Summary
The role of neutrophils in tuberculosis (TB), and whether neutrophils express granzyme B (grzB), a pro-apoptotic enzyme associated with cytotoxic T cells, is controversial. We examined neutrophils in peripheral blood (PB) and lung granulomas of Mycobacterium tuberculosis-infected cynomolgus macaques and humans to determine whether mycobacterial products or pro-inflammatory factors induce neutrophil grzB expression. We found large numbers of grzB-expressing neutrophils in macaque and human granulomas and these cells contained more grzB+ granules than T cells. Higher neutrophil, but not T cell, grzB expression correlated with increased bacterial load. Although unstimulated PB neutrophils lacked grzB expression, grzB expression increased upon exposure to M. tuberculosis bacilli, M. tuberculosis culture filtrate protein or lipopolysaccharide from Escherichia coli. Perforin is required for granzyme-mediated cytotoxicity by T cells, but was not observed in PB or granuloma neutrophils. Nonetheless, stimulated PB neutrophils secreted grzB as determined by enzyme-linked immunospot assays. Purified grzB was not bactericidal or bacteriostatic, suggesting secreted neutrophil grzB acts on extracellular targets, potentially enhancing neutrophil migration through extracellular matrix and regulating apoptosis or activation in other cell types. These data indicate mycobacterial products and the pro-inflammatory environment of granulomas up-regulates neutrophil grzB expression and suggests a previously unappreciated aspect of neutrophil biology in TB.
Introduction
Granzyme B (grzB) is a serine protease associated with cytotoxic T–lymphocyte-mediated defence against intracellular pathogens (Barry and Bleackley, 2002; Afonina et al., 2010) and cancer (Cullen et al., 2010; Rousalova and Krepela, 2010). GrzB enters target cells through a perforin-dependent mechanism and acts on intracellular substrates including pro-caspase 3 (Trapani and Smyth, 2002), thereby initiating apoptosis and pathogen clearance. Although commonly associated with T cells and natural killer cells, non-lymphoid lineage cells including chondrocytes (Horiuchi et al., 2003), keratinocytes (Hernandez-Pigeon et al., 2006; 2007) and macrophages (Choy et al., 2003; Kim et al., 2007; Vernooy et al., 2007) can also express grzB. The question of whether neutrophils can express grzB or perforin has been contentious (Grossman and Ley, 2004; Metkar and Froelich, 2004; Wagner et al., 2004; 2008; Martin et al., 2005). In addition to its intracellular activity, grzB secreted into the extracellular milieu can target a range of proteins including von Willebrand factor, cell surface receptors and extracellular matrix (ECM) components including fibronectin, vitronectin, laminin and fibrillin-1 (Hendel et al., 2010). As such, extracellular grzB could act as a modulator of cell activation by cleaving activatory or inhibitory receptors or their ligands (Boivin et al., 2009). Excessive grzB may be pathologic and extracellular grzB has been associated with abdominal aneurysm (Chamberlain et al., 2010), rheumatoid arthritis (Boivin et al., 2009) and chronic obstructive pulmonary disease (COPD) (Ngan et al., 2009).
Tuberculosis (TB) is caused by Mycobacterium tuberculosis, a pathogenic intracellular bacterium that causes pulmonary disease characterized by granulomas and associated inflammation. The granuloma represents the functional unit of bacterial containment, and is a multicellular structure where, when successful, lymphoid and myeloid cell lineages mount a coordinated response that maximizes antibacterial responses while minimizing immunopathy to uninvolved peripheral tissues. Protection against TB is strongly correlated with CD8+ T cell-mediated cytotoxicity in murine models (Flynn et al., 1992; Sousa et al., 2000). That said, the role of grzB in TB and the cell types that produce it have been difficult to ascertain. A murine model using the vaccine strain BCG indicates that grzB is up-regulated in lung cells by 14 days post-infection (Aranday-Cortes et al., 2012). Other studies using knockout mice have demonstrated that abrogating perforin expression limits CD8+ T cell-mediated cytotoxicity (Serbina et al., 2000; Woodworth et al., 2008) and impairs bacterial control (Cooper et al., 1997), but eliminating grzB expression has little effect on bacterial control (Cooper et al., 1997). Uninfected and latently infected humans have peripheral blood CD8+ T cells that produce more grzB on a per cell basis than patients with active pulmonary TB (Silva et al., 2014), suggesting that low levels of T cell grzB expression may be a risk factor for disease development. In contrast to the T cell-specific data where intracellular grzB is measured ex vivo, higher levels of soluble grzB have been measured in the serum of individuals with cavitary TB, with more grzB being correlated with larger sized cavities (Djoba Siawaya et al., 2008). Similarly, individuals with rapid responses to drug therapy had less soluble grzB in serum than individuals with slower treatment responses (Brahmbhatt et al., 2006). These data suggest that while cytotoxic T cells are important for controlling TB, excessive levels of extracellular grzB may be a negative prognosticative marker in TB. These studies do not, however, address what cell types make grzB in granulomas, what factors induce its up-regulation and how severity of disease and bacterial burden modifies grzB expression.
To better understand the role of grzB in TB, we examined granulomas from M. tuberculosis-infected cynomolgus macaques, a non-human primate that accurately models human TB, to determine how grzB is localized and which cells expressed it. To our surprise, we found that neutrophils were the most common grzB-positive cells in granulomas and expressed more grzB on a per cell basis than T cells but did not express perforin. Neutrophil grzB expression could be induced by stimulation with mycobacterial antigens or by whole M. tuberculosis, and was secreted despite the lack of perforin expression in neutrophils. Moreover, we found neutrophil grzB expression correlated with bacterial burden suggesting that neutrophil grzB expression in the granuloma may be related to antigen load. These data provide a window into a previously unappreciated aspect of neutrophil biology in TB and suggest that neutrophil-secreted grzB may be a contributing factor underlying poorly controlled TB.
Results
Preliminary experiments examining grzB expression by immunohistochemistry (IHC) indicated that granulomas contain numerous grzB-expressing cells (Fig. 1A), and many of these cells are in regions low in T cells but rich in neutrophils. A closer examination of these cells demonstrated that while CD8+ T cells can be positive for grzB, substantial numbers of the grzB+ cells did not express CD3 (Fig. 1A) or CD8 (Fig. 1B). These cells had segmented nuclei (Fig. 1B) and co-stained for calprotectin, indicating that they are neutrophils (Fig. 1C) (Mattila et al., 2013). Further analysis showed that most grzB signal co-localized in elastase-positive granules, with much less co-localization with cathelicidin- and matrix metallopeptidase 9 (MMP9)- (gelatinase) positive granules (Supplemental Fig. S3), suggesting grzB is present in the azurophilic neutrophil granules (Pham, 2006).
Fig. 1.

Neutrophils in granulomas can express grzB. Immunohistochemistry was performed on formalin-fixed paraffin-embedded granulomas to assess the localization and expression of grzB, CD3+ T cells, CD8+ lymphocytes and calprotectin-expressing neutrophils.
A. GrzB (green) localizes with T cells (red) and neutrophils (blue) in a necrotic (caseous) granuloma. The dashed line bisects the epithelioid macrophage region, highlighting a neutrophil-rich, T–cell-poor region adjacent to the caseum with an abundance of grzB expression. Scale bar represents 100 μm.
B. CD3-grzB+ cells in granulomas do not express CD8, suggesting they are not natural killer cells or γδ T cells. Scale bar represents 20 μm.
C. GrzB+ calprotectin+ cells have multilobate nuclei indicating they are neutrophils. Scale bar represents 10 μm.
We sampled a series of granulomas from animals with active TB to determine how common neutrophil grzB expression was and to compare the number and frequency of grzB+ neutrophils and T cells in macaque granulomas. Per imaging field, granulomas contained more T cells than neutrophils (Fig. 2A) and neutrophil numbers but not T cell numbers positively correlated with bacterial load [colony-forming unit (CFU)] per granuloma (neutrophils: Spearman r = 0.4135, P = 0.0358; T cells: r = 0.1995, P = 0.3285) (Fig. 2B). Of these two cell populations, more neutrophils expressed grzB than T cells (Fig. 2C) and the quantity of grzB per cell, when measured as the number of grzB-stained granules per cell (Fig. 2D), indicated that neutrophils contain significantly more grzB than CD3+ T cells. Because of the relationship between perforin and grzB-mediated cytotoxicity in T cells (Trapani and Smyth, 2002), we examined perforin expression in these cell types. T cell-rich granuloma regions contained numerous perforin-expressing cells whereas neutrophil-rich regions had relatively little perforin staining (Fig. 3A). This relationship was also borne out by a quantitative comparison of T cell and neutrophil perforin expression (Fig. 3B), suggesting that although neutrophils can express grzB, they do not engage in perforin-dependent cytolytic activities.
Fig. 2.
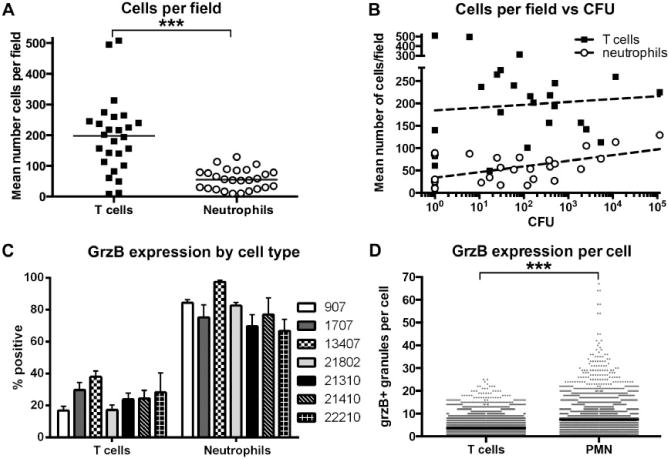
Frequency and relative quantification of grzB expression by T cells and neutrophils.
A. Granuloma fields imaged at 40× contained significantly more T cells than neutrophils. Each point represents one granuloma, n = 12 monkeys, ***P < 0.0001; Mann–Whitney test, bar represents the median.
B. Numbers of neutrophils in granulomas correlate with bacterial load per granuloma, while T-cell numbers do not. Each point represents one granuloma, n = 12 monkeys.
C. As a percent of the total population, more neutrophils than T cells express grzB in granulomas. Bars represent the mean with SEM of images from two to four granulomas per animal (monkey ID numbers indicated to the right of the graph).
D. On a per cell basis, granuloma neutrophils contain more grzB+ granules per cell than T cells. n = 4627 neutrophils and 10 722 T cells, ***P < 0.0001, Mann–Whitney test, bar represents the median.
Fig. 3.

Neutrophils do not express perforin in granulomas. Images of perforin-stained granulomas were examined for perforin expression.
A. Perforin co-localizes with grzB in CD3+ T cells but is not observed in calprotectin-stained neutrophils.
B. Quantification of perforin-expressing T cells and neutrophils in images of perforin-stained granulomas.
Human granulomas from TB patients were examined for neutrophil grzB and perforin expression. These granulomas come from individuals who have failed drug treatment and represent complex pathologies associated with repeated cycles of drug therapy and disease relapse, suggesting they may contain large quantities of M. tuberculosis antigens. GrzB+ neutrophils were present in these tissues and, as with macaque granulomas, were particularly abundant at the epithelioid macrophage–caseum interface (Fig. 4A). Quantification of the grzB expression (Fig. 4B) and perforin expression (Fig. 4C) indicated that neutrophils in human granulomas are significant contributors to grzB expression but do not express appreciable amounts of perforin.
Fig. 4.
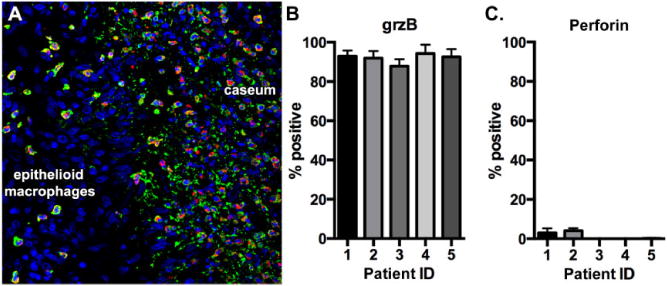
Neutrophils in human granulomas express grzB but not perforin. Human granulomas were examined for neutrophil grzB and perforin expression.
A. GrzB (red)-expressing neutrophils (green) preferentially localize at the epithelioid macrophage–caseum interface. Nuclei are indicated in blue.
B. GrzB expression. C. Perforin expression.
Identifying neutrophil grzB expression led us to investigate whether neutrophils constitutively express grzB or if expression is induced by activation. Substantial numbers of T cells in unstimulated peripheral blood expressed grzB, but very few grzB-expressing neutrophils were observed (Fig. 5A). Perforin expression by neutrophils in peripheral blood was not observed (data not shown). To determine how activation changes grzB expression by neutrophils, we stimulated cells from red blood cell (RBC)-lysed whole blood with mycobacterial peptides, bacterial ligands and non-specific cell activators [phorbol 12,13 dibutyrate (PDBu) and ionomycin] and measured grzB expression by flow cytometry. PDBu and ionomycin, a chemical cocktail that induces protein kinase C and calcium-dependent signalling pathways (Asehnoune et al., 2005), up-regulated T-cell grzB expression somewhat, but strongly up-regulated neutrophil grzB expression (Fig. 5B). Stimulating cells with ESAT6 and M. tuberculosis 38.1 (also known as CFP10), a cocktail of M. tuberculosis-specific peptides frequently used to elicit T-cell cytokine responses, did not induce significant up-regulation of grzB by either T cells (data not shown) or neutrophils (Fig. 5B). Conversely, stimulation with M. tuberculosis culture filtrate protein (CFP) and Escherichia coli lipopolysaccharide (LPS) increased neutrophil grzB expression above basal (unstimulated) and peptide-stimulated expression levels (Fig. 5B). CFP-mediated grzB expression by neutrophils was not significantly different than the expression induced by LPS stimulation (Fig. 5B). T cells did not appear to respond to LPS stimulation by up-regulating grzB, tumour necrosis factor (TNF) or interferon-γ (IFN-γ) (data not shown). These data suggest that pro-inflammatory environments containing bacterial ligands, including mycobacterial products, can stimulate grzB expression by neutrophils in the absence of pro-inflammatory T cell cytokines.
Fig. 5.

Stimulating neutrophils with bacterial ligands and pro-inflammatory activators up-regulates grzB expression. PBMCs from RBC-depleted whole blood were stimulated with a cocktail of M. tuberculosis ESAT6 and 38.1 peptides, CFP, LPS and P + I and grzB expression was assessed by flow cytometry.
A. Representative FACS plots showing CD4+ and CD8+ T cell and neutrophil activation by CFP and P + I relative to media-only controls.
B. Quantification of grzB expression by neutrophils. Results from PDBu-stimulated cells are represented on the right axis. Each marker represents an individual monkey with n = 23 monkeys from five independent experiments depicted, **P < 0.005, ***P < 0.0005, Wilcoxon matched-pairs signed-rank test for pairwise comparison.
GrzB-mediated T cell cytotoxicity requires perforin (Trapani and Smyth, 2002), but neutrophils do not express perforin, raising the question of whether neutrophils secrete grzB, and if they do, is grzB secretion antigen dependent? To answer these questions, we performed grzB enzyme-linked immunospots (ELISPOTs) on paired samples of neutrophil-depleted (buffy coat) peripheral blood mononuclear cells (PBMCs) and purified neutrophils using three categories of stimulators: T cell-specific stimulators (ESAT6+38.1 peptides), bacterial toll-like receptor (TLR) ligands (mycobacterial CFP or LPS) and non-specific cell activators (PDBu and ionomycin). ELISPOT analysis of grzB secretion identified distinct differences between buffy coat (non-neutrophil) PBMCs and neutrophils (Fig. 6A). Small numbers of buffy coat cells secreted grzB, but there was little evidence of antigen-specific or protein kinase C (PDBu and ionomycin) stimulated grzB expression (Fig. 6B). In contrast, neutrophil grzB secretion was up-regulated by CFP-and PDBu + ionomycin stimulation and LPS-stimulated cells also showed a slight, but not statistically significant, increase in grzB expression (Fig. 6C).
Fig. 6.
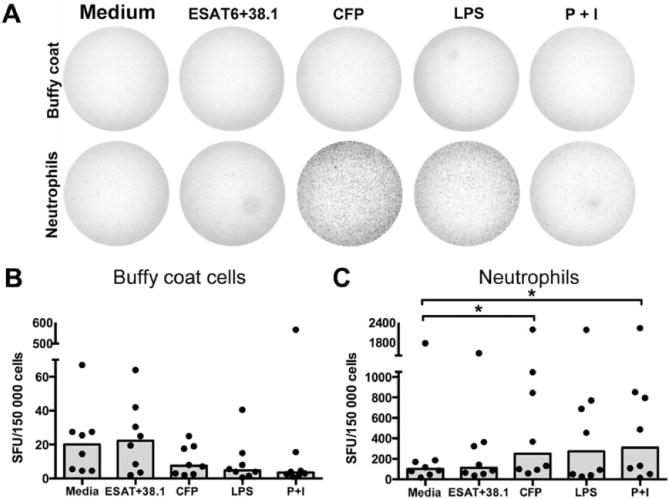
Mycobacterial products and pro-inflammatory stimuli elicit grzB secretion by peripheral blood neutrophils. Paired sets of neutrophil-depleted buffy coat PBMCs and purified neutrophils were subject to stimulation in ELISPOT assay and the number of spot-forming units (SFU) were quantified.
A. Representative ELISPOT wells showing the differences between cell fractions and appearance of spots.
B. Quantification of grzB expression. Each spot represents the mean of two wells per cell fraction from each monkey. Bars represent the median of results from two independent experiments, four monkeys per experiment. *P < 0.05, Wilcoxon matched-pairs signed-rank test for pairwise comparison between unstimulated and stimulated samples.
Our data indicate that M. tuberculosis antigens can elicit grzB expression from macaque neutrophils. We next sought to determine whether live M. tuberculosis can induce grzB expression by neutrophils. Flow cytometry-based experiments identified that exposure to viable M. tuberculosis induced grzB expression in neutrophils from RBC-lysed whole blood (Fig. 7A). Likewise, we noted that isolated neutrophils secreted grzB following incubation with M. tuberculosis whereas neutrophil-depleted PBMCs did not (Fig. 7B). Granulomas can vary significantly in both bacterial burden (Lin et al., 2014) and neutrophil abundance (Mattila et al., 2013), suggesting that exposure of neutrophils to bacterial products in granulomas varies over a wide range. This led us to investigate whether neutrophils, which are exquisitely sensitive to bacterial products, have grzB expression that is influenced by bacterial burden in vivo. To investigate this, we stained granulomas of known bacterial burden (from plating for CFU at necropsy) and correlated the number of bacteria per granuloma with the mean number of T cells and neutrophils in the images for each granuloma, and the number of grzB+ granules per cell (Fig. 7C). We found a trend in the relationship between numbers of bacteria per granuloma and grzB expression by neutrophils (Spearman r = 0.3468, P = 0.0827), but not for T cells (Spearman r = −0.1115, P = 0.5878), suggesting that bacterial products can drive neutrophil grzB expression in vivo. There was variability in grzB expression by neutrophils and one monkey whose granulomas had normal numbers of granuloma neutrophils and T cells (Fig. 7C, open markers) had very low neutrophil grzB expression, but normal T cell grzB expression. The consequence of this variability is not known.
Fig. 7.

GrzB expression is up-regulated by M. tuberculosis in an antigen-dependent manner. FACS experiments using whole blood co-cultured with M. tuberculosis, ELISPOT assays comparing buffy coat PBMCS with purified neutrophils and immunohistochemistry on tissues with known bacterial burdens demonstrate that M. tuberculosis can cause up-regulated grzB expression in neutrophils.
A. FACS experiments using neutrophils from RBC-free PBMCs. Bars represent mean with SEM. *P < 0.05; Wilcoxon matched-pairs signed-rank test.
B. ELISPOT assays indicate M. tuberculosis does not significantly up-regulate grzB expression by buffy coat PBMCs but does significantly up-regulate grzB expression by neutrophils. *P < 0.05; Wilcoxon matched-pairs signed-rank test.
C. Bacterial burdens correlate with higher numbers of grzB+ granules per cell for neutrophils (Spearman r = 0.3468, P = 0.0827) but not for CD3+ T cells (Spearman r = −0.1115, P = 0.5878). One monkey (indicated by open markers) had normal numbers of neutrophils and T cells per image, but had neutrophils with very few grzB+ granules per cell, but normal T-cell grzB expression.
Discussion
Neutrophils are among the first cells to respond to injury or infection and can use a formidable range of effectors for host defence, including phagocytosis and the ability to generate reactive oxygen intermediates and antimicrobial peptides (Nathan and Shiloh, 2000; Winterbourn and Kettle, 2013; Mayadas et al., 2014). Additionally, neutrophils contribute to cytokine, chemokine and growth factor production (Kemp et al., 2005; Doz et al., 2013; Wang et al., 2013); transport microbes to lymph nodes (Abadie et al., 2005); and participate in T cell priming (Morel et al., 2008; Blomgran and Ernst, 2011; Blomgran et al., 2012), but are generally not considered to engage in T cell-like cytotoxic activity. Although neutrophils can be abundant in tuberculous granulomas, we know very little about what they do (Lowe et al., 2012). Evidence from zebrafish infected with M. marinum suggests that neutrophils play a protective role and can restrict bacterial growth during early infection by killing bacteria in apoptotic macrophages (Yang et al., 2012). A rat-based model of early M. tuberculosis infection where recruiting neutrophils to the lungs via inhaled LPS leads to decreased viable bacterial viability suggests that neutrophils may have a similar function in mammalian lungs (Sugawara et al., 2004). In contrast, data from murine and non-human primate models, and from human TB patients at later stages of infection, associate large numbers of neutrophils with poorly controlled disease (Ozaki et al., 1992; Law et al., 1996; Ashitani et al., 2002; Brahmbhatt et al., 2006; Lin et al., 2010; Nandi and Behar, 2011). In humans, a neutrophil-derived interferon signature is one of the first indicators of active TB (Berry et al., 2010), and neutrophils are the most common M. tuberculosis-infected cell type in human airways and can contain viable, replicating bacteria (Eum et al., 2010). These data imply that neutrophils may not be protective beyond the earliest stages of infection, but instead may engage in activities that modify the granuloma microenvironment in favour of mycobacterial survival, or even provide an alternative replicative niche for M. tuberculosis. Considering these disparate observations, neutrophils may play different roles at different phases of infection, being beneficial in very early stages of infection but becoming progressively more pathologic in situations of dysregulated cytokine production, excessive inflammation or higher bacterial burdens. These contradictions beg for further exploration into what neutrophils are doing in granulomas, how these activities may influence granuloma homeostasis and bacterial containment, and whether neutrophils can be manipulated in ways that promote bacterial control.
Like the role of neutrophils in TB, grzB and perforin expression by neutrophils is controversial. Wagner et al. (2004) were among the first to identify constitutive neutrophil grzB expression with some variability among different individuals. Metkar and Froelich (2004) dispute this and were unable to identify grzB expression, attributing Wagner et al.’s results to methodological errors in their antibody-based assays and lymphocyte contamination in reverse transcription polymerase chain reaction, Western blots and biochemical assays. Grossman and Ley (2004) and Martin et al. (2005) corroborated this lack of grzB expression in mice and human volunteers. Although these studies come to different conclusions, it should be noted that the neutrophils used by these groups were obtained from healthy human donors except for Martin et al.’s study (2005), which included lymphoma and leukaemia patients. Cells from individuals with inflammatory conditions or infections (such as TB), or from tissues, may give different results. Wagner et al. noted that neutrophil grzB expression could be up-regulated by co-incubation with purified IFN-γ (2004), suggesting the inflammatory milieu may contribute to grzB expression. In subsequent work, this group described grzB expression not only in mature neutrophils but also in myeloid origin cell lines and stem cell-derived neutrophils (Wagner et al., 2008). GrzB has been identified in a wide variety of non-lymphocyte cell types in recent years, indicating grzB expression is more broadly distributed than previously thought (Hendel et al., 2010). Perforin expression by neutrophils has been equally contentious (Metkar and Froelich, 2004; Wagner et al., 2004; Martin et al., 2005), and although we looked for perforin-expressing neutrophils, our data suggest that in macaque and human granulomas, or in stimulated macaque blood, neutrophils do not express appreciable amounts of perforin.
Our data demonstrate that large numbers of neutrophils in cynomolgus macaque and human granulomas express grzB. Moreover, granulomas with greater bacterial burdens contained more neutrophils, and more of those neutrophils express grzB, than granulomas with lower bacterial burdens, suggesting a dose-dependent relationship between neutrophil grzB expression and M. tuberculosis in vivo. Neutrophil grzB expression was induced by mycobacterial CFP, which is an undefined mixture of secreted proteins and lipids, in a manner that was very similar to what we observed with E. coli LPS, a known TLR4 agonist that signals through protein kinase C (Yang et al., 2007), suggesting an analogous mechanism of activation may be occurring with CFP. Unstimulated peripheral blood neutrophils are grzB negative, but TB usually presents as a highly localized disease and the granuloma microenvironment is rich in mycobacterial antigens that appear to stimulate a robust, but localized, neutrophil grzB response. Moreover, our in vitro experiments did not find that CFP or LPS up-regulated IFN-γ or TNF expression by neutrophils or T cells, suggesting that neutrophil grzB expression can be independent of these pro-inflammatory T cell cytokines. Our ELISPOT data using T cell-depleted neutrophil populations further suggest that T cell-produced cytokines or T cell: neutrophil contact is not required for neutrophil grzB expression. Immunohistochemical data from macaque granulomas indicate that neutrophils in granulomas are negative for phosphorylated STAT1 and STAT3 (unpublished) indicators of interferon or IL-6 and IL-10 signalling, respectively, implying that cytokines using these signalling pathways are not likely to be involved in neutrophil grzB expression.
Serine proteases are important components of the neutrophil armamentarium that can have diverse functions. These proteases assist neutrophil migration through tissues, regulate activation of other cells by cleaving cell-surface receptors, proteolytically activate cytokines and antimicrobial peptides, and have their own intrinsic antimicrobial activity in extracellular and intraphagosomal environments (Raptis and Pham, 2005; Pham, 2006; 2008; Standish and Weiser, 2009; Omoto et al., 2010). In murine TB models, containment of M. tuberculosis requires serine protease activity (Reece et al., 2010). GrzB is a serine protease, and although best known for its targeted perforin-dependent cytotoxicity, grzB in the extracellular milieu is implicated in proteolytic activation of the pro-inflammatory cytokines IL-1α (Afonina et al., 2011) and IL-18 (Omoto et al., 2010), and anti-thrombotic activity (Buzza et al., 2008). GrzB can attack a variety of ECM components including fibronectin, fibrinogen, vitronectin, laminin, fibrillin-1 and collagen IV (Boivin et al., 2009; Cullen et al., 2010; Hendel et al., 2010; Hiebert and Granville, 2012). This can result in release of ECM-bound growth factors and cytokines (Boivin et al., 2009; Hiebert and Granville, 2012), production of chemotactic peptide fragments or increased expression of pro-inflammatory cytokines such as IL-6, IL-8 and TNF (Birdsall et al., 2004; Kuhns et al., 2007; Boivin et al., 2009; Cullen et al., 2010; Hendel et al., 2010; Hiebert and Granville, 2012). Moreover, loss of adhesion to the ECM can lead to apoptosis (anoikis) in contact-dependent cells (Choy et al., 2004). We predict that neutrophils in the granuloma can secrete grzB into the extracellular environment, but we have not observed perforin expression, a molecule required for T cell-mediated cytotoxicity (Trapani and Smyth, 2002), suggesting that neutrophil-expressed grzB may be capable of inducing anoikis in nearby cells. Cathepsin G, a serine protease in the same granule as grzB, has been implicated in neutrophil-mediated cardiac myocyte anoikis (Rafiq et al., 2006; 2008). It is not known whether this happens in the granuloma, but an accumulation of grzB-secreting neutrophils in the region adjacent to caseum conceivably could be sufficient to degrade cell: cell adhesions between epithelioid macrophages, thereby inducing anoikis in these cells.
Dys-regulated protease expression, and secretion into the extracellular environment, can have maladaptive consequences that lead to pathology. Although neutrophil serine proteases may play protective roles in some models of mycobacterial disease (Reece et al., 2010; Jena et al., 2012; Steinwede et al., 2012), neutrophil proteases are also associated with pathology including acute lung injury (Zhou et al., 2012), COPD (Owen, 2008; Hoenderdos and Condliffe, 2013), emphysema (Wright and Churg, 2007) and M. avium infection (Yamazaki et al., 1998). Secreted (extracellular) grzB is associated with conditions that include pathologic tissue remodelling including rheumatoid arthritis (Boivin et al., 2009), aortic aneurysm (Chamberlain et al., 2010) and COPD (Ngan et al., 2009). In addition to directly causing pathology, cleavage of ECM, generation of chemotactic factors and cytokine activation, grzB may lead to an excessive inflammatory response that no longer helps contain M. tuberculosis, but promotes dissemination instead. Moreover, in vitro assays demonstrated that recombinant human grzB is not bactericidal for M. tuberculosis nor does it alter bacterial growth kinetics (Supplemental Fig. S4), suggesting that secreted grzB does not have direct bactericidal or bacteriostatic properties. Taken together, these factors suggest that neutrophil grzB production may not be associated with bacterial killing, but may function to facilitate neutrophil migration through the granuloma’s dense cellular network.
Historically, neutrophils have been considered to be short-lived phagocytes that are critically important for protection against acute infections, but have less involvement in chronic disease. More recent data have suggested these cells are considerably more dynamic than previously thought, and may have surprising and unexpected functions that can be consequential in a wide range of conditions. The relationship between neutrophils and pathology in TB continues to be complex. Identification of grzB as an additional factor produced by neutrophils presents another possible target for therapeutic intervention that may increase the options for treating TB.
Experimental procedures
Animal husbandry and ethics assurance
Cynomolgus macaques (Macaca fascicularis) were housed and maintained by the University of Pittsburgh’s Department of Laboratory Animals. All procedures were performed in accordance with protocols approved by the University of Pittsburgh’s Institutional Animal Care and Use committee. Experiments were performed on samples from M. tuberculosis-infected animals enrolled as untreated controls in unrelated ongoing studies. These animals were not vaccinated, undergoing anti-mycobacterial treatments or experiencing immunomodulatory therapies. For human samples, anonymized lung tissue containing granulomas were collected from patients with treatment refractory TB during therapeutic lung resection surgery at National Masan Hospital. The collection was approved by the hospital’s institutional review board, an exemption from National Institutes of Health and with written consent of the subjects.
IHC
Multiparameter IHC was performed on formalin-fixed paraffin-embedded (FFPE) tissue sections from granuloma-containing lung samples as previously described (Mattila et al., 2013). Purified antibodies for IHC were anti-human grzB (clone Grb7; DAKO), anti-human CD8 (clone 1A5; Leica Microsystems), anti-human perforin (clone Ab-2; ThermoFisher), anti-human CD3 (rabbit polyclonal; DAKO), anti-human cathelicidin (rabbit polyclonal; Abcam), anti-human MMP9 (rabbit polyclonal; Abcam) and anti-human neutrophil elastase (rabbit polyclonal; Abcam). A rabbit polyclonal anti-grzB antibody (Abcam) was used for staining human tissue sections. Anti-human calprotectin (clone MAC387; ThermoFisher) was used to identify neutrophils (Mattila et al., 2013). For detection, antibodies were stained with fluorochrome-labelled secondary antibodies purchased either from Life Technologies or Jackson ImmunoResearch. In applications where two mouse antibodies were needed to stain antigens in the same tissue section (calprotectin and grzB), the least abundant antigen (grzB) was stained using indirect immunofluorescence while and the more abundant antigen (calprotectin) was stained using antibodies labelled with Zenon antibody-labelling reagents (Life Technology). CD8 staining was identified by tyramide amplification (Perkin Elmer) and performed as per the manufacturer’s protocol. After staining, coverslips were mounted over the tissue sections with Prolong Gold Antifade Mounting Medium with DAPI (Life Technologies) and the tissue sections were imaged with an Olympus Fluoview laser scanning confocal microscope. For granulomas that were too large to be imaged in a single 20× field, multiple overlapping images were acquired and assembled into a single montage using Photoshop CS4 (Adobe Systems).
Image analysis
Cells in immunofluorescently stained FFPE tissue sections were counted manually to determine the frequency of grzB-positive neutrophils and T cells using Photoshop CS4. For studies comparing grzB expression and bacterial burden, FFPE granulomas with known bacterial burden were stained. These granulomas, and their bacterial burdens, were obtained as previously indicated (Lin et al., 2009; 2012; 2014). Briefly, granulomas obtained at necropsy were bisected; half was mechanically homogenized and plated on 7H11 agar plates for bacterial enumeration and the other half fixed in formalin and embedded in paraffin. CellProfiler 2.0 (Carpenter et al., 2006) was used to quantify the area per cell cross-section and number of granules per cell in these granulomas. To quantify these metrics, 40× fields were acquired and individual cells with diameters similar to what we expect for an intact cell’s cross section, and not touching other cells, were selected and analysed by CellProfiler. For analysis of granules per cell, 40× fields that contained neutrophils and T cells were selected for analysis while fields that contained caseum or heavily clustered cells were excluded from analysis. Granulomas with autofluorescent artefacts were also excluded from analysis. Granulomas can differ significantly in size and between one and four fields were used included in the final analysis. A CellProfiler pipeline that identified the number of grzB+ granules, T cells, neutrophils per image and then overlaid the grzB on top of the masked cells to quantify the number of grzB+ granules per cell was used for analysis.
Neutrophil stimulations and flow cytometry
Erythrocytes in whole blood were lysed using RBC lysing solution (BD Biosciences), and the nucleated cells resuspended in Roswell Park Memorial Institute (RPMI) medium (Sigma Aldrich) containing 10% human AB serum (Gemini Bio-Products), 1% N-[2-hydroxyethyl]piperazine-N’-[2-ethanesulphonic acid] (Sigma) and 1% L-glutamine (Sigma). A total of 106 cells were transferred to sterile polystyrene tubes and incubated 37°C/5% CO2 in the presence of antigens including mycobacterial ESAT6 and 38.1 (also known as CFP10) (chemically synthesized overlapping peptide pools, 10 μg ml−1 each protein, GenScript USA), mycobacterial CFP (10 μg ml−1; GenScript USA), purified lipopolysaccharide from E. coli (LPS) (20 EU ml−1; Sigma) or a cocktail of PDBu and ionomycin (25 nM and 5 μM final concentrations, respectively; Sigma). Recombinant ESAT6 and 38.1 proteins were not used because they contained small amounts of endotoxin, although preliminary studies indicated there was not a quantitative difference between neutrophil responses to chemically synthesized peptides or E. coli-expressed recombinant proteins (Supplemental Fig. S1). For cells incubated with viable M. tuberculosis, virulent Erdman strain M. tuberculosis were cultured to mid-log growth phase in 7H9 medium and quantified with a spectrophotometer. A volume equivalent to 106 bacteria (multiplicity of infection = 1, based on the OD of cultures with known CFU) was centrifuged and the bacteria were resuspended in RPMI before being added to the cells under BSL3 conditions. After 30 min of incubation with stimulators or bacteria, brefeldin A (BD Biosciences) was added at a 1× concentration to the tubes to inhibit protein secretion and the cells were subsequently cultured for another 3.5 h at 37°C/5% CO2 before being stained for CD11b (clone ICRF44), CD3 (clone SP34-2), CD4 (clone SP34) and CD8 (clone SK1). As flow cytometry for activated caspase 3 (clone C92-605) indicated that culture with brefeldin A and/or M. tuberculosis did not increase the frequency of apoptotic cells (data not shown). Antibodies for flow cytometry were fluorochrome-conjugated antibodies purchased through BD Biosciences. Surface-stained cells were fixed and permeabilized using Fix-Perm/Perm-Wash reagents (BD Biosciences) and stained intracellularly for grzB (clone GB11). After staining, cells were washed, fixed and read on a FACSCalibur (BD Biosciences) or LSRII (BD Biosciences) flow cytometer and data were analysed with FlowJo (Treestar).
PBMC and neutrophil grzB ELISPOT
Percoll (GE Healthcare Biosciences) gradient centrifugation was used to separate plasma-free whole blood into a lymphocyte-rich buffy coat fraction (buffy coat cells) and a neutrophil-enriched erythrocyte pellet. The erythrocyte pellet was subsequently lysed of RBCs, yielding a neutrophil-rich cell population. This procedure yielded cell preparations that were significantly enriched for neutrophils (Supplemental Fig. S2) and was used instead of other multistep procedures to minimize the potential for nonspecific neutrophil activation. For ELISPOT assays, lymphocytes or neutrophils (150 000 cells per well) were added to wells of 0.45 um MultiScreen plates (Millipore, Billerica, MA) and incubated at 37°C/5% CO2 in stimulator- or M. tuberculosis-containing medium. Cells were stimulated with Erdman strain M. tuberculosis, anti-CD3 antibody (a positive control for T cell responses) ESAT6/CFP10, CFP, LPS or PDBu and ionomycin at the previously indicated concentrations. An 18-h incubation period was chosen because of the short life span associated with neutrophils. With the exception of the incubation period, ELISPOT assays were performed according to manufacturer’s instructions and developed with the AEC Development Kit (Vector Laboratories). Spots on plates were read with an ELISPOT reader (CTL) and stimulated cell responses compared against stimulator-free media-only wells.
Statistical analysis
Statistical comparisons were performed with GraphPad Prism 6.0 using the non-parametric Mann–Whitney test for independent data. All pairwise comparisons were tested using the nonparametric Wilcoxon signed-rank test for matched pairs and unadjusted P-values were reported. P-values were not adjusted to reflect the inflated error rate caused by multiple comparisons. Non-parametric Spearman’s correlation was performed to analyse the correlation between T cells, neutrophils, grzB and bacterial load. The threshold for statistical significance was set at P < 0.05.
Supplementary Material
Fig. S1 Mycobacterial peptides and proteins induce similar responses in isolated neutrophils. Isolated neutrophils were stimulated with cocktails of recombinant ESAT6 and 38.1 proteins or synthesized ESAT6 and 38.1 peptides and grzB expression assessed by flow cytometry. PDBu and ionomycin treatment (P + I) was used as a positive control. *P < 0.05, Mann–Whitney test. Bar represents the median.
Fig. S2 Representative isolation efficiency for neutrophils and T cells. Percoll gradients were used to separate whole blood into two fractions: a T–cell-rich buffy coat and a neutrophil-rich RBC pellet. Erythrocytes in the RBC pellet were lysed and the fractions were analysed by flow cytometry in terms frequency of (A) neutrophil-sized or lymphocyte-sized objects, or (B) the % of CD11b+ neutrophils compared with % of CD3+ T cells presented in terms of % of the total population (left panel) or as fold enrichment compared with the corresponding fraction (right panel).
Fig. S3 GrzB colocalizes with elastase in azurophilic neutrophil granules. Tissue sections were imaged at 60× magnification by confocal microscopy after being stained for grzB and proteins expressed in azurophilic granules (elastase), secondary granules (cathelicidin) and gelatinase granules (MMP9).
Fig. S4 GrzB does not kill M. tuberculosis in liquid culture. Replicate cultures were grown in 7H9 media with or without recombinant human grzB for 7 days and bacterial growth was measured by spectrophotometry. GrzB addition did not change bacterial doubling time or the final number of bacteria per culture.
Acknowledgments
The authors gratefully acknowledge technical assistance provided by Mark Rodgers, Carolyn Bigbee, Chelsea Chedrick and the Flynn Lab veterinary staff. We are also grateful to the staff of the National Masan Hospital and the pathologist Dr Seokyong Eum of the International Tuberculosis Research Center for their assistance in tissue collection. Support for this study was provided by NIH grants AI077183 (J.T.M.), HL110811 (J.L.F.), EB012579 (J.L.F.); the Bill and Melinda Gates Foundation (J.L.F.); and the Heiser Program for Research Leprosy (J.T.M.). Support for human tissue collection was provided in part by the Division of Intramural Research, NIAID, NIH and the Korean Ministry of Health and Welfare through the Korean CDC (Clifton E. Barry 3rd and L.E.V.).
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Conflict of interest
The authors declare no competing financial interests.
References
- Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJ, Tanguy M, et al. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106:1843–1850. doi: 10.1182/blood-2005-03-1281. [DOI] [PubMed] [Google Scholar]
- Afonina IS, Cullen SP, Martin SJ. Cytotoxic and non-cytotoxic roles of the CTL/NK protease granzyme B. Immunol Rev. 2010;235:105–116. doi: 10.1111/j.0105-2896.2010.00908.x. [DOI] [PubMed] [Google Scholar]
- Afonina IS, Tynan GA, Logue SE, Cullen SP, Bots M, Luthi AU, et al. Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1alpha. Mol Cell. 2011;44:265–278. doi: 10.1016/j.molcel.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranday-Cortes E, Hogarth PJ, Kaveh DA, Whelan AO, Villarreal-Ramos B, Lalvani A, Vordermeier HM. Transcriptional profiling of disease-induced host responses in bovine tuberculosis and the identification of potential diagnostic biomarkers. PLoS ONE. 2012;7:e30626. doi: 10.1371/journal.pone.0030626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asehnoune K, Strassheim D, Mitra S, Yeol Kim J, Abraham E. Involvement of PKCalpha/beta in TLR4 and TLR2 dependent activation of NF-kappaB. Cell Signal. 2005;17:385–394. doi: 10.1016/j.cellsig.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Ashitani J, Mukae H, Hiratsuka T, Nakazato M, Kumamoto K, Matsukura S. Elevated levels of alpha-defensins in plasma and BAL fluid of patients with active pulmonary tuberculosis. Chest. 2002;121:519–526. doi: 10.1378/chest.121.2.519. [DOI] [PubMed] [Google Scholar]
- Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol. 2002;2:401–409. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsall HH, Porter WJ, Green DM, Rubio J, Trial J, Rossen RD. Impact of fibronectin fragments on the transendothelial migration of HIV-infected leukocytes and the development of subendothelial foci of infectious leukocytes. J Immunol. 2004;173:2746–2754. doi: 10.4049/jimmunol.173.4.2746. [DOI] [PubMed] [Google Scholar]
- Blomgran R, Ernst JD. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J Immunol. 2011;186:7110–7119. doi: 10.4049/jimmunol.1100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host Microbe. 2012;11:81–90. doi: 10.1016/j.chom.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin WA, Cooper DM, Hiebert PR, Granville DJ. Intracellular versus extracellular granzyme B in immunity and disease: challenging the dogma. Lab Invest. 2009;89:1195–1220. doi: 10.1038/labinvest.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmbhatt S, Black GF, Carroll NM, Beyers N, Salker F, Kidd M, et al. Immune markers measured before treatment predict outcome of intensive phase tuberculosis therapy. Clin Exp Immunol. 2006;146:243–252. doi: 10.1111/j.1365-2249.2006.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzza MS, Dyson JM, Choi H, Gardiner EE, Andrews RK, Kaiserman D, et al. Antihemostatic activity of human granzyme B mediated by cleavage of von Willebrand factor. J Biol Chem. 2008;283:22498–22504. doi: 10.1074/jbc.M709080200. [DOI] [PubMed] [Google Scholar]
- Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain CM, Ang LS, Boivin WA, Cooper DM, Williams SJ, Zhao H, et al. Perforin-independent extracellular granzyme B activity contributes to abdominal aortic aneurysm. Am J Pathol. 2010;176:1038–1049. doi: 10.2353/ajpath.2010.090700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy JC, McDonald PC, Suarez AC, Hung VH, Wilson JE, McManus BM, Granville DJ. Granzyme B in atherosclerosis and transplant vascular disease: association with cell death and atherosclerotic disease severity. Mod Pathol. 2003;16:460–470. doi: 10.1097/01.MP.0000067424.12280.BC. [DOI] [PubMed] [Google Scholar]
- Choy JC, Hung VH, Hunter AL, Cheung PK, Motyka B, Goping IS, et al. Granzyme B induces smooth muscle cell apoptosis in the absence of perforin: involvement of extracellular matrix degradation. Arterioscler Thromb Vasc Biol. 2004;24:2245–2250. doi: 10.1161/01.ATV.0000147162.51930.b7. [DOI] [PubMed] [Google Scholar]
- Cooper AM, D’Souza C, Frank AA, Orme IM. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun. 1997;65:1317–1320. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen SP, Brunet M, Martin SJ. Granzymes in cancer and immunity. Cell Death Differ. 2010;17:616–623. doi: 10.1038/cdd.2009.206. [DOI] [PubMed] [Google Scholar]
- Djoba Siawaya JF, Bapela NB, Ronacher K, Veenstra H, Kidd M, Gie R, et al. Immune parameters as markers of tuberculosis extent of disease and early prediction of anti-tuberculosis chemotherapy response. J Infect. 2008;56:340–347. doi: 10.1016/j.jinf.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Doz E, Lombard R, Carreras F, Buzoni-Gatel D, Winter N. Mycobacteria-infected dendritic cells attract neutrophils that produce IL-10 and specifically shut down Th17 CD4 T cells through their IL-10 receptor. J Immunol. 2013;191:3818–3826. doi: 10.4049/jimmunol.1300527. [DOI] [PubMed] [Google Scholar]
- Eum SY, Kong JH, Hong MS, Lee YJ, Kim JH, Hwang SH, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137:122–128. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman WJ, Ley TJ. Granzymes A and B are not expressed in human neutrophils. Blood. 2004;104:906–907. doi: 10.1182/blood-2004-03-0858. [DOI] [PubMed] [Google Scholar]
- Hendel A, Hiebert PR, Boivin WA, Williams SJ, Granville DJ. Granzymes in age-related cardiovascular and pulmonary diseases. Cell Death Differ. 2010;17:596–606. doi: 10.1038/cdd.2010.5. [DOI] [PubMed] [Google Scholar]
- Hernandez-Pigeon H, Jean C, Charruyer A, Haure MJ, Titeux M, Tonasso L, et al. Human keratinocytes acquire cellular cytotoxicity under UV-B irradiation. Implication of granzyme B and perforin. J Biol Chem. 2006;281:13525–13532. doi: 10.1074/jbc.M512694200. [DOI] [PubMed] [Google Scholar]
- Hernandez-Pigeon H, Jean C, Charruyer A, Haure MJ, Baudouin C, Charveron M, et al. UVA induces granzyme B in human keratinocytes through MIF: implication in extracellular matrix remodeling. J Biol Chem. 2007;282:8157–8164. doi: 10.1074/jbc.M607436200. [DOI] [PubMed] [Google Scholar]
- Hiebert PR, Granville DJ. Granzyme B in injury, inflammation, and repair. Trends Mol Med. 2012;18:732–741. doi: 10.1016/j.molmed.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Hoenderdos K, Condliffe A. The neutrophil in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2013;48:531–539. doi: 10.1165/rcmb.2012-0492TR. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Saito S, Sasaki R, Tomatsu T, Toyama Y. Expression of granzyme B in human articular chondrocytes. J Rheumatol. 2003;30:1799–1810. [PubMed] [Google Scholar]
- Jena P, Mohanty S, Mohanty T, Kallert S, Morgelin M, Lindstrom T, et al. Azurophil granule proteins constitute the major mycobactericidal proteins in human neutrophils and enhance the killing of mycobacteria in macrophages. PLoS ONE. 2012;7:e50345. doi: 10.1371/journal.pone.0050345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp TJ, Ludwig AT, Earel JK, Moore JM, Vanoosten RL, Moses B, et al. Neutrophil stimulation with Mycobacterium bovis bacillus Calmette-Guerin (BCG) results in the release of functional soluble TRAIL/Apo-2L. Blood. 2005;106:3474–3482. doi: 10.1182/blood-2005-03-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WJ, Kim H, Suk K, Lee WH. Macrophages express granzyme B in the lesion areas of atherosclerosis and rheumatoid arthritis. Immunol Lett. 2007;111:57–65. doi: 10.1016/j.imlet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Kuhns DB, Priel DA, Gallin JI. Induction of human monocyte interleukin (IL)-8 by fibrinogen through the toll-like receptor pathway. Inflammation. 2007;30:178–188. doi: 10.1007/s10753-007-9035-1. [DOI] [PubMed] [Google Scholar]
- Law K, Weiden M, Harkin T, Tchou-Wong K, Chi C, Rom WN. Increased release of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha by bronchoalveolar cells lavaged from involved sites in pulmonary tuberculosis. Am J Respir Crit Care Med. 1996;153:799–804. doi: 10.1164/ajrccm.153.2.8564135. [DOI] [PubMed] [Google Scholar]
- Lin PL, Rodgers M, Smith L, Bigbee M, Myers A, Bigbee C, et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun. 2009;77:4631–4642. doi: 10.1128/IAI.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PL, Myers A, Smith L, Bigbee C, Bigbee M, Fuhrman C, et al. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 2010;62:340–350. doi: 10.1002/art.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PL, Dartois V, Johnston PJ, Janssen C, Via L, Goodwin MB, et al. Metronidazole prevents reactivation of latent Mycobacterium tuberculosis infection in macaques. Proc Natl Acad Sci USA. 2012;109:14188–14193. doi: 10.1073/pnas.1121497109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PL, Ford CB, Coleman MT, Myers AJ, Gawande R, Ioerger T, et al. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat Med. 2014;20:75–79. doi: 10.1038/nm.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DM, Redford PS, Wilkinson RJ, O’Garra A, Martineau AR. Neutrophils in tuberculosis: friend or foe? Trends Immunol. 2012;33:14–25. doi: 10.1016/j.it.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Martin P, Wallich R, Pardo J, Mullbacher A, Munder M, Modolell M, Simon MM. Quiescent and activated mouse granulocytes do not express granzyme A and B or perforin: similarities or differences with human polymorphonuclear leukocytes? Blood. 2005;106:2871–2878. doi: 10.1182/blood-2005-04-1522. [DOI] [PubMed] [Google Scholar]
- Mattila JT, Ojo OO, Kepka-Lenhart D, Marino S, Kim JH, Eum SY, et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J Immunol. 2013;191:773–784. doi: 10.4049/jimmunol.1300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metkar SS, Froelich CJ. Human neutrophils lack granzyme A, granzyme B, and perforin. Blood. 2004;104:905–906. doi: 10.1182/blood-2004-03-0888. [DOI] [PubMed] [Google Scholar]
- Morel C, Badell E, Abadie V, Robledo M, Setterblad N, Gluckman JC, et al. Mycobacterium bovis BCG-infected neutrophils and dendritic cells cooperate to induce specific T cell responses in humans and mice. Eur J Immunol. 2008;38:437–447. doi: 10.1002/eji.200737905. [DOI] [PubMed] [Google Scholar]
- Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208:2251–2262. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngan DA, Vickerman SV, Granville DJ, Man SF, Sin DD. The possible role of granzyme B in the pathogenesis of chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2009;3:113–129. doi: 10.1177/1753465809341965. [DOI] [PubMed] [Google Scholar]
- Omoto Y, Yamanaka K, Tokime K, Kitano S, Kakeda M, Akeda T, et al. Granzyme B is a novel interleukin-18 converting enzyme. J Dermatol Sci. 2010;59:129–135. doi: 10.1016/j.jdermsci.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Owen CA. Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease. Int J Chronic Obstruct Pulmon Dis. 2008;3:253–268. doi: 10.2147/copd.s2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki T, Nakahira S, Tani K, Ogushi F, Yasuoka S, Ogura T. Differential cell analysis in bronchoalveolar lavage fluid from pulmonary lesions of patients with tuberculosis. Chest. 1992;102:54–59. doi: 10.1378/chest.102.1.54. [DOI] [PubMed] [Google Scholar]
- Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- Pham CT. Neutrophil serine proteases fine-tune the inflammatory response. Int J Biochem Cell Biol. 2008;40:1317–1333. doi: 10.1016/j.biocel.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiq K, Kolpakov MA, Abdelfettah M, Streblow DN, Hassid A, Dell’Italia LJ, Sabri A. Role of protein-tyrosine phosphatase SHP2 in focal adhesion kinase down-regulation during neutrophil cathepsin G-induced cardiomyocytes anoikis. J Biol Chem. 2006;281:19781–19792. doi: 10.1074/jbc.M513040200. [DOI] [PubMed] [Google Scholar]
- Rafiq K, Hanscom M, Valerie K, Steinberg SF, Sabri A. Novel mode for neutrophil protease cathepsin G-mediated signaling: membrane shedding of epidermal growth factor is required for cardiomyocyte anoikis. Circ Res. 2008;102:32–41. doi: 10.1161/CIRCRESAHA.107.150573. [DOI] [PubMed] [Google Scholar]
- Raptis SZ, Pham CT. Neutrophil-derived serine proteases in immune complex-mediated diseases. Immunol Res. 2005;32:211–215. doi: 10.1385/IR:32:1-3:211. [DOI] [PubMed] [Google Scholar]
- Reece ST, Loddenkemper C, Askew DJ, Zedler U, Schommer-Leitner S, Stein M, et al. Serine protease activity contributes to control of Mycobacterium tuberculosis in hypoxic lung granulomas in mice. J Clin Invest. 2010;120:3365–3376. doi: 10.1172/JCI42796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousalova I, Krepela E. Granzyme B-induced apoptosis in cancer cells and its regulation (review) Int J Oncol. 2010;37:1361–1378. doi: 10.3892/ijo_00000788. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Liu CC, Scanga CA, Flynn JL. CD8+ CTL from lungs of Mycobacterium tuberculosis-infected mice express perforin in vivo and lyse infected macrophages. J Immunol. 2000;165:353–363. doi: 10.4049/jimmunol.165.1.353. [DOI] [PubMed] [Google Scholar]
- Silva BD, Trentini MM, da Costa AC, Kipnis A, Junqueira-Kipnis AP. Different phenotypes of CD8+ T cells associated with bacterial load in active tuberculosis. Immunol Lett. 2014;160:23–32. doi: 10.1016/j.imlet.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Sousa AO, Mazzaccaro RJ, Russell RG, Lee FK, Turner OC, Hong S, et al. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc Natl Acad Sci USA. 2000;97:4204–4208. doi: 10.1073/pnas.97.8.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standish AJ, Weiser JN. Human neutrophils kill Streptococcus pneumoniae via serine proteases. J Immunol. 2009;183:2602–2609. doi: 10.4049/jimmunol.0900688. [DOI] [PubMed] [Google Scholar]
- Steinwede K, Maus R, Bohling J, Voedisch S, Braun A, Ochs M, et al. Cathepsin G and neutrophil elastase contribute to lung-protective immunity against mycobacterial infections in mice. J Immunol. 2012;188:4476–4487. doi: 10.4049/jimmunol.1103346. [DOI] [PubMed] [Google Scholar]
- Sugawara I, Udagawa T, Yamada H. Rat neutrophils prevent the development of tuberculosis. Infect Immun. 2004;72:1804–1806. doi: 10.1128/IAI.72.3.1804-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- Vernooy JH, Moller GM, van Suylen RJ, van Spijk MP, Cloots RH, Hoet PH, et al. Increased granzyme A expression in type II pneumocytes of patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:464–472. doi: 10.1164/rccm.200602-169OC. [DOI] [PubMed] [Google Scholar]
- Wagner C, Iking-Konert C, Denefleh B, Stegmaier S, Hug F, Hansch GM. Granzyme B and perforin: constitutive expression in human polymorphonuclear neutrophils. Blood. 2004;103:1099–1104. doi: 10.1182/blood-2003-04-1069. [DOI] [PubMed] [Google Scholar]
- Wagner C, Stegmaier S, Hansch GM. Expression of granzyme B in peripheral blood polymorphonuclear neutrophils (PMN), myeloid cell lines and in PMN derived from haematopoietic stem cells in vitro. Mol Immunol. 2008;45:1761–1766. doi: 10.1016/j.molimm.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhou X, Pan B, Yang L, Yin X, Xu B, Zhao D. Investigation of the effect of Mycobacterium bovis infection on bovine neutrophils functions. Tuberculosis. 2013;93:675–687. doi: 10.1016/j.tube.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, Kettle AJ. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid Redox Signal. 2013;18:642–660. doi: 10.1089/ars.2012.4827. [DOI] [PubMed] [Google Scholar]
- Woodworth JS, Wu Y, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J Immunol. 2008;181:8595–8603. doi: 10.4049/jimmunol.181.12.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JL, Churg A. Current concepts in mechanisms of emphysema. Toxicol Pathol. 2007;35:111–115. doi: 10.1080/01926230601059951. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Kubo K, Sekiguchi M, Honda T. Analysis of BAL fluid in M. avium-intracellulare infection in individuals without predisposing lung disease. Eur Respir J. 1998;11:1227–1231. doi: 10.1183/09031936.98.11061227. [DOI] [PubMed] [Google Scholar]
- Yang CS, Lee JS, Song CH, Hur GM, Lee SJ, Tanaka S, et al. Protein kinase C zeta plays an essential role for Mycobacterium tuberculosis-induced extracellular signal-regulated kinase 1/2 activation in monocytes/macrophages via Toll-like receptor 2. Cell Microbiol. 2007;9:382–396. doi: 10.1111/j.1462-5822.2006.00797.x. [DOI] [PubMed] [Google Scholar]
- Yang CT, Cambier CJ, Davis JM, Hall CJ, Crosier PS, Ramakrishnan L. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe. 2012;12:301–312. doi: 10.1016/j.chom.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Dai Q, Huang X. Neutrophils in acute lung injury. Front Biosci. 2012;17:2278–2283. doi: 10.2741/4051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Mycobacterial peptides and proteins induce similar responses in isolated neutrophils. Isolated neutrophils were stimulated with cocktails of recombinant ESAT6 and 38.1 proteins or synthesized ESAT6 and 38.1 peptides and grzB expression assessed by flow cytometry. PDBu and ionomycin treatment (P + I) was used as a positive control. *P < 0.05, Mann–Whitney test. Bar represents the median.
Fig. S2 Representative isolation efficiency for neutrophils and T cells. Percoll gradients were used to separate whole blood into two fractions: a T–cell-rich buffy coat and a neutrophil-rich RBC pellet. Erythrocytes in the RBC pellet were lysed and the fractions were analysed by flow cytometry in terms frequency of (A) neutrophil-sized or lymphocyte-sized objects, or (B) the % of CD11b+ neutrophils compared with % of CD3+ T cells presented in terms of % of the total population (left panel) or as fold enrichment compared with the corresponding fraction (right panel).
Fig. S3 GrzB colocalizes with elastase in azurophilic neutrophil granules. Tissue sections were imaged at 60× magnification by confocal microscopy after being stained for grzB and proteins expressed in azurophilic granules (elastase), secondary granules (cathelicidin) and gelatinase granules (MMP9).
Fig. S4 GrzB does not kill M. tuberculosis in liquid culture. Replicate cultures were grown in 7H9 media with or without recombinant human grzB for 7 days and bacterial growth was measured by spectrophotometry. GrzB addition did not change bacterial doubling time or the final number of bacteria per culture.


