Abstract
Kinetoplast maxicircle DNA of trypanosomatids encodes eighteen proteins. RNA editing is required to confer translatability to mRNA for twelve of these. Sequence conservation of the predicted hydrophobic polypeptides indicates that they represent functional components of the respiratory chain. Yet, so far only two of those, cytochrome c oxidase subunit I and apocytochrome b of cytochrome c reductase, have been identified with biochemical methods. Here we report on identification of A6 subunit of F1FO ATPase encoded by a pan-edited mRNA in Trypanosoma brucei. The polypeptide was present among the 35S-labeled mitochondrial translation products characterized by anomalous migration in denaturing 2D gels. It was identified as an ATPase subunit by co-migration with this complex in Blue Native 2D gels. A partial N-terminal sequence of the corresponding polypeptide present in the gel-purified ATPase complex from Leishmania tarentolae was consistent with the predicted A6 sequence.
Keywords: mitochondrial translation, RNA editing, subunit 6, F1FO ATPase, Trypanosoma brucei
The trypanosomatid-type RNA editing is a complex and costly process which serves to produce translatable mRNA out of pre-edited genome-encoded sequences, with the end result of making a set of subunits for mitochondrial respiratory complexes and one ribosomal protein [1]. The extent of the required editing varies greatly among the genes for different subunits of the same complex, or for the same gene in different species, and it does so without an apparent rationale [2]. However, the protein sequences are remarkably conserved indicating that the final products of this cumbersome process are functional. Numerous studies have indicated that mitochondrial gene expression is indispensable and subject to regulation during the life cycle. In Trypanosoma brucei, the agent of Human African Trypanosomiasis, also known as 'sleeping sickness', the mitochondrion is fully up-regulated in procyclic cells, the form found in the tsetse fly vector, and down-regulated in bloodstream (BS) 'long slender' trypanosomes which proliferate in a mammal [3]. The upregulation involves the mitochondrial synthesis of subunits I – III (COI-COIII) for cytochrome c oxidase and apocytochrome b (Cyb) of cytochrome c reductase (cytochrome bc1 complex), while the downregulation leads to cessation of their production. According to the current knowledge of the mitochondrial metabolism in BS trypanosomes, some other proteins have to be expressed constitutively: these include several subunits of NADH dehydrogenase, ribosomal protein S12, and, most notably, subunit 6 (A6, formerly MURF4) of F1FO ATPase [4,5]. This enzymatic complex is active throughout the life cycle but plays the opposite roles in procyclic and BS cells [6,7]. Subunit 6 (also known as subunit a in other organisms) is an integral membrane protein forming part of the transmembrane proton channel and interfacing the multimeric subunit 9 (subunit c) [8]. In T. brucei the predicted A6 protein (231 amino acids, 28.7 kDa) is translated from the pan-edited mRNA [9] and contains six transmembrane α-helices (as determined by TMpred, Supplementary Fig. 1). Although this protein is crucial for the function of the F1FO ATPase, the respective polypeptide was not detected during the previous analyses of this enzyme [10].
A similar problem had been encountered at the analysis of cytochrome c oxidase and cytochrome c reductase complexes which contain three and one mitochondrially encoded subunits, respectively [11,12]. It was resolved by employing an extreme hydrophobicity of these proteins, the very property which makes them refractory to the standard approach, to separate the mitochondrial subunits from the nuclear-encoded, less hydrophobic subunits [13,14]. The anomalous electrophoretic migration of hydrophobic polypeptides in SDS gels results in their positioning off the main diagonal in two-dimensional (2D) gels. Such separation is crucial because hydrophobic polypeptides show tendency to precipitate, aggregate and appear in gels as diffuse poorly stained bands or spots, which would, therefore, be effectively masked by 'normal' polypeptides. The identity of the major off-diagonal spots observed in the 2D gel analysis of the purified cytochrome c oxidase and cytochrome c reductase complexes in Leishmania tarentolae (COI and Cyb, respectively) was determined by N-terminal sequencing (Edman degradation following removal of the N-terminal formyl group) [13,14]. The COII polypeptide was later found among the minor components of cytochrome c oxidase, apparently this polypeptide, as well as COIII, is more prone to aggregation compared to COI. By combining the 2D gel approach with metabolic labeling of cells with 35S-amino acids in the presence of cycloheximide it was possible to detect some of the de novo synthesized mitochondrial proteins [15]. The two most abundant labeled spots represented COI and Cyb polypeptides.
A similar approach has resulted in detection of several hydrophobic polypeptides in T. brucei [16]. The two most abundant labeled components were identified as COI and Cyb by comigration with the off-diagonal components observed in the purified cytochrome c oxidase and reductase complexes, respectively (I. Š. and D. A. M., unpublished observations). This identification proved to be invaluable to investigate the interface of RNA editing/maturation and translation in T. brucei [16–18]. However, the dearth of identifiable mitochondrial translation products represents an impediment to furthering such studies. As a partial remedy, in this work we have identified an additional mitochondrially encoded product - subunit A6 of F1FO ATPase, a constitutively expressed protein defined by a pan-edited mRNA.
In order to detect the products of mitochondrial translation in procyclic T. brucei, strain 29-13, 107 cells were labeled with [35S]-amino acid mix in the presence of 100 ug/ml cycloheximide to inhibit the cytosolic translation for 1 h and then chased with the excess of cold methionine and cysteine for 2 h [15,16]. Proteins in the total cell lysate were separated using a 2D (9% vs 14%) polyacrylamide Tris-glycine-SDS gel. The labeled products were detected by fluorography (Fig. 1A). In addition to COI and Cyb, several additional labeled polypeptides were also present. With the purpose of identification of these products, we then studied the incorporation of the labeled products into mitochondrial respiratory complexes. Crude mitochondrial fraction was obtained from 0.5 × 109 cells labeled with 35S as described above. Mitochondrial membranes were lysed with 1% dodecyl maltoside and the complexes were separated in a 3–13% Blue Native / 10% Tris-tricine-SDS 2D gel [19] (Fig. 1B). Renografin-purified cold mitochondria were analyzed in parallel (Fig. 1C). The respiratory complexes in gels were identified by their relative migration in the native dimension, a characteristic banding pattern in the denaturing dimension and by probing with antibodies against the F1FO ATPase subunit p18, cytochrome c reductase Rieske protein and cytochrome c oxidase trCOIV subunit as described previously [20]. It should be mentioned that the gradient 3–13% BN gel shown in Fig. 1B and 1C is a trade-off chosen for its optimal resolution of the oligomeric ATPase, as opposed to F1 moiety of the enzyme and the cytochrome c oxidase and reductase complexes which are better resolved in a 6% uniform BN gel (see below). Under these conditions, the bulk of the radioactivity associated with the COI spot is found in the BN gel region encompassing cytochrome c oxidase indicating that most of the newly synthesized COI protein gets assembled into this respiratory complex (Fig. 1B). A fraction of the radioactive COI material is also seen at the origin of the BN dimension testifying to the presence of the incompletely solubilized or aggregated material. Additional labeled material present in the cytochrome c oxidase may represent oligomerized COI (migrating slower than monomeric COI in Tris-tricine dimension), as well as COII and/or COIII subunits (migrating faster than monomeric COI). The radioactivity found in the Cyb spot, which represents the most intensively labeled spot in Fig. 1A, can be only partially chased into the assembled cytochrome c reductase. A broad streak formed by the Cyb labeled material in the BN dimension suggests that this polypeptide, probably the one synthesized in excess, is found in partially assembled and/or chaperon complexes; however, this has not been investigated further.
Figure 1.
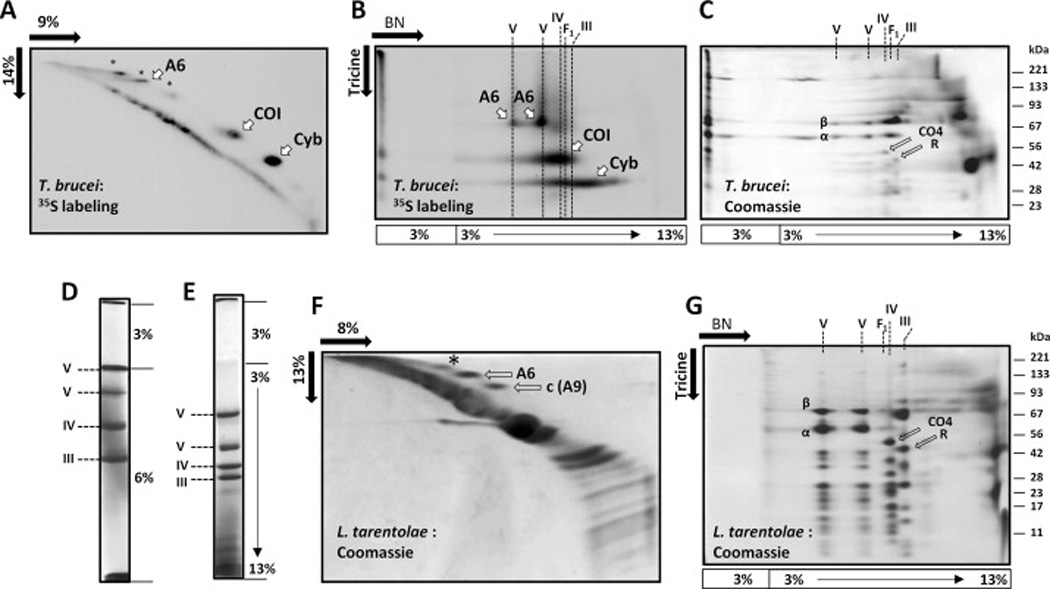
Identification of subunit A6 by incorporation of a mitochondrial translation product into F1FO ATPase in T. brucei (A – C) and finding this subunit in the purified ATPase complex in L. tarentolae (D –G). (A) Separation of the mitochondrial translation products in 2D (9% vs 14% polyacrylamide) Tris-glycine-SDS gel in T. brucei. Cells were labeled with [35S] EasyTag Express Labeling Mix (PerkinElmer Life Sciences) in the presence of 100 ug/ml cycloheximide for 1 h and chased with cold cysteine and methionine for 2 h. Labeled products were revealed by fluorography. COI - cytochrome c oxidase subunit I, Cyb - apocytochome b, A6 - ATPase subunit 6. The nature of the labeled material on the main diagonal remains uncertain; it likely represents the products of aberrant mitochondrial translation. (B) Incorporation of the labeled mitochondrial products into respiratory complexes. Crude mitochondrial fraction was isolated from the cells labeled as described in A. Mitochondrial respiratory complexes were solubilized with 1% dodecyl maltoside and separated in 3–13% Blue Native gel (BN dimension). The resolved protein complexes were denatured with 1% SDS in situ and separated in 10% Tris-tricine polyacrylamide gel (Tricine dimension). Labeled products were revealed by fluorography. Respiratory complexes are designated as follows: III - cytochrome c reductase (cytochrome bc1, Complex III), IV - cytochrome c oxidase (Complex IV), V - F1FO ATPase (Complex V). F1 - F1 moiety of F1FO ATPase. The lower panel shows position of the stacking and resolving gels of BN dimension. (C) Separation of mitochondrial respiratory complexes of T. brucei in a 2D Blue Native / Tris-tricine-SDS gel. Mitochondria were purified using standard renografin flotation method. Respiratory complexes were extracted with 1% dodecyl maltoside (using the equivalent of 400 µg mitochondrial protein per assay) and separated in a 2D BN gel in parallel with the experiment shown in B. The gel was stained with Coomassie. Positions of characteristic subunits used to identify the respiratory complexes are shown as follows: α and β - subunits of the catalytic F1 moiety of F1FO ATPase; CO4 - subunit trCOIV of cytochrome c oxidase; R - Rieske subunit of cytochrome c reductase. Other designations are as in B. (D) Respiratory complexes from mitochondria of L. tarentolae. Mitochondria were purified using a standard renografin flotation procedure. Mitochondria equivalent to 200 µg of mitochondrial protein were lysed with 1% dodecyl maltoside and the lysate was fractionated in 6% Blue Native gel. The gel was additionally stained with Coomassie for a better visualization of the bands after electrophoresis (the respiratory complexes are designated to the left). (E) As in D, except that the lysate was fractionated in 3–13% gradient Blue Native gel. (F) Identification of A6 and A9 subunits of F1FO ATPase of L. tarentolae. The ATPase complex was electroeluted (Model 422 Electro-Eluter, Bio-Rad) from several preparative gels (Supplementary Fig. 1). The combined material was resolved in a 2D (8% vs 13% polyacrylamide) Tris-glycine-SDS gel. The gel was blotted onto a PVDF membrane [13, 14], which was then stained with Coomassie. The off-diagonal spots of interest were excised from the membrane, the N-terminal formyl group was removed by incubating the membrane in 0.5 N HCl / methanol at room temperature for 90 min. Partial N-terminal amino acid sequences were determined by Edman degradation. (G) Separation of mitochondrial respiratory complexes of L. tarentolae in a 2D Blue Native / Tris-tricine-SDS gel. All designations are as in C. The size markers (BenchMark™ Pre-stained Protein Ladder, Invitrogen) are shown to the right.
The F1FO ATPase complex is easily detectable by the characteristic presence of the two abundant subunits from its F1 moiety: α and β (Fig. 1C). The fastest migrating form of the complex, found in the vicinity of the cytochrome c oxidase and reductase complexes represents F1, and two additional forms seen in gel represent oligomeric forms of the holoenzyme [21]. It is remarkable that the radioactivity represented by the putative A6 labeled spot in Fig. 1A is nearly completely chased into the F1FO ATPase complex (Fig. 1B). Due to its absence in F1 it is likely part of FO with A6 being the most likely candidate.
In order to verify this tentative conclusion, the polypeptide with properties of the predicted A6 proteins was searched using the isolated F1FO ATPase complex. N-terminal sequencing by Edman degradation was chosen for sequence analysis instead of masss-pectrometry because mitochondrially encoded polypeptides were conspicuously absent in the recent analyses on mitochondrial proteome in trypanosomatids [22]. We used L. tarentolae instead of T. brucei because it was easier to obtain a large amount of the material necessary for analysis of this polypeptide which was expected to be inefficiently blotted and poorly retained on the sequencing membrane, as was the case with other studied mitochondrial polypeptides. Additional advantages of using L. tarentolae is that mitochondria are usually isolated in high purity and solubilization of membranes with dodecyl maltoside is highly effective. Examples of Blue Native gel separation of L. tarentolae respiratory complexes are shown in Fig 1D and 1F, and that of a BN/Tricine-SDS 2D gel in Fig. 1G. The high level of similarity in the composition of respiratory complexes in these two species is obvious (see also Fig. 5 of reference [20]). Preparative BN gels, each loaded with 3.2 mg of mitochondrial protein were employed to purify the ATPase complex from renografin-purified mitochondria of L. tarentolae. The electro-eluted material from several such gels was pooled and analyzed in denaturing 2D gels as shown in Fig. 1F. Stainable off-diagonal spots were present in the area approximately matching position of the labeled A6 spot of T. brucei (Fig. 1A). After blotting on a PVDF membrane and the N-terminal deblocking, a partial N-terminal sequence of two of those polypeptides was determined. The sequence of one of them (xTVAISxQGL), where x denoted unidentified residues, matched the predicted sequence of ATPase subunit 9 (subunit c) from the related organism L. major, strain Friedlin, as determined by Blastp (http://www.genedb.org/blast/submitblast/GeneDB_Lmajor). This subunit represents a hydrophobic component of the FO moiety [23] and is, therefore, also prone to anomalous gel migration. If the cleavage site of the A9 (c) polypeptide is conserved among species, this result indicates that the N-terminal signal sequence of 28 amino acids is cleaved off in L. major, and that of 39–40 amino acids is removed in T. brucei (Supplementary Fig. 3).
The partial N-terminal sequence of another spot was xFV(a/f)IvxDLV(h/i)M, where x denoted unidentifiable residues and low case letters denote residues identified with lower confidence. Remarkably, this sequence was consistent with the predicted sequence of the mitochondrially encoded A6 polypeptide: MFVFFVCDLVIM (considering that cysteine residues are not identifiable by the utilized sequencing protocol). We concluded from this analysis that A6 polypeptide was indeed present in the specific area of the electrophoretic gel supporting the identification based on the gel migration a labeled translation product described above.
Identification of mitochondrially encoded proteins represents an important pre-requisite to investigation of mechanisms coordinating mRNA processing and translation in trypanosomatids, as well as, at least in T. brucei, the developmental aspects of this interaction. Until now, our ability to directly investigate the impact of genetic manipulations, such as RNA interference, on translation was limited to just two proteins, COI and Cyb. These proteins are encoded by the mRNAs with no (COI) or little (Cyb) editing; they show the same developmental pattern of expression and, therefore, may be controlled by similar mechanisms. The identification of A6, a protein which unlike the other two is constitutively expressed throughout the life cycle and is encoded by a pan-edited mRNA, may facilitate uncovering additional aspects of the interface between editing and translation.
Supplementary Material
Highlights.
A6, a mitochondrial subunit of F1FO ATPase encoded by pan-edited mRNA in Trypanosoma brucei, for long time had eluded direct identification due to its extreme hydrophobicity
This subunit is detected by chasing a mitochondrial translation product into the ATPase complex
A partial N-terminal amino acid sequencing of the matching protein in Leishmania tarentolae has confirmed the presence of A6 in purified F1FO ATPase
Acknowledgements
This work was supported by the EMBO fellowship ASTF 7-4014 to I.Š., the Slovak Research and Development Agency grant APVV-0286-12, the Scientific Grant Agency grant 1/0664/13 to A.H. and the NIH grant AI088292 to D.A.M. We thank Z. Voburka for conducting the N-terminal sequencing and A. Ziková and B. Panicucci for discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aphasizhev R, Aphasizheva I. Mitochondrial RNA editing in trypanosomes: small RNAs in control. Biochimie. 2014;100:125–131. doi: 10.1016/j.biochi.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson L, Thiemann OH, Savill NJ, Alfonzo JD, Maslov DA. Evolution of RNA editing in trypanosome mitochondria. Proc Natl Acad Sci USA. 2000;97:6986–6993. doi: 10.1073/pnas.97.13.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenn K, Matthews KR. The cell biology of Trypanosoma brucei differentiation. Curr Opin Microbiol. 2007;10:539–546. doi: 10.1016/j.mib.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opperdoes FR, Michels PA. Complex I of Trypanosomatidae: does it exist? Trends Parasitol. 2008;24:310–317. doi: 10.1016/j.pt.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Bringaud F, Riviere L, Coustou V. Energy metabolism of trypanosomatids: adaptation to available carbon sources. Mol Biochem Parasitol. 2006;149:1–9. doi: 10.1016/j.molbiopara.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Brown SV, Chi TB, Williams N. The Trypanosoma brucei mitochondrial ATP synthase is developmentally regulated at the level of transcript stability. Mol Biochem Parasitol. 2001;115:177–187. doi: 10.1016/s0166-6851(01)00282-1. [DOI] [PubMed] [Google Scholar]
- 7.Schnaufer A, Clark-Walker GD, Steinberg AG, Stuart K. The F1-ATP synthase complex in bloodstream stage trypanosomes has an unusual and essential function. EMBO J. 2005;24:4029–4040. doi: 10.1038/sj.emboj.7600862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Ballmoos C, Cook GM, Dimroth P. Unique rotary ATP synthase and its biological diversity. Annu Rev Biophys. 2008;37:43–64. doi: 10.1146/annurev.biophys.37.032807.130018. [DOI] [PubMed] [Google Scholar]
- 9.Bhat GJ, Koslowsky DJ, Feagin JE, Smiley BL, Stuart K. An extensively edited mitochondrial transcript in kinetoplastids encodes a protein homologous to ATPase subunit 6. Cell. 1990;61:885–894. doi: 10.1016/0092-8674(90)90199-o. [DOI] [PubMed] [Google Scholar]
- 10.Ziková A, Schnaufer A, Dalley RA, Panigrahi AK, Stuart KD. The F0F1-ATP synthase complex contains novel subunits and is essential for procyclic Trypanosoma brucei. PLoS Pathog. 2009;5:e1000436. doi: 10.1371/journal.ppat.1000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speijer D, Breek CKD, Muijsers AO, Hartog AF, Berden JA, Albracht SPJ, et al. Characterization of the respiratory chain from cultured Crithidia fasciculata. Mol Biochem Parasitol. 1997;85:171–186. doi: 10.1016/s0166-6851(96)02823-x. [DOI] [PubMed] [Google Scholar]
- 12.Horváth A, Berry EA, Huang LS, Maslov DA. Leishmania tarentolae: A parallel isolation of cytochrome bc1 and cytochrome c oxidase. Exp Parasitol. 2000;96:160–167. doi: 10.1006/expr.2000.4564. [DOI] [PubMed] [Google Scholar]
- 13.Horváth A, Kingan TG, Maslov DA. Detection of the mitochondrially encoded cytochrome c oxidase subunit I in the trypanosomatid protozoan Leishmania tarentolae. J Biol Chem. 2000;275:17160–17165. doi: 10.1074/jbc.M907246199. [DOI] [PubMed] [Google Scholar]
- 14.Horváth A, Berry EA, Maslov DA. Translation of the edited mRNA for cytochrome b in trypanosome mitochondria. Science. 2000;287:1639–1640. doi: 10.1126/science.287.5458.1639. [DOI] [PubMed] [Google Scholar]
- 15.Horváth A, Neboháčová M, Lukeš J, Maslov DA. Unusual polypeptide synthesis in the kinetoplast-mitochondria from Leishmania tarentolae. Identification of individual de novo translation products. J Biol Chem. 2002;277:7222–7230. doi: 10.1074/jbc.M109715200. [DOI] [PubMed] [Google Scholar]
- 16.Aphasizheva I, Maslov DA, Wang X, Huang L, Aphasizhev R. Pentatricopeptide repeat proteins stimulate mRNA adenylation/uridylation to activate mitochondrial translation in trypanosomes. Mol Cell. 2011;42:106–117. doi: 10.1016/j.molcel.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aphasizheva I, Maslov DA, Aphasizhev R. Kinetoplast DNA-encoded ribosomal protein S12: a possible functional link between mitochondrial RNA editing and translation in Trypanosoma brucei. RNA Biol. 2013;10:1679–1688. doi: 10.4161/rna.26733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridlon L, Škodová I, Pan S, Lukeš J, Maslov DA. The importance of the 45 S ribosomal small subunit-related complex for mitochondrial translation in Trypanosoma brucei. J Biol Chem. 2013;288:32963–32978. doi: 10.1074/jbc.M113.501874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schagger H, Cramer WA, von Jagow G. Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal Biochem. 1994;217:220–230. doi: 10.1006/abio.1994.1112. [DOI] [PubMed] [Google Scholar]
- 20.Maslov DA, Ziková A, Kyselová I, Lukeš J. A putative novel nuclear-encoded subunit of the cytochrome c oxidase complex in trypanosomatids. Mol Biochem Parasitol. 2002;125:113–125. doi: 10.1016/s0166-6851(02)00235-9. [DOI] [PubMed] [Google Scholar]
- 21.Hashimi H, Benkovičová V, Čermáková P, Lai DH, Horváth A, Lukeš J. The assembly of F1FO-ATP synthase is disrupted upon interference of RNA editing in Trypanosoma brucei. Int J Parasitol. 2010;40:45–54. doi: 10.1016/j.ijpara.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Panigrahi AK, Ogata Y, Ziková A, Anupama A, Dalley RA, Acestor N, et al. A comprehensive analysis of Trypanosoma brucei mitochondrial proteome. Proteomics. 2009;9:434–450. doi: 10.1002/pmic.200800477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chi TB, Brown SV, Williams N. Subunit 9 of the mitochondrial ATP synthase of Trypanosoma brucei is nuclearly encoded and developmentally regulated. Mol Biochem Parasitol. 1998;92:29–38. doi: 10.1016/s0166-6851(97)00222-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


