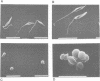
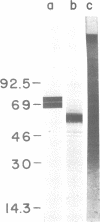
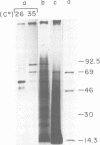
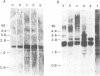
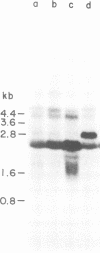
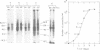Abstract
We have studied the effects of growth phase and temperature on the morphological and molecular processes that occur during stage differentiation of Leishmania. Parasites which differ in their ability to transform axenically were compared. A typical heat shock response is observed in strains that transform axenically. Heat shock proteins (HSPs) of 70 and 83 kd are transcribed and synthesized along with a decrease in cellular protein synthesis, including tubulin. Changes in the transcription of the tubulin gene are also noted. In strains which do not transform in culture HSPs are induced, but cellular protein synthesis is unaffected and no differences are observed in tubulin transcription and expression. HSPs 70 and 83 remain constitutively expressed in amastigotes. HSP 83 transcription increases along the promastigote growth curve. beta-Tubulin transcription is also affected by growth phase and temperature increase, though alpha-tubulin remains unaltered. An amastigote-like hybridization pattern is induced in heat-shocked promastigotes, in which a larger transcript for beta-tubulin (2.8 kb) becomes dominant and the promastigote transcript (2.4 kb) decreases. Tubulin expression is susceptible to temperature control in Leishmania mexicana amazonensis, though direct correlation to HSP expression was not demonstrated.
Full text
PDF
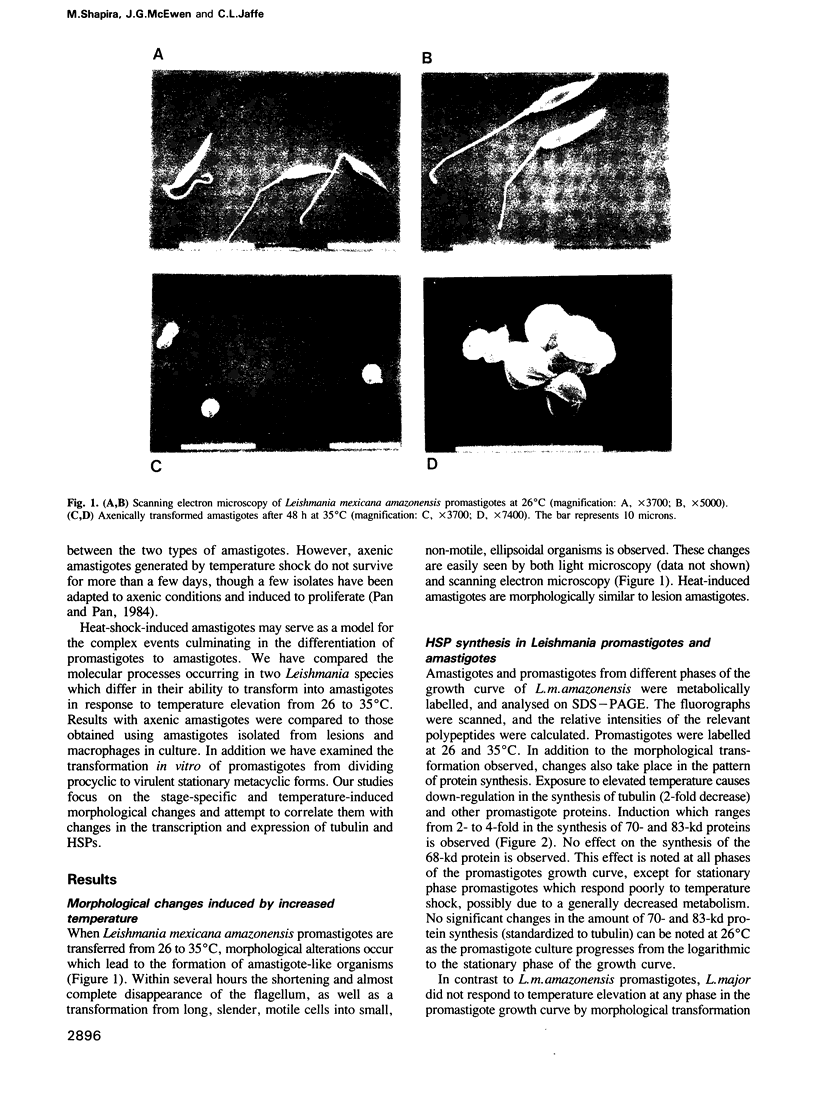
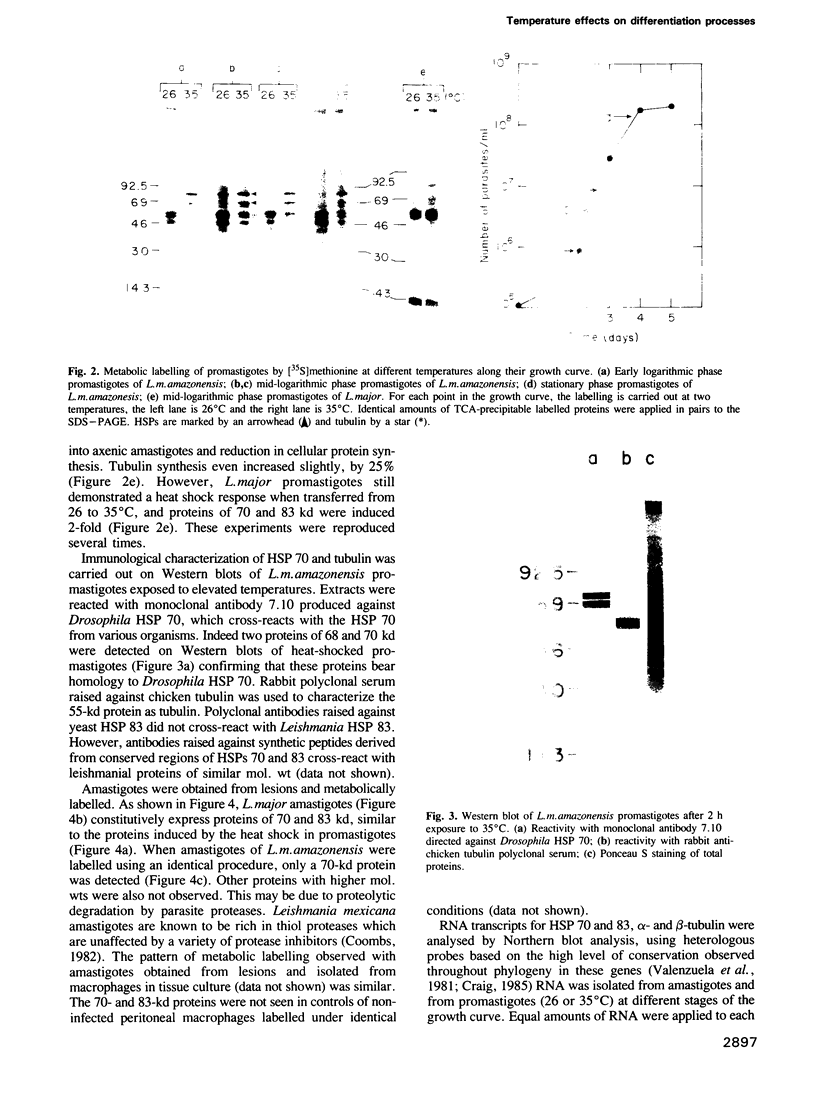
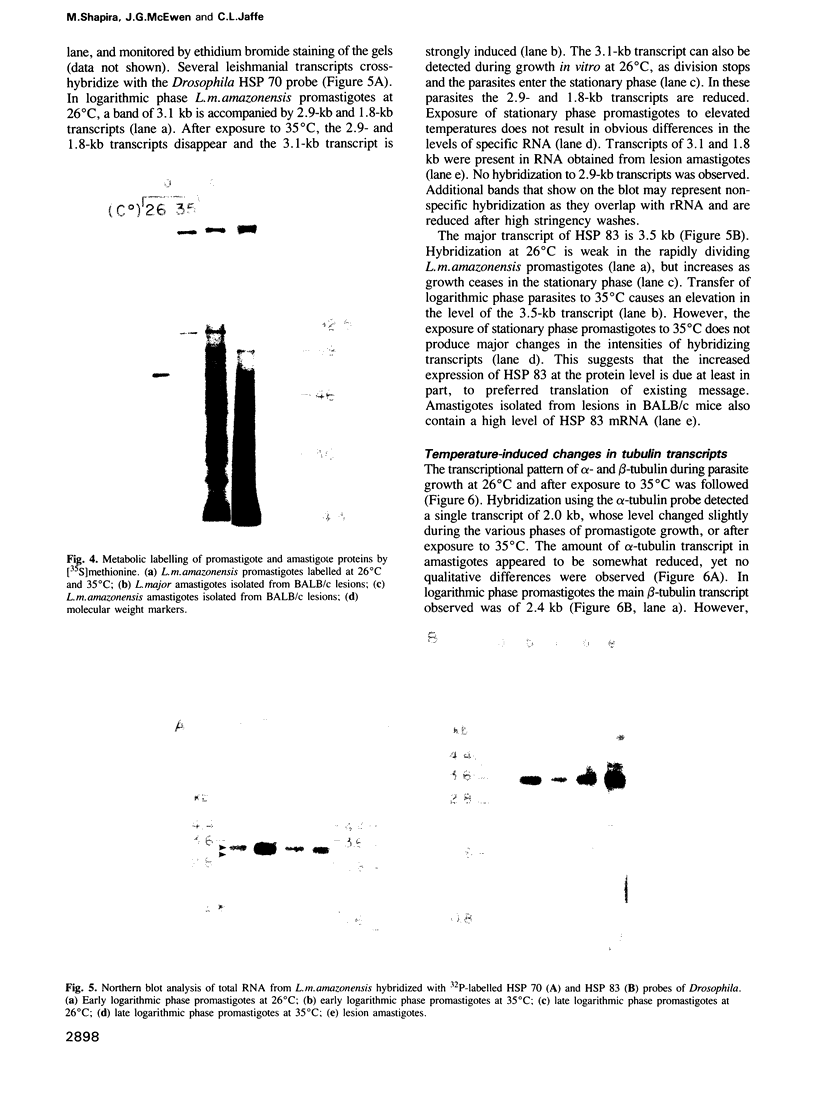
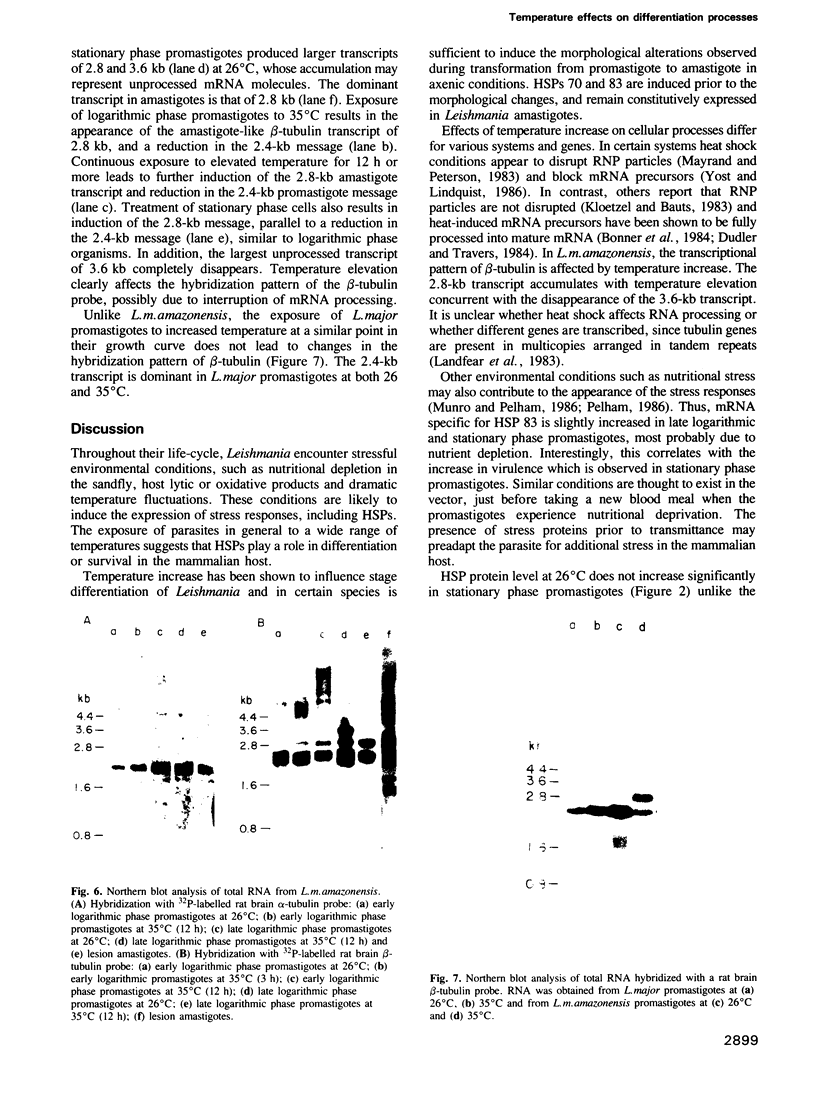


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardeshir F., Flint J. E., Richman S. J., Reese R. T. A 75 kd merozoite surface protein of Plasmodium falciparum which is related to the 70 kd heat-shock proteins. EMBO J. 1987 Feb;6(2):493–499. doi: 10.1002/j.1460-2075.1987.tb04780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. J., Parks C., Parker-Thornburg J., Mortin M. A., Pelham H. R. The use of promoter fusions in Drosophila genetics: isolation of mutations affecting the heat shock response. Cell. 1984 Jul;37(3):979–991. doi: 10.1016/0092-8674(84)90432-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coombs G. H. Proteinases of Leishmania mexicana and other flagellate protozoa. Parasitology. 1982 Feb;84(1):149–155. doi: 10.1017/s003118200005174x. [DOI] [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Darling T. N., Blum J. J. In vitro reversible transformation of Leishmania braziliensis panamensis between promastigote and ellipsoidal forms. J Protozool. 1987 May;34(2):166–168. doi: 10.1111/j.1550-7408.1987.tb03154.x. [DOI] [PubMed] [Google Scholar]
- Dudler R., Travers A. A. Upstream elements necessary for optimal function of the hsp 70 promoter in transformed flies. Cell. 1984 Sep;38(2):391–398. doi: 10.1016/0092-8674(84)90494-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fong D., Chang K. P. Tubulin biosynthesis in the developmental cycle of a parasitic protozoan, Leishmania mexicana: changes during differentiation of motile and nonmotile stages. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7624–7628. doi: 10.1073/pnas.78.12.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong D., Wallach M., Keithly J., Melera P. W., Chang K. P. Differential expression of mRNAs for alpha- and beta-tubulin during differentiation of the parasitic protozoan Leishmania mexicana. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5782–5786. doi: 10.1073/pnas.81.18.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini M. S. Effects of promastigote growth phase, frequency of subculture, and host age on promastigote-initiated infections with Leishmania donovani in the golden hamster. J Protozool. 1974 Oct;21(4):521–527. doi: 10.1111/j.1550-7408.1974.tb03692.x. [DOI] [PubMed] [Google Scholar]
- Ginzburg I., Behar L., Givol D., Littauer U. Z. The nucleotide sequence of rat alpha-tubulin: 3'-end characteristics, and evolutionary conservation. Nucleic Acids Res. 1981 Jun 25;9(12):2691–2697. doi: 10.1093/nar/9.12.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg I., Teichman A., Dodemont H. J., Behar L., Littauer U. Z. Regulation of three beta-tubulin mRNAs during rat brain development. EMBO J. 1985 Dec 30;4(13B):3667–3673. doi: 10.1002/j.1460-2075.1985.tb04133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Jarvis H. M., Mitchell G. F. Leishmania major: identification of stage-specific antigens and antigens shared by promastigotes and amastigotes. Parasite Immunol. 1984 May;6(3):223–233. doi: 10.1111/j.1365-3024.1984.tb00795.x. [DOI] [PubMed] [Google Scholar]
- Hansen B. D., Webster H. K., Hendricks L. D., Pappas M. G. Leishmania mexicana: purine metabolism in promastigotes, axenic amastigotes, and amastigotes derived from Vero cells. Exp Parasitol. 1984 Aug;58(1):101–109. doi: 10.1016/0014-4894(84)90025-0. [DOI] [PubMed] [Google Scholar]
- Hedstrom R., Culpepper J., Harrison R. A., Agabian N., Newport G. A major immunogen in Schistosoma mansoni infections is homologous to the heat-shock protein Hsp70. J Exp Med. 1987 May 1;165(5):1430–1435. doi: 10.1084/jem.165.5.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren R., Livak K., Morimoto R., Freund R., Meselson M. Studies of cloned sequences from four Drosophila heat shock loci. Cell. 1979 Dec;18(4):1359–1370. doi: 10.1016/0092-8674(79)90246-0. [DOI] [PubMed] [Google Scholar]
- Hunter K. W., Cook C. L., Hayunga E. G. Leishmanial differentiation in vitro: induction of heat shock proteins. Biochem Biophys Res Commun. 1984 Dec 14;125(2):755–760. doi: 10.1016/0006-291x(84)90603-x. [DOI] [PubMed] [Google Scholar]
- Hunter K. W., Jr, Cook C. L., Hensen S. A. Temperature-induced in vitro transformation of Leishmania mexicana. I. Ultrastructural comparison of culture-transformed and intracellular amastigotes. Acta Trop. 1982 Jun;39(2):143–150. [PubMed] [Google Scholar]
- L'Hernault S. W., Rosenbaum J. L. Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry. 1985 Jan 15;24(2):473–478. doi: 10.1021/bi00323a034. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landfear S. M., McMahon-Pratt D., Wirth D. F. Tandem arrangement of tubulin genes in the protozoan parasite Leishmania enriettii. Mol Cell Biol. 1983 Jun;3(6):1070–1076. doi: 10.1128/mcb.3.6.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence F., Robert-Gero M. Induction of heat shock and stress proteins in promastigotes of three Leishmania species. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4414–4417. doi: 10.1073/pnas.82.13.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Mayrand S., Pederson T. Heat shock alters nuclear ribonucleoprotein assembly in Drosophila cells. Mol Cell Biol. 1983 Feb;3(2):161–171. doi: 10.1128/mcb.3.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Pan A. A., Honigberg B. M. Leishmania mexicana pifanoi: in vivo and in vitro interactions between amastigotes and macrophages. Z Parasitenkd. 1985;71(1):3–13. doi: 10.1007/BF00932913. [DOI] [PubMed] [Google Scholar]
- Pan A. A. Leishmania mexicana pifanoi: analysis of the antigenic relationships between promastigotes and amastigotes by gel diffusion, immunoelectrophoresis, and immunoprecipitation. J Protozool. 1986 May;33(2):192–197. doi: 10.1111/j.1550-7408.1986.tb05588.x. [DOI] [PubMed] [Google Scholar]
- Pan A. A. Leishmania mexicana: serial cultivation of intracellular stages in a cell-free medium. Exp Parasitol. 1984 Aug;58(1):72–80. doi: 10.1016/0014-4894(84)90022-5. [DOI] [PubMed] [Google Scholar]
- Pan A. A., Pan S. C. Leishmania mexicana: comparative fine structure of amastigotes and promastigotes in vitro and in vivo. Exp Parasitol. 1986 Oct;62(2):254–265. doi: 10.1016/0014-4894(86)90030-5. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Wheeler D. A., Harrison L. H., Kay H. D. The immunobiology of leishmaniasis. Rev Infect Dis. 1983 Sep-Oct;5(5):907–927. doi: 10.1093/clinids/5.5.907. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Pratt D. M., David J. R. Monoclonal antibodies recognizing determinants specific for the promastigote state of Leishmania mexicana. Mol Biochem Parasitol. 1982 Nov;6(5):317–327. doi: 10.1016/0166-6851(82)90064-0. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Perkins P. V. Identification of an infective stage of Leishmania promastigotes. Science. 1984 Mar 30;223(4643):1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- Sadick M. D., Raff H. V. Differences in expression and exposure of promastigote and amastigote membrane molecules in Leishmania tropica. Infect Immun. 1985 Feb;47(2):395–400. doi: 10.1128/iai.47.2.395-400.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Theodorakis N. G., Morimoto R. I. Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol. 1987 Dec;7(12):4357–4368. doi: 10.1128/mcb.7.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986 Apr 25;45(2):185–193. doi: 10.1016/0092-8674(86)90382-x. [DOI] [PubMed] [Google Scholar]
- Yuckenberg P. D., Poupin F., Mansour T. E. Schistosoma mansoni: protein composition and synthesis during early development; evidence for early synthesis of heat shock proteins. Exp Parasitol. 1987 Jun;63(3):301–311. doi: 10.1016/0014-4894(87)90177-9. [DOI] [PubMed] [Google Scholar]