, EGFP-rod or EGFP alone before surface biotinylated. α11.2 was extracted before IP and IB with anti-α11.2 to measure total α11.2 levels, and re-probed with Streptavidin-HRP to measure levels of α11.2 at the cell surface. Head and Rod domain significantly decreased α11.2 biotinylation (Figure 3G,H) indicating a reduction in CaV1.2 surface expression.
Figure 3. Dominant Negative α-Actinins Reduce Surface Expression of CaV1.2.
HEK293 cells were transfected with α11.2, α2δ1, β2a, and either GFP, EGFP-α-actinin-1 or dominant negative EGFP-α-actinin-Head or -Rod constructs.
(A, E) Ba2+ (A) and Ca2+ (E) currents from GFP-positive cells were elicited from a holding potential of −80 mV by depolarization to +10 mV for 1 s. Current traces were normalized to their peak values and overlaid for comparison in A.
(B, F) Summary of the ratios of the Ba2+ (B) and Ca2+ (F) currents at the end of the 1 s depolarizing pulse to their peak values.
(C) I/V curves of Ba2+ peak currents activated from a holding potential of −70 mV by depolarization to the marked potentials for 300 ms (interpulse intervals were 10 s; p<0.05 for head and for rod vs. GFP control between −10 and +40 mV; t-test).
(D) Steady-state inactivation curves. Depolarizing pulses to the indicated potentials for 3 s were followed by a test pulse for 100 ms to the potential that elicited maximal peak currents (see I/V curves in C). Current values were normalized to the maximal current observed in each set of measurements, averaged, and fitted to the Boltzmann equation.
(B, C, D, F) Means ± SEM from n independent but interleaved transfection experiments.
(G) Surface biotinylation of α11.2 in HEK293 cells. CaV1.2 was extracted with Triton X-100 before IP with anti-α 11.2, IB with anti-α 11.2, stripping, and reprobing with streptavidin. Mix, combination of lysates from the three samples after mock IP with nonspecific control IgG. Lanes for each probing were from the same immunoblot and exposure and rearranged for clarity.
(H) Means ± SD of relative ratios of biotinylated to total α11.2 signals, normalized to control (GFP; n, number of independent samples from 10 different experiments; * p<0.05, ANOVA).
See also Figure S2 for lack of effect of head and rod domains on F actin.
To control for perturbation of cortical F-actin by these constructs, parallel cultures were stained with phalloidin, which specifically binds to F-actin. Cortical actin structures were intact in cells transfected with either the Head or Rod domain, though the EGFP-Head domain formed intracellular clusters that also stained for F-actin (Figure S2). As we did not observe a detectable change in cortical F-actin with the Head construct, the Head domain clusters may sequester some F-actin in the cell interior without causing a substantial alteration in the content of dynamic and cortical actin. Modest amounts of the head domain outside the clustered structures apparently suffice to act as dominant negative constructs without grossly disturbing cortical F-actin. The EGFP-Rod domain, which does not bind F-actin, showed a diffuse distribution and did not colocalize with F-actin, as expected (Pavalko and Burridge, 1991; Zhang and Gunst, 2006). Accordingly, the effects of the Head and Rod domains on Cav1.2 surface expression are not due to disruption of F-actin.
The above electrophysiological, IF, and IP experiments indicate that the dominant negative α-actinin constructs reduce surface expression of CaV1.2 in HEK293 cells under equilibrium conditions.
Knockdown of α-Actinin Reduces CaV1.2 Surface Expression in HEK293 Cells
We performed knock down experiments to further test the role of α-actinin. We developed shRNA expressing vectors that specifically and individually knocked down the different α-actinin isoforms in humans and rodents (Table S1). To test these constructs, we expressed EGFP-tagged α-actinin-1 through -4 with or without the corresponding shRNA. Coexpression of each shRNA decreased the EGFP-α-actinin band of the respective isoform (Figure 4A) but not of other isoforms (Schnizler et al., 2009). RT-PCR showed that HEK293 cells express α-actinin-1 and -4, but not -2 or -3 (Figure S3A). Coexpression of shRNAs against these two α-actinins in HEK293 cells decreased CaV1.2 peak currents when compared to coexpression of shRNAs against α-actinins-2 and -3, which served as negative controls (Figure 4D). α-Actinin-1 plus -4 shRNA expression did not affect activation or inactivation with either Ba2+ or Ca2+ as charge carrier (Figure 4B,C,E,F,G). Parallel cultures were labeled with phalloidin to verify that α-actinin-1 and -4 knock-down had no significant effect on cortical F-actin. Cells transfected with the shRNA vectors against α-actinin-1 and -4 showed virtually the same pattern of cortical F-actin staining as their untransfected neighbors and as cells transfected with either the parental pSilencer-DsRed vector or vectors against α-actinin-2 and -3 (Figure S3B).
Figure 4. Knockdown of Endogenous α-Actinins Reduces CaV1.2 Current Density.
(A) Knockdown of α-actinin isoforms with isoform-specific shRNA encoding vectors. HEK293 cells were cotransfected with EGFP-tagged α-actinins-1, -2, -3, or -4 and pSilencer-DsRed encoding no shRNA (−) or shRNA against the respective α-actinin isoforms (+). Lysate samples containing equal amounts of protein were analyzed by immunoblotting with anti-GFP antibodies for detection of the corresponding EGFP-tagged α-actinins.
(B–G) HEK293 cells were transfected with α11.2, α2δ1, β2a and a combination of pSilencer-DsRed with shRNA against α-actinins-1 and -4 to knock down endogenous α-actinin (Figure S3). A combination of shRNA against α-actinins-2 and -3 served as negative control.
(B, F) Ba2+ (B) and Ca2+ (F) currents from DsRed-positive cells were elicited from a holding potential of −80 mV by depolarization to +10 mV for 1 s. Current traces were normalized to their peak values and overlaid for comparison in B.
(C, G) Summary of the ratios of the Ba2+ (C) and Ca2+ (G) currents at the end of the 1 s depolarizing pulse to their peak values.
(D) I/V curves of Ba2+ peak currents activated from a holding potential of −70 mV by depolarization to the marked potentials for 300 ms (interpulse intervals were 10 s; p<0.05 for α-actinin-1+4 shRNA vs. α-actinin-2+3 shRNA control between +10 and +40 mV; t-test).
(E) Steady-state inactivation curves. Depolarizing pulses to the indicated potentials for 3 s were followed by a test pulse for 100 ms to the potential that elicited maximal peak currents (see I/V curves in D). Current values were normalized to the maximal current observed in each set of measurements, averaged, and fitted to the Boltzmann equation.
(C, D, E, G) Values are given as means ± SEM and are derived from n independent but interleaved transfection experiments.
See also Figure S3 for α-actinin isoforms in HEK293 cells and for lack of effect of shRNA on F-actin.
Knockdown of α-Actinin Impairs Synaptic Localization of CaV1.2
CaV1.2 (Bloodgood and Sabatini, 2007; Davare et al., 2001; Hell et al., 1996; Obermair et al., 2004; Tippens et al., 2008) and α-actinin (Allison et al., 1998; Wyszynski et al., 1998; Wyszynski et al., 1997) are enriched in postsynaptic dendritic spines, where they colocalize (Figure 1, S4). We found by RT-PCR that neurons express α-actinins-1, -2, and -4 (Schnizler et al., 2009) (see also (Peng et al., 2004; Walikonis et al., 2000; Walikonis et al., 2001; Wyszynski et al., 1998)). To examine the role of α-actinin in neurons, hippocampal cultures were transfected after 4 days in vitro (DIV) with parental pSilencer (pSil), a combination of shRNA expressing vectors against α-actinins-1, -2 and -4, or against α-actinin-3 as negative control. At 21 DIV colocalization of endogenous α11.2 and the postsynaptic markers PSD-95 (Tseng and Hell, unpublished data) and Shank were significantly reduced upon knock-down compared to control (empty pSil plasmid or α-actn-3 shRNA) (Figure 5). Ectopic expression of α-actinin-1 with silent shRNA-resistant mutations completely rescued the loss of α11.2 from spines.
Figure 5. α-Actinin Knockdown Reduces CaV1.2 in Spines.
Hippocampal cultures were transfected with parental pSilencer-DsRed, α-actinin-3 shRNA, or shRNAs against α-actinins-1, -2, and -4 alone or in combination with the α-actinin-1 rescue vector at 4 DIV and stained with FP1 and anti-Shank at 21 DIV.
(A) Confocal immunofluorescence micrographs.
(B) Cumulative fraction plot for α11.2 intensity of puncta colocalized with the postsynaptic marker Shank. A total of n images were analyzed from 5 independent experiments (p < 0.01 for sh1/2/4 vs. control, vs. sh3, and vs. rescue by ANOVA; error bars indicate SEM).
See also Figure S4 for colocalization of α11.2 and α-actinin.
CaM Competes with α-Actinin for the α11.2 C-terminus in a Ca2+-dependent Manner
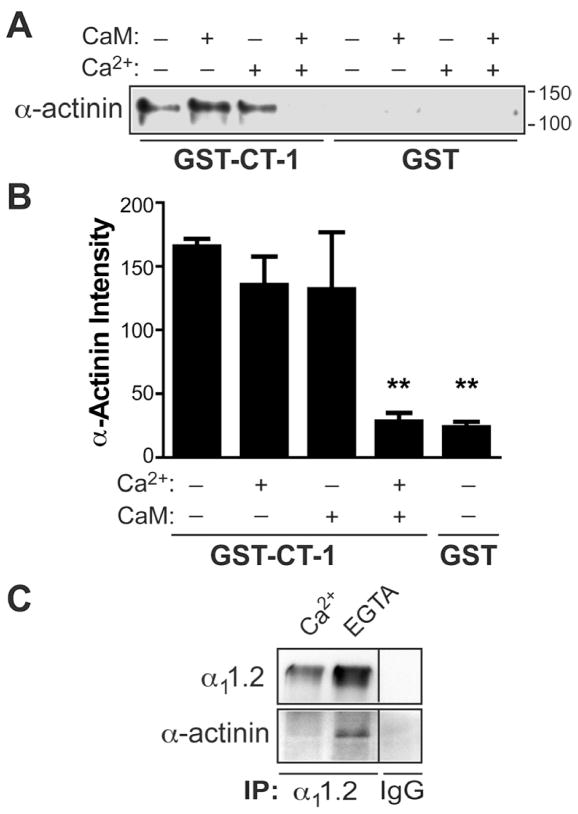
Because α-actinin binds to the same region of α11.2 as CaM, we tested whether the two proteins compete for binding to this site. As before (Figure 2C, lane 6), MPB-tagged α-actinin-1 bound to immobilized CT-1-GST (Figure 6A, lane 1) but not to GST alone (lanes 5–8). Addition of Ca2+-free CaM (“apo-CaM”) or Ca2+ individually did not alter α-actinin-1 binding (lanes 2,3), but Ca2+ and CaM together displaced α-actinin-1 from GST-CT-1 (lane 4). Endogenous α-actinin co-precipitated with α11.2 from brain extracts in the absence but not presence of free Ca2+ (Figure 6C), as endogenous CaM likely displaced α-actinin from the α11.2 complex when Ca2+ was available. Like Ca2+-CaM, apo-CaM also binds to the IQ locus in the C-terminus of α11.2, but with much lower affinity, indicating that CaM rearranges itself on the α11.2 C-terminus upon addition of Ca2+ to form additional interactions that stabilize the association ((Halling et al., 2006) and Ref therein). Furthermore, apo-CaM and α-actinin can simultaneously bind GST-CT-1 (Malik, Shea, and Hell, unpublished data). These observations suggest that apo-CaM, α11.2, and α-actinin form a ternary complex under low basal intracellular Ca2+ conditions. When Ca2+ concentrations increase, CaM binds Ca2+ and displaces α-actinin from α11.2 (Figure 9H, upper panels).
Figure 6. Both Ca2+ and CaM are Required to Displace α-Actinin from α11.2.
(A,B) Purified GST or GST-CT-1 were immobilized on glutathione-Sepharose and incubated with MBP-α-actinin-1 and subsequently ± CaM in the presence of either 5 mM Ca2+ (Ca2+ +) or 500 μM EGTA (Ca2+ -) before IB for MBP (A) and densitometric quantification (B). Only combined Ca2+-CaM, but not Ca2+ or CaM alone, displaced α-actinin. (** p<0.01, ANOVA; n=6).
(C) CoIP of α-actinin and α11.2 is Ca2+ sensitive. Rat brain was extracted with Triton X-100 in the presence of 2.5 mM Ca2+ or 5 mM EGTA before IP. Pooled homogenates were used for control IP with non-specific IgG. IBs were probed for α11.2 (top) and α-actinin (bottom). α-actinin co-precipitated with α11.2 only in the absence of free Ca2+.
Figure 9. CaM is Necessary for CaV1.2 Endocytosis upon Ca2+ Influx.
(A, B) Hippocampal cultures (21 DIV) were treated with vehicle, FPL ± NBQX, or glutamate for 5 min and stained with FP1, anti-Shank, and anti-bassoon.
(A) Confocal immunofluorescence micrographs.
(B) Cumulative fraction plots for α11.2 intensity of puncta colocalized with the postsynaptic marker Shank (left panels in A). Shank in turn colocalized with the presynaptic marker bassoon (right panels in A). A total of n images were analyzed from 5 independent experiments (Control vs. FPL and vs. glutamate: p<0.001; FPL+NBQX vs. FPL: p < 0.05; bars: SEM).
(C–G) L-type Ca2+ currents (ICa,L) were recorded from hippocampal neurons 14–21 DIV during depolarizing steps from a holding potential of −60 mV to the indicated potentials. 1 μM ω-conotoxin (CTx) MVIIC and 1 μM ω-CTxGVIA were present to block P/Q-type and N-type Ca2+ channels, respectively.
(C) I/V curves before and after perfusion with 10 μM of the L-type blocker isradipine. Inset shows recordings obtained during 100 ms-long depolarizations from −60 mV to 0 mV before and after application of 10 μM isradipine. The I/V curves are typical for L-type currents and the block of these currents by isradipine (inset) pharmacologically identifies those as L-type.
(D) Run down of ICa,L in hippocampal neurons 14–21 DIV with 10 mM extracellular Ca2+ upon repeated 100 ms - depolarizations from −60 to 0 mV (Control) was largely prevented by expression of the Ca2+ binding deficient CaM1234 and inclusion of the CaM blocker calmidazolium and the endocytosis blocker dynasore. For each time course, peak ICa,L values were normalized to the first trace.
(E, F) Complete set of current recordings over full time course of run down (red, initial trace; blue, last trace) for Control (E) and CaM1234 (F) which, as expected, also inhibited CDI but not VDI.
(G) Complete set of initial peak current densities, which were not different between control and various treatment conditions.
(H) Model of Ca2+/CaM-mediated displacement of α-actinin and Ca2+-induced endocytosis of CaV1.2. Under resting conditions α-actinin binds simultaneously with apo-CaM to the IQ motif of α11.2, tethering Cav1.2 to the cortical F-actin at the plasma membrane. Upon Ca2+ influx apo-CaM will bind Ca2+, inducing a conformational change in Cav1.2 that will result in CDI within ms. Concurrently, Ca2+/CaM will dislodge α-actinin from Cav1.2. At the same time eIF3e will bind to the loop between domains II and III of α11.2, which could, but does not have to, be regulated by CaM and α-actinin. If α-actinin remains detached from Cav1.2, the endocytic adaptor protein AP2 or other functionally related proteins bind to Cav1.2 to initiate endocytosis. This binding step might depend on eIF3e. After endocytosis, Cav1.2 can be reinserted into the plasma membrane or degraded.
See also Figure S5 for additional quantitative analysis of spine localization of α11.2.
Ca2+ Influx Induces CaV1.2 Internalization
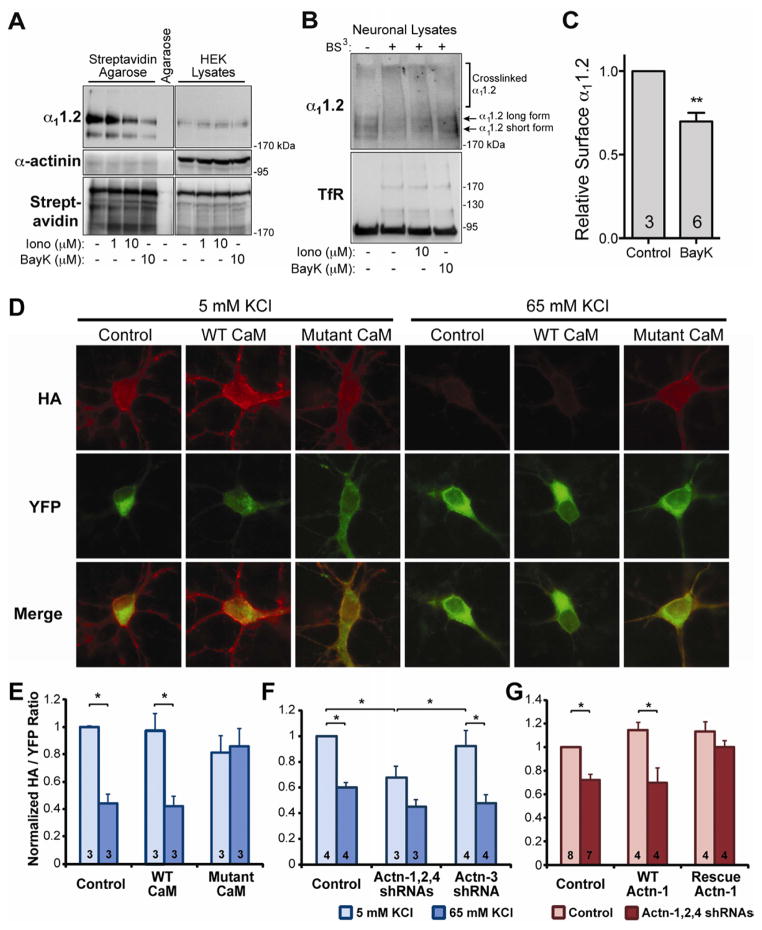
If this Ca2+-dependent displacement of α-actinin occurred in intact cells, it would sever the interaction between CaV1.2 and its surface anchor, α-actinin, triggering the reversible removal of L-type channels from the cell surface upon elevation of intracellular Ca2+ described earlier (Green et al., 2007; Liu et al., 1994). To test this hypothesis, HEK293 cells expressing CaV1.2 were treated with the Ca2+ ionophore ionomycin or the L-type channel activity-enhancing BayK8644 for 10 min before surface biotinylation. Both 10 μM ionomycin and BayK8644 clearly reduced surface biotinylation of α11.2 (Figure 7A, top left panel, lanes 2 and 3) without affecting total amounts of α11.2 detected in lysate samples (top right). Accordingly, α11.2 had redistributed away from the cell surface without being degraded. The relative levels of isolated and total biotinylated proteins were similar, as indicated by the streptavidin reprobing (Figure 7A bottom). Intracellular proteins were not biotinylated, as indicated by the α-actinin blots (Figure 7A middle left). The small amount of α-actinin in the streptavidin pull-downs likely reflects its interaction with various surface proteins such as integrins.
Figure 7. Ca2+ Influx Decreases CaV1.2 on the Surface of HEK293 Cells and Neurons.
(A) HEK293 cells were transfected with α11.2, β2a, and α2δ1 and treated with ionomycin or BayK8644 for 10 min. Surface proteins were biotinylated, extracted with Triton X-100, and pulled down with streptavidin Agarose before IB for α11.2 (top), α-actinin (middle), and total biotinylated proteins (bottom).
(B) IB of surface vs. internal α11.2 (top) and transferrin receptor (TfR, bottom) from 21 DIV primary cortical neurons treated with ionomycin and BayK8644. Surface proteins crosslinked by BS3 are shifted towards the top of blots.
(C) Quantification of α11.2 from BayK8644 treated and BS3 crosslinked neurons from 3 independent experiments. BayK8644 significantly induced CaV1.2 internalization as measured by the decrease in crosslinked to uncrosslinked α11.2 signal (p<0.01, t-test; n, number of independent samples from 3 experiments).
(D–G) Hippocampal neurons were cotransfected with CaV1.2 (YFP-HA-α11.2) and CaM, shRNA, or α-actinin constructs as indicated. After 48 h, neurons were depolarized with control (5 mM) or high (65 mM) KCl for 15 min, fixed, and stained for surface-expressed CaV1.2 with anti-HA antibody.
(D) Surface-labeled (HA, red) vs total expression (YFP, green) of HA-α11.2-YFP after coexpression with none, WT or mutant CaM1234 ± depolarization with 65 mM KCl.
(E–G) Quantification of CaV1.2 surface expression as the HA:YFP signal ratio normalized to basal expression with control vector cotransfection. Thirty neurons were analyzed from each of 3–8 independent experiments (indicated within bars; * p < 0.05, ANOVA; bars: SEM).
(E) Mutant CaM1234 but not WT CaM prevents depolarization-triggered internalization of CaV1.2.
(F) Knockdown of α-actinin with combined shRNA plasmids against α-actinin-1/2/4 but not shRNA against α-actinin-3, which neurons lack, reduces basal CaV1.2 surface expression and KCl-induced CaV1.2 endocytosis.
(G) Rescue of shRNA-induced decrease in basal CaV1.2 surface expression by ectopic expression of shRNA-insensitive α-actinin-1.
To determine whether Ca2+ influx through CaV1.2 triggers endocytosis in neurons, mature rat cortical cultures (21 DIV) were treated for 10 min with either ionomycin or BayK8644 and surface proteins cross-linked with the membrane impermeant crosslinker BS3 (Boudreau and Wolf, 2005; Grosshans et al., 2002). Equivalent amounts of protein in lysates were separated on SDS-PAGE gels and immunoblotted for α11.2 (Figure 7B, top) or the transferrin receptor (TfR) as a control (Figure 7B, bottom). α11.2 and TfR in lysates from cultures that had not been crosslinked were of the expected molecular mass (MR), including long and short α11.2 forms, which appear as weak bands due to low endogenous expression (Figure 7B, top). BS3 resulted in a reduction of the free α11.2 long and short forms and a concomitant increase in higher MR species at the top of the gel (Figure 7B top, second lane). A modest amount of the TfR was also crosslinked and migrated in two clearly detectable bands above the non-crosslinked form. Ionomycin or BayK8644 induced re-appearance of non-crosslinked α11.2 long and short forms, indicating that more CaV1.2 had become inaccessible to BS3 due to endocytosis upon Ca2+ influx. BayK8644 increases Ca2+ flux by L-type channels by 2–3 fold, matching physiological increases during heightened neuronal activity (Tavalin et al., 2004; Xu et al., 2010). Thus we tested more systematically whether BayK8644 reduces surface localization of CaV1.2, which was the case (Figure 7C, p<0.05).
To investigate whether Ca2+ influx leads to CaV1.2 endocytosis in hippocampal neurons by CaM-dependent displacement of α-actinin, we heterologously expressed α11.2 with an extracellular HA tag and an N-terminal YFP tag (Green et al., 2007). Cells were surface labeled with anti-HA and the ratio of HA to YFP signal monitored following membrane depolarization. Depolarization for 15 min with 65 mM KCl reduced this ratio by 40–60%, indicating that it triggered endocytosis of CaV1.2 (Figure 7D–F). Expression of a CaM mutant that contained a point mutation in each of its 4 EF hands to abrogate Ca2+ binding (CaM1234) prevents endogenous CaM from causing CDI of CaV1.2 (Peterson et al., 1999; Pitt et al., 2001) (see also Figure 9E vs F). CaM1234 did not affect basal CaV1.2 surface expression but averted internalization of CaV1.2 upon membrane depolarization (Figure 7D,E). This effect was not due to excess CaM per se, as overexpression of WT CaM had no effect. These results reveal a new physiological role for Ca2+ and CaM in dynamically regulating CaV1.2 surface localization after insertion into the plasma membrane.
We used the same approach to evaluate the role of α-actinin in CaV1.2 surface targeting under basal and depolarizing conditions by knocking down α-actinin-1, -2, and -4. Neurons were cotransfected with the combination of the corresponding shRNA vectors plus YFP- and HA-tagged α11.2. Knockdown reduced basal CaV1.2 surface expression and KCl-induced reduction in plasma membrane CaV1.2 (Figure 7F). Some KCl-triggered CaV1.2 endocytosis appears to have remained (although it did not reach statistical significance) likely due to incomplete α-actinin knock-down. Cotransfection with shRNA against α-actinin-3, which is not present in neurons, affected neither surface expression nor depolarization-induced internalization of CaV1.2, compared to non-shRNA plasmid controls.
Off-target effects of shRNAs were excluded by rescue. Overexpression of WT α-actinin-1 together with YFP- and HA-tagged α11.2 led to a small, statistically insignificant increase in CaV1.2 surface expression (Figure 7G). Knockdown of neuronal α-actinins with a combination of shRNAs against α-actinin-1, -2, and -4, which also knocks down the ectopically expressed α-actinin-1, reduced surface expression of YFP- and HA-tagged α11.2 by 30–40% whether wt α-actinin-1 was or was not co-expressed. For the rescue experiment, silent mutations were introduced into the shRNA recognition site of α-actinin-1 to prevent silencing. Neurons expressing shRNA-insensitive α-actinin-1 showed a small, statistically insignificant increase in CaV1.2 surface expression similar to neurons transfected with the unmodified α-actinin-1 (Figure 7G). Expression of this shRNA-insensitive α-actinin-1 prevented the reduction of surface expression of CaV1.2 by α-actinin knock down. We conclude that the shRNA effects are specific and that the α-actinin-1 isoform is sufficient for proper surface expression of CaV1.2.
Ca2+ Influx Displaces α-Actinin from CaV1.2 in Acute Forebrain Slices
Like BayK8644, FPL64176 increases Ca2+ flux through L-type channels by 2–3 fold but by a different mechanism (Tavalin et al., 2004; Xu et al., 2010; Zhang et al., 2011). Incubation of acute forebrain slices with ionomycin, glutamate, or FPL reduced α-actinin coIP with CaV1.2 by >50% (Figure 8A,B). Remarkably, the glutamate effect was completely blocked by the L-type antagonist isradipine but not by two different NMDAR antagonists (pore blocking MK801 and glycine competing 5,7-dichlorokynurenic acid (DCKA); Figure 8B,C; we confirmed activity of the MK801 batch by testing its effect on LTP, which was blocked; Qian and Hell, unpublished). Accordingly, induction of massive Ca2+ influx via NMDAR by glutamate does not contribute to CaV1.2 endocytosis, which is rather specifically triggered by Ca2+ influx through L-type channels even though a substantial portion of L-type channels resides in dendritic spines where NMDAR are also concentrated (Figure 1B,C) (Davare et al., 2001). FPL prolongs L-type channel openings but does not itself open the channels and thus depends on network activity. α-Actinin - CaV1.2 CoIPs indicate that inhibition of network activity with either the Na+ channel blocker TTX or the AMPAR antagonist NBQX prevented the dissociation of α-actinin from CaV1.2 by FPL treatment (Figure 8D). FPL treatment as short as 60 s was sufficient to displace α-actinin from CaV1.2 (Figure 8E). Accordingly, an increase of Ca2+ influx through L-type channels within physiological range is sufficient to displace α-actinin from CaV1.2. Furthermore, dissociation of α-actinin from CaV1.2 by FPL treatment was blocked by three different CaM antagonists (W7, trifluoperazine (TFP), calmidazolium (CMZ); Figure 8F). Although none of those drugs are entirely specific for CaM, they differ in their side effects (detailed in (Bartos et al., 2010)). Collectively these data thus indicate that removal of α-actinin from CaV1.2 requires CaM.
Ca2+ Influx Displaces CaV1.2 from Dendritic Spines
Like slices, hippocampal cultures were treated with FPL and glutamate to enhance basal L-type-mediated Ca2+ influx and network activity, respectively. Cultures were then analyzed by IF triple labeling for endogenous CaV1.2, the presynaptic marker bassoon, and the postsynaptic marker Shank to define postsynaptic CaV1.2 puncta. FPL and glutamate incubations lead to a statistically highly significant reduction in co-localization of CaV1.2 with Shank (Figure 9A,B). The AMPAR antagonist NBQX, which blocks the spontaneous synaptic and thereby neuronal activity in these cultures, inhibits FPL-induced loss of CaV1.2 from spines. Accordingly, network activity is required for the FPL effect. We conclude that Ca2+ influx via L-type channels induces loss of CaV1.2 from spines.
Ca2+ Influx Causes Run-down of L-type Currents by CaM-dependent Endocytosis of L-type Channels
Endocytosis of CaV1.2 upon Ca2+ influx should result in loss of L-type currents (ICa,L). In fact, whole cell patch recordings of ICa,L upon 100 ms depolariziations at 0.2 Hz with extracellular Ca2+ in hippocampal cultures showed a strong run down over 5 min (Figure 9D,E). N- and P/Q-type Ca2+ currents were blocked by ω-Conotoxin (CTx) GVIA and MVIIC to effectively isolate ICa,L as indicated by its complete elimination by isradipine (Figure 9C). The run down of ICa,L was largely abrogated by CMZ and ectopic expression of the Ca2+ -binding deficient CaM1234 mutant (Figure 9D,F), which also blocked CDI (Figure 9F) as expected (Peterson et al., 1999). The inactivation remaining in CaM1234-transfected neurons reflects Ca2+/CaM-independent voltage-dependent inactivation (VDI). To confirm that run down was due to endocytosis rather than induction of long lasting silent states of the L-type channels, we included dynasore, an endocytosis inhibitor, in the recording electrode. Dynasore also inhibited the run down. Importantly, none of the treatments reduced peak currents (Figure 9G) excluding the possibility that such a reduction could have resulted in decreased run-down. These data indicating that the Ca2+ influx through L-type channels triggers endocytosis of CaV1.2 via CaM.
DISCUSSION
Surface expression of CaV1.2 depends on the efficacy of trafficking of this channel from the ER to the plasma membrane and how well it is retained there. The auxiliary α2δ and β subunits and, when overexpressed, CaM can promote trafficking of CaV1.2 to the surface, probably by facilitating its release from the ER (Dai et al., 2009; Dolphin, 2009; Ravindran et al., 2008; Wang et al., 2007). How CaV1.2 is anchored at defined surface localizations has hitherto been unknown. Pull down assays show that α-actinin binds at or near the IQ motif in α11.2 (residues 1644–1666; Figure 2). Surface and IF labeling upon expression of dominant negative fragments of α-actinin and knock down of α-actinin show that α-actinin localizes CaV1.2 in the plasma membrane and dendritic spines (Figures 3–5), possibly by linking CaV1.2 to F-actin as depolymerization of F-actin causes a run down of L-type current (Johnson and Byerly, 1993). Accordingly, mutations in the shared CaM/α-actinin binding region of α11.2 that impair surface expression of α11.2 (Ravindran et al., 2008; Wang et al., 2007) likely act by affecting α-actinin rather than CaM binding.
CaM pre-associates in its Ca2+-free apo form with the IQ motif of α11.2 to mediate rapid CDI (Fallon et al., 2005; Kim et al., 2004; Peterson et al., 1999; Van Petegem et al., 2005; Zuhlke et al., 1999). Given that the interaction between CaM and the IQ region controls channel inactivation, binding of α-actinin to this region could also affect gating. However, neither overexpression of the dominant negative α-actinin Head or Rod domain (Figure 3) nor knockdown of α-actinin altered activation or inactivation of Ca2+ or Ba2+ currents through CaV1.2 (Figure 4). It thus appears that α-actinin does not affect the structural determinants of CaV1.2 gating. However, if α-actinin is absolutely essential for CaV1.2 surface expression, the currents through CaV1.2 that remain after expression of α-actinin shRNA or dominant negative constructs would reflect CaV1.2 that maintained binding to endogenous α-actinin that escaped knock-down or that successfully competed with the dominant negative rod or head domain. Accordingly, the remaining channels would not be different from CaV1.2 under control conditions. We thus cannot exclude that α-actinin affects channel properties.
α-Actinin binds to the IQ motif simultaneously with CaM only in the absence but not presence of Ca2+ (Figure 6; Malik, Shea, and Hell, unpublished data). These results indicate that under basal conditions α-actinin and CaM are both associated with the IQ region of native α11.2 (Figure 9H). Ca2+ in conjunction with CaM displaces α-actinin from the IQ motif in vitro (Figure 6) and from endogenous CaV1.2 in intact neurons (Figure 8). Ca2+ influx decreases surface expression of CaV1.2 in HEK293 cells (Figure 7A) and neurons in a CaM-dependent manner (Figure 7B–E). Also, earlier work on the functional coupling between L-type channels and Ca2+-activated K+ channels found that chelating intracellular Ca2+ with BAPTA increases the prevalence of L-type channels at the surface of hippocampal pyramidal neurons over a period of 30 min (Marrion and Tavalin, 1998). This earlier work provides independent support for a role of intracellular Ca2+ in regulating abundance of L-type channels in the plasma membrane.
Ca2+-induced binding of eIF3e to loop II/III in Cav1.2 is important for Ca2+-driven endocytosis of Cav1.2 (Green et al., 2007). It is possible that displacement of α-actinin by Ca2+/CaM from the C-terminus of Cav1.2 and eIF3e binding to loop II/III of Cav1.2 are two completely independent yet individually necessary steps for endocytosis of Cav1.2 to occur. Alternatively, the conformational change of the C-terminus of Cav1.2 that results from Ca2+ associating with CaM could affect the conformation of loop II/III and thereby binding of loop II/III to eIF3e, linking the two events. It is also possible that α-actinin bound to the C-terminus of Cav1.2 obstructs access of eIF3e to loop II/III until α-actinin is displaced upon Ca2+influx by Ca2+/CaM.
Collectively, our previous and current data support the following model (Figure 9H): under basal conditions, apo-CaM and α-actinin bind to the same segment in Cav1.2, with the latter anchoring Cav1.2 via the cortical F-actin at the cell surface. Ca2+ influx has three effects: 1. Apo-CaM will bind Ca2+ and trigger a conformational change in Cav1.2 that will cause CDI within milliseconds. 2. At the same time Ca2+/CaM will dislodge α-actinin from Cav1.2. In parallel eIF3e will bind to the loop between domains II and III of Cav1.2, which may or may not be influenced by CaM and α-actinin. If α-actinin displacement lasts long enough (e.g., prolonged Ca2+ influx), the endocytic machinery (exemplified by the adaptor protein AP2) will bind to Cav1.2, thereby initiating endocytosis. This step might also depend on eIF3e. After endocytosis, Cav1.2 can be reinserted into the plasma membrane (see (Liu et al., 1994)) or degraded.
Ca2+ overload is detrimental to neuronal function and has been implicated in various neurological diseases (Disterhoft et al., 1994; Lee et al., 1999). Chronic increase in L-type channel activity contributes to the etiology of senile symptoms and Alzheimer’s disease (Deyo et al., 1989; Disterhoft et al., 1994; Thibault and Landfield, 1996). Our work uncovered an unexpected, complex mechanism for down regulation of CaV1.2 via Ca2+-CaM-driven displacement of α-actinin and the ensuing internalization of CaV1.2. Dysregulation of this mechanism could contribute to those neurological diseases. These findings provide the basis for further mechanistic studies on surface localization of CaV1.2 under physiological and pathological conditions.
EXPERIMENTAL PROCEDURES
Animal Handling
All animal procedures were consistent with NIH guidelines and approved by the University of Iowa, UC Davis, University of North Carolina, and Stanford University Institutional Animal Care and Use Committees.
Immunoprecipitation and Immunoblotting from Brain Extract and Acute Forebrain Slices
Acute forebrain slices were prepared and handled as described (Halt et al., 2012). Rat brain tissue, forebrain slices, or HEK293 cells were extracted with 1% Triton X-100, cleared by 15–30 min ultracentrifugation (250,000 × g), and analyzed by IP and IB as described (Hall et al., 2007; Hall et al., 2006; Hell et al., 1993a).
Cell culture and imaging
Primary hippocampal neuronal cultures were prepared from E17–19 embryonic rats as described (Chen et al., 2008). HEK293 cells and neurons were seeded onto poly-L-lysine coated coverslips, transfected after 24 hrs and 6–12 DIV, respectively, using standard transfection reagents, and fixed after an additional 1–10 days for immunofluorescence analysis or used for recording of ICa,L after 1 day.
Perfusion and immunofluorescence imaging of brain sections
Adult male rats were deeply anesthetized with sodium pentobarbital and perfused with 0.5% paraformaldehyde. Immunofluorescence was performed on Vibratome brain sections and images collected on a Leica SP2 confocal microscope.
In vitro pulldown assays
Expression and purification of MBP-tagged α-actinin-1 was according to the manufacturer’s protocol for the pMalE-c2 vector (New England Biolabs). Expression, purification, and pull down of GST fusion proteins were as described previously (Hall et al., 2007; Hall et al., 2006).
Electrophysiology
For recording of IBa from HEK cells, extracellular solution contained (in mM) 125 NaCl, 10 tetraethylammonium chloride (TEA-Cl), 5 BaCl2, 5.4 CsCl, 1,4-aminopyridine, 1 MgCl2, 10 HEPES, 10 glucose (pH 7.4 with NaOH) and intracellular solution 120 CsCl, 10 TEA-Cl, 10 EGTA, 1 MgCl2, 3 MgATP, 0.5 Na3GTP, 10 HEPES (pH 7.3 with CsOH). For ICa, extracellular solution contained (in mM) 150 Tris, 1 MgCl2, and 10 CaCl2 and intracellular solution 140 N-methyl-D-glucamine, 10 HEPES, 1 MgCl2, 2 Mg-ATP, 5 EGTA (pH 7.3 with methanesulfonic acid(MeSO4)). IBa or ICa were determined during a pulse protocol from a holding potential −70 mV to 0 or 10 mV.
For recording of ICa,L from hippocampal neurons (14–21 DIV) extracellular solution contained (in mM) 130 N-methyl-D-glucamine (NMDG) Cl, 10 CaCl2, 10 HEPES, 1 MgCl2, and 10 glucose, (pH 7.4 with NMDG) plus 1 μM ω-conotoxin (CTx) MVIIC, 1 μM ω-CTxGVIA, and 30 μM niflumic acid and intracellular solution 110 Cs-MeSO4, 20 TEA-Cl, 10 HEPES, 5 Mg-ATP, 0.5 GTP (pH 7.3 with CsOH).
Supplementary Material
HIGHLIGHTS.
α-Actinin binding to CaV1.2 IQ region maintains CaV1.2 surface and spine localization
Ca2+ influx via L channels, not NMDAR, displaces α-actinin from CaV1.2 via calmodulin
Ca2+ influx via L channels results in loss of CaV1.2 from dendritic spines
This Ca2+ influx causes run down of L current by calmodulin-induced endocytosis.
Acknowledgments
This work was supported by the NIH training grants HL07121, DK07759, and AG00213 to DDH; AG017502 to JWH; NS35527 to RJW; DC009433 and HL087120 to AL; and the American Heart Association grant 0535235N (DDH). The authors thank Jason Ulrich, Audrey Dickey, Ivar Stein, and Coleen Cowan for technical support and M. Navedo, UC Davis, for critically reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier C, Garcia-Caballero A, Simms B, You H, Chen L, Walcher J, Tedford HW, Hermosilla T, Zamponi GW. The Cav beta subunit prevents RFP2-mediated ubiquitination and proteasomal degradation of L-type channels. Nat Neurosci. 2011;14:173–180. doi: 10.1038/nn.2712. [DOI] [PubMed] [Google Scholar]
- Bartos JA, Ulrich JD, Li H, Beazely MA, Chen Y, Macdonald JF, Hell JW. Postsynaptic clustering and activation of Pyk2 by PSD-95. J Neurosci. 2010;30:449–463. doi: 10.1523/JNEUROSCI.4992-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, et al. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314:615–620. doi: 10.1126/science.1132915. [DOI] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Nonlinear regulation of unitary synaptic signals by Cav(2.3) voltage-sensitive calcium channels located in dendritic spines. Neuron. 2007;53:249–260. doi: 10.1016/j.neuron.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Stevens B, Chang J, Milbrandt J, Barres BA, Hell JW. NS21: redefined and modified supplement B27 for neuronal cultures. J Neurosci Methods. 2008;171:239–247. doi: 10.1016/j.jneumeth.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Hall DD, Hell JW. Supramolecular Assemblies and Localized Regulation of Voltage-gated Ion Channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science. 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Straube KT, Disterhoft JF. Nimodipine facilitates associative learning in aging rabbits. Science. 1989;243:809–811. doi: 10.1126/science.2916127. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Gispen WH, Traber J, Khachaturian ZS, editors. Calcium Hypothesis of Aging and Dementia. 1994. [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr Op Neurobiol. 2009;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Dzhura I, Wu Y, Colbran RJ, Corbin JD, Balser JR, Anderson ME. Cytoskeletal disrupting agents prevent calmodulin kinase, IQ domain and voltage-dependent facilitation of L-type Ca2+ channels. J Physiol. 2002;545:399–406. doi: 10.1113/jphysiol.2002.021881. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fallon JL, Halling DB, Hamilton SL, Quiocho FA. Structure of calmodulin bound to the hydrophobic IQ domain of the cardiac Ca(v)1.2 calcium channel. Structure. 2005;13:1881–1886. doi: 10.1016/j.str.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Gao T, Bunemann M, Gerhardstein BL, Ma H, Hosey MM. Role of the C terminus of the alpha 1C (Cav1.2) subunit in membrane targeting of cardiac L-type calcium channels. J Biol Chem. 2000;275:25436–25444. doi: 10.1074/jbc.M003465200. [DOI] [PubMed] [Google Scholar]
- Graef IA, Mermelstein PG, Stankunas K, Neilson JR, Deisseroth K, Tsien RW, Crabtree GR. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature. 1999;401:703–708. doi: 10.1038/44378. [DOI] [PubMed] [Google Scholar]
- Green EM, Barrett CF, Bultynck G, Shamah SM, Dolmetsch RE. The tumor suppressor eIF3e mediates calcium-dependent internalization of the L-type calcium channel Cav1.2. Neuron. 2007;55:615–632. doi: 10.1016/j.neuron.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nature Neurosci. 2002;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- Grover LM, Teyler TJ. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990;347:477–479. doi: 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]
- Hall DD, Davare MA, Shi M, Allen ML, Weisenhaus M, McKnight GS, Hell JW. Critical role of cAMP-dependent protein kinase anchoring to the L-type calcium channel Cav1.2 via A-kinase anchor protein 150 in neurons. Biochem. 2007;46:1635–1646. doi: 10.1021/bi062217x. [DOI] [PubMed] [Google Scholar]
- Hall DD, Feekes JA, Arachchige Don AS, Shi M, Hamid J, Chen L, Strack S, Zamponi GW, Horne MC, Hell JW. Binding of protein phosphatase 2A to the L-type calcium channel Cav1.2 next to Ser1928, its main PKA site, is critical for Ser1928 dephosphorylation. Biochem. 2006;45:3448–3459. doi: 10.1021/bi051593z. [DOI] [PubMed] [Google Scholar]
- Halling DB, Aracena-Parks P, Hamilton SL. Regulation of voltage-gated Ca2+ channels by calmodulin. Sci STKE. 2006;2006:er1. doi: 10.1126/stke.3182006er1. [DOI] [PubMed] [Google Scholar]
- Halt AR, Dallapiazza R, Yu H, Stein IS, Qian H, Junti S, Wojcik S, Brose N, Sliva A, Hell JW. CaMKII binding to GluN2B is Critical During Memory Consolidation. The EMBO J. 2012;31:1203–1216. doi: 10.1038/emboj.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Breeze LJ, Wang KKW, Chavkin C, Catterall WA. N-methyl-D-aspartate receptor-induced proteolytic conversion of postsynaptic class C L-type calcium channels in hippocampal neurons. Proc Natl Acad Sci USA. 1996;93:3362–3367. doi: 10.1073/pnas.93.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel α1 subunits. J Cell Biol. 1993a;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW, Yokoyama CT, Wong ST, Warner C, Snutch TP, Catterall WA. Differential phosphorylation of two size forms of the neuronal class C L-type calcium channel α1 subunit. J Biol Chem. 1993b;268:19451–19457. [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Calcium signaling in dendrites and spines: practical and functional considerations. Neuron. 2008;59:902–913. doi: 10.1016/j.neuron.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Hoogland TM, Saggau P. Facilitation of L-type Ca2+ channels in dendritic spines by activation of beta2 adrenergic receptors. J Neurosci. 2004;24:8416–8427. doi: 10.1523/JNEUROSCI.1677-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Byerly L. A cytoskeletal mechanism for Ca2+ channel metabolic dependence and inactivation by intracellular Ca2+ Neuron. 1993;10:797–804. doi: 10.1016/0896-6273(93)90196-x. [DOI] [PubMed] [Google Scholar]
- Kepplinger KJ, Kahr H, Forstner G, Sonnleitner M, Schindler H, Schmidt T, Groschner K, Soldatov NM, Romanin C. A sequence in the carboxy-terminus of the alpha(1C) subunit important for targeting, conductance and open probability of L-type Ca(2+) channels. FEBS Lett. 2000;477:161–169. doi: 10.1016/s0014-5793(00)01791-9. [DOI] [PubMed] [Google Scholar]
- Kim J, Ghosh S, Nunziato DA, Pitt GS. Identification of the components controlling inactivation of voltage-gated Ca2+ channels. Neuron. 2004;41:745–754. doi: 10.1016/s0896-6273(04)00081-9. [DOI] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Interactions of calmodulin and alpha-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors. J Neurosci. 1999;19:1165–1178. doi: 10.1523/JNEUROSCI.19-04-01165.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lader AS, Kwiatkowski DJ, Cantiello HF. Role of gelsolin in the actin filament regulation of cardiac L-type calcium channels. Am J Physiol. 1999;277:C1277–1283. doi: 10.1152/ajpcell.1999.277.6.C1277. [DOI] [PubMed] [Google Scholar]
- Lee J-M, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399(Suppl):A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Bayer KU, Merrill MA, Lim IA, Shea MA, Schulman H, Hell JW. Regulation of calcium/calmodulin-dependent protein kinase II docking to N-methyl-D-aspartate receptors by calcium/calmodulin and a-actinin. J Biol Chem. 2002;277:48441–48448. doi: 10.1074/jbc.M205164200. [DOI] [PubMed] [Google Scholar]
- Liu G, Shi J, Yang L, Cao L, Park SM, Cui J, Marx SO. Assembly of a Ca(2+)-dependent BK channel signaling complex by binding to beta2 adrenergic receptor. EMBO J. 2004;23:2196–2205. doi: 10.1038/sj.emboj.7600228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Bangalore R, Rutledge A, Triggle DJ. Modulation of L-type Ca2+ channels in clonal rat pituitary cells by membrane depolarization. Mol Pharmacol. 1994;45:1198–1206. [PubMed] [Google Scholar]
- Lu L, Zhang Q, Timofeyev V, Zhang Z, Young JN, Shin HS, Knowlton AA, Chiamvimonvat N. Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via alpha-actinin2. Circ Res. 2007;100:112–120. doi: 10.1161/01.RES.0000253095.44186.72. [DOI] [PubMed] [Google Scholar]
- Marrion NV, Tavalin ST. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- Merrill MA, Malik Z, Akyol Z, Bartos JA, Leonard AS, Hudmon A, Shea MA, Hell JW. Displacement of alpha-Actinin from the NMDA Receptor NR1 C0 Domain By Ca(2+)/Calmodulin Promotes CaMKII Binding. Biochem. 2007;46:8485–8497. doi: 10.1021/bi0623025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Muller J, Stiess M, Marais E, Schulla V, Lacinova L, et al. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Sunagawa M, Kosugi T, Sperelakis N. Actin filament disruption inhibits L-type Ca(2+) channel current in cultured vascular smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C480–487. doi: 10.1152/ajpcell.2000.279.2.C480. [DOI] [PubMed] [Google Scholar]
- Obermair GJ, Szabo Z, Bourinet E, Flucher BE. Differential targeting of the L-type Ca2+ channel alpha1C (Cav1.2) to synaptic and extrasynaptic compartments in hippocampal neurons. Eur J Neurosci. 2004;19:2109–2122. doi: 10.1111/j.0953-816X.2004.03272.x. [DOI] [PubMed] [Google Scholar]
- Pavalko FM, Burridge K. Disruption of the actin cytoskeleton after microinjection of proteolytic fragments of alpha-actinin. J Cell Biol. 1991;114:481–491. doi: 10.1083/jcb.114.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M. Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J Biol Chem. 2004;279:21003–21011. doi: 10.1074/jbc.M400103200. [DOI] [PubMed] [Google Scholar]
- Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- Pitt GS, Zuhlke RD, Hudmon A, Schulman H, Reuter H, Tsien RW. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels. J Biol Chem. 2001;276:30794–30802. doi: 10.1074/jbc.M104959200. [DOI] [PubMed] [Google Scholar]
- Ravindran A, Lao QZ, Harry JB, Abrahimi P, Kobrinsky E, Soldatov NM. Calmodulin-dependent gating of Ca(v)1.2 calcium channels in the absence of Ca(v)beta subunits. Proc Natl Acad Sci USA. 2008;105:8154–8159. doi: 10.1073/pnas.0711624105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Schnizler MK, Schnizler K, Zha XM, Hall DD, Wemmie JA, Hell JW, Welsh MJ. The cytoskeletal protein alpha-actinin regulates acid-sensing ion channel 1a through a C-terminal interaction. J Biol Chem. 2009;284:2697–2705. doi: 10.1074/jbc.M805110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, Kuhbandner S, Striessnig J, Klugbauer N, Feil R, Hofmann F. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J Biol Chem. 2000;275:39193–39199. doi: 10.1074/jbc.M006467200. [DOI] [PubMed] [Google Scholar]
- Sinnegger-Brauns MJ, Hetzenauer A, Huber IG, Renstrom E, Wietzorrek G, Berjukov S, Cavalli M, Walter D, Koschak A, Waldschutz R, et al. Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by L-type Ca2+ channels. J Clin Invest. 2004;113:1430–1439. doi: 10.1172/JCI20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavalin SJ, Shepherd D, Cloues RK, Bowden SE, Marrion NV. Modulation of single channels underlying hippocampal L-type current enhancement by agonists depends on the permeant ion. J Neurophysiol. 2004;92:824–837. doi: 10.1152/jn.00700.2003. [DOI] [PubMed] [Google Scholar]
- Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272:1017–1020. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- Tippens AL, Pare JF, Langwieser N, Moosmang S, Milner TA, Smith Y, Lee A. Ultrastructural evidence for pre- and postsynaptic localization of Cav1.2 L-type Ca2+ channels in the rat hippocampus. J Comp Neurol. 2008;506:569–583. doi: 10.1002/cne.21567. [DOI] [PubMed] [Google Scholar]
- Van Petegem F, Chatelain FC, Minor DL., Jr Insights into voltage-gated calcium channel regulation from the structure of the Cav1.2 IQ domain-Ca2+/calmodulin complex. Nat Struct Mol Biol. 2005;12:1108–1115. doi: 10.1038/nsmb1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waithe D, Ferron L, Page KM, Chaggar K, Dolphin AC. β-Subunits Promote the Expression of Cav2.2 Channels by Reducing Their Proteasomal Degradation. J Biol Chem. 2011;286:9598–9611. doi: 10.1074/jbc.M110.195909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walikonis RS, Jensen ON, Mann M, Provance DW, Jr, Mercer JA, Kennedy MB. Identification of proteins in the postsynaptic density fraction by mass spectrometry. J Neurosci. 2000;20:4069–4080. doi: 10.1523/JNEUROSCI.20-11-04069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walikonis RS, Oguni A, Khorosheva EM, Jeng CJ, Asuncion FJ, Kennedy MB. Densin-180 forms a ternary complex with the (alpha)-subunit of Ca2+/calmodulin-dependent protein kinase II and (alpha)-actinin. J Neurosci. 2001;21:423–433. doi: 10.1523/JNEUROSCI.21-02-00423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HG, George MS, Kim J, Wang C, Pitt GS. Ca2+/calmodulin regulates trafficking of Ca(V)1.2 Ca2+ channels in cultured hippocampal neurons. J Neurosci. 2007;27:9086–9093. doi: 10.1523/JNEUROSCI.1720-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski M, Kharazia V, Shanghvi R, Rao A, Beggs AH, Craig AM, Weinberg R, Sheng M. Differential regional expression and ultrastructural localization of alpha-actinin-2, a putative NMDA receptor-anchoring protein, in rat brain. J Neurosci. 1998;18:1383–1392. doi: 10.1523/JNEUROSCI.18-04-01383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, Sheng M. Competitive binding of α-actinin and calmodulin to the NMDA receptor. Nature. 1997;385:439–442. doi: 10.1038/385439a0. [DOI] [PubMed] [Google Scholar]
- Xu X, Marx SO, Colecraft HM. Molecular mechanisms, and selective pharmacological rescue, of Rem-inhibited Cav1.2 channels in heart. Circ Res. 2010;107:620–630. doi: 10.1161/CIRCRESAHA.110.224717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Bal M, Bierbower S, Zaika O, Shapiro MS. AKAP79/150 Signal Complexes in G-Protein Modulation of Neuronal Ion Channels. J Neurosci. 2011;31:7199–7211. doi: 10.1523/JNEUROSCI.4446-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Ehlers MD, Bernhardt JP, Su CT, Huganir RL. Calmodulin mediates calcium-dependent inactivation of N-methyl-D-aspartate receptors. Neuron. 1998;21:443–453. doi: 10.1016/s0896-6273(00)80553-x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Gunst SJ. Dynamic association between alpha-actinin and beta-integrin regulates contraction of canine tracheal smooth muscle. J Physiol. 2006;572:659–676. doi: 10.1113/jphysiol.2006.106518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Olcese R, Qin N, Noceti F, Birnbaumer L, Stefani E. Feedback inhibition of Ca2+ channels by Ca2+ depends on a short sequence of the C terminus that does not include the Ca2+ -binding function of a motif with similarity to Ca2+ -binding domains. Proc Natl Acad Sci USA. 1997;94:2301–2305. doi: 10.1073/pnas.94.6.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.