Abstract
Increased concentrations of secreted phospholipase A2 type IIA (sPLA2-IIA), have been found in the synovial fluid of patients with rheumatoid arthritis. It has been shown that sPLA2-IIA specifically binds to integrin αvβ3, and initiates a signaling pathway that leads to cell proliferation and inflammation. Therefore, the interaction between integrin and sPLA2-IIA could be a potential therapeutic target for the treatment of proliferation or inflammation-related diseases. Two one-bead-one-compound peptide libraries were co nstructed and screened, and seven target hits were identified. Herein we report the identification, synthesis, and biological testing of two pyrazolylthiazole-tethered peptide hits and their analogs. Biological assays showed that these compounds were able to suppress the sPLA2-IIA-integrin interaction and sPLA2-IIA-induced migration of monocytic cells and that the blockade of the sPLA2-IIA-integrin binding was specific to sPLA2-IIA and not to the integrin.
Keywords: Inflammation, Inhibition of protein-protein interaction, sPLA2-IIA, Integrin
Graphical Abstract

Secreted phospholipase A2 group IIA (sPLA2-IIA) is an enzyme that hydrolyzes the sn-2 ester in the glyceroacyl phospholipids present in lipoproteins and cell membranes, resulting in release of arachidonic acid and lysophospho-lipids.1 These products are subjected to further metabolism to form potent mediators which induce disease-related cellular processes, including inflammation, apoptosis, and atherogenesis.2 As a result, sPLA2-IIA has been considered an important player in the inflammation-related diseases as well as a therapeutic target.3 However, catalytically inactive sPLA2-IIA mutants are still able to enhance inflammatory processes via signal transduction involving kinases such as the extracellular responsive kinase 1 and 2 (ERK1/2), p38, and NF-κB.4 Furthermore, potent inhibitors of the catalytic activity of sPLA2-IIA fail to demonstrate significant therapeutic effect for the treatment of inflammatory diseases such as rheumatoid arthritis5 and asthma.6 Thus, the paradox of the well known pro-inflammatory mechanism of the enzymatic function of sPLA2-IIA with the poor efficacy of its inhibitors in treating inflammatory diseases has prompted investigations into its other pathogenic roles.7-10
One plausible explanation for the paradox is that sPLA2-IIA may function as an “inflammation-mediating” ligand by interacting with certain cell receptors through its non-catalytic site, causing retained pro-inflammatory signaling in cells. Indeed, there have been reports of interaction of sPLA2-IIA with cell receptors or cell surface substances to support the above hypothesis: for example, sPLA2-IIA binds to rodent M-type receptor7 (human sPLA2-IIA does not bind to human M-type receptor)8 and to heparan sulfate proteoglygans like glypican-19 and decorin in apoptotic human T-cells.10 In addition, we recently discovered that sPLA2-IIA could interact with integrin αvβ3 and α4β1 and induce the pro-inflammatory activation of ERK1/2, as well as cell proliferation.11 Therefore, based on our recent discovery of the signaling function of sPLA2-IIA, we postulate that interactions between sPLA2-IIA and integrins may serve as novel therapeutic targets for inflammation intervention and the treatment of related diseases, and that identification of the inhibitors of the interaction would be a practical stride towards employment of the new target. Hence, we initiated an inhibitor discovery effort in which we implemented peptide library synthesis, hit-identification via on-bead screening, and re-synthesis and validation of active compounds in biological assays. As a result, we identified small compounds that are able to inhibit the sPLA2-IIA-integrin αvβ3 interaction. These confirmed compounds and their inhibition activity could lead to a new strategy to treat diseases that stem from detrimental inflammation processes.
The One-Bead-One-Compound (OBOC) library is a powerful tool for identification of biologically active compounds or hit generation in drug discovery.12,13a,14 The molecular diversity of the library can be easily increased simply by repetitive employment of the split-and-mix procedure and peptidyl chain elongation, and the resulting large molecular diversity would increase the possibility of active compounds present in the library. The identity of a compound on a bead can be conveniently established by Edman degradation13b or mass spectrometry protocol, depending upon the coding strategy utilized for the OBOC library. To further increase opportunities in binding to the target molecules as well as drug-likability of hit compounds, we capped the peptide chain with heteroatom-rich heterocyclic acids 1-10 (see Figure 1 for structures of the capping agents). Two of them, 1 and 8 were synthesized in our laboratory.15 The rest of these acids were obtained from commercial sources.
Figure 1.
Capping agents employed in the target library.
Two sets of OBOC libraries were prepared on 90 μm-diameter TentaGel beads according to a literature method (their general formula is shown in Figure 2).13a Compounds in Set I consist of a tetrapeptide chain and compounds in Set II employ a hexapeptide one. Both libraries were synthesized on two-layered Tentagel beads with either L or D amino acids (see supporting information for the isomer specification). The outer and inner layers on each bead contain almost the same peptide constitution except for the N-terminal residues: the terminal amino acid in the inner layer encodes for the heterocyclic capping moiety of the peptide chain on the outer layer. Considering that nineteen amino acids (D or L, cysteine not included) and ten different heterocyclic carboxylic acids were used to construct the libraries, the molecular diversity of the tetrapeptide library can reach ~1.3 million (10×194). For the hexapeptide library, the molecular diversity can reach ~about 40 million due to the limited number of beads in five grams of the Tentagel resin used for the library construction.
Figure 2.
General formulas (GF) of the two sets of libraries used for on-bead screening (X = amino acid residue, D-isomers of R, N, L, F and T were used. Cap = heterocyclic carboxylic acid, Xcode = amino acid coded for a specific capping heterocycle).
The synthesis of each library is executed in three phases as shown in Scheme 1. In phase I, the peptide chain is elongated from the free amine on bead to the desired length by repetition of split-coupling-mix operational cycles. In phase II, topological segregationof the two layers was achieved to give 14 by swelling of 13 in water followed by partial protection of the outer layer with an amount of FmocOSu inadequate for complete protection in the mixed solvent (DCM/Et2O). Following layer formation, the inner layer of 14 was selectively Boc-protected giving 15. Finally in phase III, the peptide chain in the outer layer was deprotected and capped with the heterocyclic acids shown in Figure 1 respectively in ten separate tubes. The hexapeptide OBOC library was synthesized in the same way except two more split-coupling-mix cycles were employed to gain the hexapeptide chain in the phase I stage.
Scheme 1.
The three phases of library synthesis.
With these encoded OBOC libraries in hand, a differential assay was conducted to identify hits that only bind to wild-type sPLA2-IIA, ideally only at its integrin-binding site. This assay consists of sequential incubations with an integrin-binding defective mutant sPLA2-IIA(R74E/R100E) and wild-type sPLA2-IIA, and image subtraction12,13a to selectively visualize the beads that only contain the desired hits. The mutant sPLA2-IIA (R74E/R100E) was previously demonstrated to be unable to bind to integrin αvβ3.12,13a Our screening process involves a lengthy procedure, the principles of which are based on our already published strategies12,13a and briefly illustrated in Scheme 2. In the petri dish shown in Scheme 2, all beads can be categorized into four types (A, B, C or D) according to the binding selectivity of the peptide on each bead. After incubating with biotinylated mutant sPLA2-IIA, there are two binding possibilities: some beads bind this protein and some do not. In this example, beads C and D bind the mutant protein and are stained blue by sequential treatment with antibiotin-alkaline phosphatase conjugate and 5-bromo-4-chloro-3-indolyl-phosphate (BCIP), as shown in image 1 of Scheme 2. Image 1 was then recorded using a high-resolution scanner. Beads A and B do not bind mutant sPLA2-IIA. Because of that, beads A and B cannot be stained blue due to lack of the biotinylated mutant protein and therefore do not show up as blue beads in image 1. All the immobilized beads are then washed with PBS buffer solution to clear out the mutant protein free in solution and further incubated with wild-type sPLA2-IIA, resulting in three possibilities: some beads bind wild-type sPLA2-IIA only, like beads B; some bind both the mutant and wild-type protein, like beads D; and some do bind not either, like beads A. Since the mutant protein has only two amino acid mutations, much of its surface area might be similar to that of the wild-type sPLA2-IIA, including the catalytic site. The majority of the stained beads in image 2 can be categorized as beads D. However, our interest lies only in peptides that can recognize the uniqueness of the wild type sPLA2-IIA, the integrin-binding site by fitting into it. Since the mutant has lost its integrin-binding site, beads B, which do not bind the mutant and only bind the wild-type, are most likely to contain peptides that can recognize the integrin-binding site. After visualization and scanning, image 2 was obtained showing the above binding possibilities. By subtracting immobilized image 1 from immobilized image 2, only beads that bind with wild-type sPLA2-IIA remain visible in the differential image 3. Guided by image 3 in Scheme 2, immobilized beads B were selectively picked out of the petri dish and submitted for decoding of the peptide chain using an automated Edman degradation sequencer.
Scheme 2.
Flowchart of the on-bead screening process.
Although >100 beads showed up in the differential image (image 3), only twenty-four beads were selected for a quick decoding process. Upon Edman degradation some of the beads did not release residues above the instrument detection limit, but seven gave unambiguous decoding results upon sequencing (Table 1). A quick statistical analysis on the occurrence of the four main classes of amino acids (basic, acidic, polar and nonpolar) reveals that 33% of the residues of these hit compounds are basic in nature. The less abundant residues are nonpolar, 27%; polar, 20%; and acidic, 20%. This order of amino acid occurrences of different types of amino acid does not seem to be in agreement with the differences between wild-type sPLA2-IIA and its mutant: at pH=7.4, the arginine of the wild type is protonated and should repel the positively-charged basic residue-rich peptides. Therefore, these hits could be false positives and needed to be re-synthesized and confirmed in secondary biological assays.
Table 1.
The structure formula and inhibition activity of compounds. Inhibitory effect of compounds on sPLA2-IIA-integrin interaction was assessed in adhesion assay using αvβ3-K562 cells and immobilized sPLA2-IIA. Wells of 96-well microtiter plates were coated with sPLA2-IIA, and the remaining protein-binding sites were blocked with BSA. sPLA2-IIA coated wells were incubated with different concentrations of compounds (10 to 500 μM) for 30 min. αvβ3-K562 cells were added to the wells and incubated for 1 h at 37 °C, and bound cells were quantified by using phosphatase assays. The IC50 and maximum inhibition was calculated with adhesion without inhibitors as 100% and adhesion to BSA only as 0%. Data are shown as means ± S.E. (n=3). N/A, “not active’ (<30% inhibition). Natural amino acids are designated by the standard single-letter code. Amino acids with an asterisk stand for D-amino acids.
| IC50 (μM) |
Max. Inhibition (%) |
||
|---|---|---|---|
|
|
|||
| Cmpd2 |

|
- | N/A |
| Cmpd3 |

|
- | N/A |
| Cmpd4 |
|
85±16 | 71±11 |
| Cmpd8 |

|
20±1.3 | 85±1.5 |
| Cmpd10 |

|
- | N/A |
| Cmpd16 |

|
- | N/A |
| Cmpd21 |
|
71±18 | 93±10 |
All hits were re-synthesized on Rink amide MBHA resin following standard solid-phase synthesis procedures. Their purity and chemical identities were confirmed with LC/MS (see supporting information). And then all hits were screened in a cell adhesion inhibition assay. The assay was conducted to check whether transfected K562 erythroleukemia cells, which have integrin αvβ3 over-expressed on the cell surface, could still adhere to the sPLA2-IIA immobilized in a well in the presence of a hit compound. If the number of cells adhering to the immobilized sPLA2-IIA is decreased in the presence of a hit, the sPLA2-IIA-integrin interaction is inhibited by the hit. The decrease in the adhering cell should correlate with inhibition strength of the hit. The IC50 and maximum inhibition effect of the hits are derived from dose-dependent experiments with the hits and the results are listed in Table 1.
As Table 1 indicate, hits Cmpd4, Cmpd8 and Cmpd21 all can have more than 70% inhibition effect against the interaction between sPLA2-IIA and integrin αvβ3, while Cmpd2, Cmpd3, Cmpd10 and Cmpd16 have little or no inhibition effect. Among the three active hits, Cmpd8 and Cmpd21 are more interesting or exciting than Cmpd4. First of all, they are much more active than Cmpd4. For instance, Cmpd8 and Cmpd21 can reach 85% and 93% maximum inhibition level respectively while Cmpd4 inhibits the interaction at 71% at most; Cmpd8 and Cmpd21 have IC50 values of 20 and 71 μM respectively while Cmpd4 gets IC50 = 85 μM. Secondly, both hits carry the same pyrazolylthiazole heterocyclic moiety. Since ten different capping agents were used, and the pyrazolylthiazole motif is repeated among the most active hits, we infer that the pyrazolythiazole might be able to well fit the integrin-binding site of sPLA2-IIA or at least a part of the site.
A control assay was conducted to confirm that inhibition of the sPLA2-IIA-integrin interaction is attributed to binding to sPLA2-IIA by the hit compounds, and not to binding to the integrin (Figure 3). Disintegrin and metalloproteinase domain-containing protein 15 (ADAM15) is a known ligand of integrin αvβ316 and is an enzyme that in humans is encoded by the ADAM15 gene.17 Through its disintegrin-like domain, this protein specifically interacts with the integrin beta chain, beta 3.18 We previously showed that RGD-containing peptide inhibits the binding of both ADAM-15 and sPLA-IIA to integrin αvβ3 11, 16, suggesting that the ADAM-15 and sPLA2-IIA-binding sites in αvβ3 overlap. If a hit compound inhibits the adhesion by binding to the integrin, the number of cells left in the wells should decrease no matter what protein is present in the well. The experimental result in Figure 3 shows that Cmpd8 and Cmpd21 suppressed cell adhesion when sPLA2-IIA was coated, but did not suppress cell adhesion when ADAM15 protein was coated on the wells. Therefore, this suggests that the suppression of cell adhesion by Cmpd8 and Cmpd21 is specific to sPLA2-IIA, and that Cmpd8 and Cmpd21 specifically bind to sPLA2-IIA, but not to ADAM15 or integrins.
Figure 3.
Effect of compounds on sPLA2-IIA-integrin or ADAM15-integrin interaction in adhesion assays using αvβ3-K562 cells and immobilized sPLA2-IIA or ADAM15. 200 μM of Cmpd8 and Cmpd21 were used. Cmpd8 and Cmpd21 specifically suppressed adhesion of αvβ3-K562 cells to sPLA2-IIA, but not to ADAM15, suggesting that the compounds are specific to sPLA2-IIA and not to the αvβ3 integrin. Data are shown as means +/− S.E. (n=3).
sPLA2-IIA is a potent chemoattractant.19 We first show that WT sPLA2-IIA induced migration of U937 human monocytic leukemia cells in a modified Boyden chamber, but the integrin-binding defective sPLA2-IIA mutant (R74E/R100E) did not (Figure 4 top).
Figure 4.
Cmpd 8 inhibits sPLA2-IIA induced cell migration in a dose-dependent manner. The lower chamber of the modified Boyden chamber contains sPLA2-IIA (40 ng/ml). In inhibition experiments, Cmpd8 (4 to 16 μM) was placed in the lower chamber of Transwell. U937 cells were plated in the upper chamber, and incubated for 20 h at 37 °C. The number of migrated cells was counted. Data is shown as means +/− SE (n=4).
These results indicate that sPLA2-IIA-integrin interaction plays a role in sPLA2-IIA-induced monocyte migration. We tested if Cmpd8, the most potent inhibitor of the sPLA2-IIA-integrin interaction, suppresses sPLA2-IIA-induced migration of U937 cells; indeed, Cmpd8 suppressed sPLA2-IIA-induced migration in a dose-dependent manner (Figure 4 bottom). These findings suggest that Cmpd8 suppresses sPLA2-IIA-induced chemotaxis through blocking integrin binding to sPLA2-IIA.
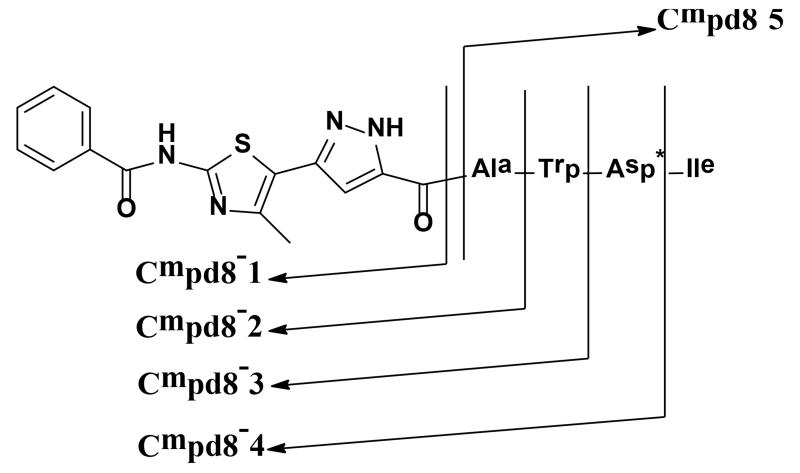
Active compounds Cmpd8 and Cmpd21 are composed of two major motifs: the heterocyclic moiety and the peptide chain. Since peptides are susceptible to decomposition effected by proteolytic enzymes, it would be beneficial to the stability of an active compound if it has minimal peptide linkages. As there is no structural data for a sPLA2-IIA-inhibitor complex, a trial-and-error approach was used to investigate the essential structure components of the two hit compounds for their affinity to the integrin-binding site of sPLA2-IIA. Analogs of these compounds were designed by truncating Cmpd8 and Cmpd21, i.e, by “removing” one amino acid at a time (as demonstrated for Cmpd8 in Figure 5). The structure formulas and inhibition activity of the analogs are listed in Table 2.
Figure 5.
Disconnections for designing structure-truncated analogs of Cmpd8. The truncated versions of Cmpd 8 and Cmpd 21 were synthesized and tested in the cell adhesion assay; structure and activities for this series are listed in Table 2. Asp* stands for D-Asp.
Table 2.
Inhibition activities of Cmpd8, Cmpd21, and truncated analogs. Inhibitory effect of compounds on sPLA2-IIA-integrin interaction was assessed in adhesion assays using αvβ3-K562 cells and immobilized sPLA2-IIA. The IC50 and maximum inhibition was calculated with adhesion without inhibitors as 100% and adhesion to BSA only as 0%. Data are shown as means ± S.E. (n=3).
| Compound | Structural Formulaa | Activity (IC50, μM) |
Maximum Inhibition (%) |
|---|---|---|---|
| Cmpd8-1 | R-OH | - | N/A |
| Cmpd8-2 | R-Ala | - | N/A |
| Cmpd8-3 | R-Ala-Trp | - | N/A |
| Cmpd8-4 | R-Ala-Trp-Asp* | - | N/A |
| Cmpd8-5 | Ala-Trp-Asp*-Ile | - | N/A |
| Cmpd8 | R-Ala-Trp-Asp*-Ile | 20±1.3 | 85±1.5 |
| Cmpd21-1 | R-Gly | 241±33 | 98±2.4 |
| Cmpd21-2 | R-Gly-Arg* | 262±6.8 | 77±1.8 |
| Cmpd21-3 | R-Gly-Arg*-Gly | 217±14 | 81±3.0 |
| Cmpd21-4 | R-Gly-Arg*-Gly-Asp* | 155±3.8 | 87±1.7 |
| Cmpd21-5 | R-Gly-Arg*-Gly-Asp*-Asp* | - | N/A |
| Cmpd21-6 | Gly-Arg*-Gly-Asp*-Asp*-Asp* | - | N/A |
| Cmpd21 | R-Gly-Arg*-Gly-Asp*-Asp*-Asp* | 71±18 | 93±10 |

|
A terminal residue has an ending NH2 group replacing the OH group of its carboxylic acid functionality. Amino acids with an asterisk stand for D-amino acids.
Table 2 indicates that the heterocyclic capping agent (Cmpd8-1) and simple peptide chains (Cmpd8-5 and Cmpd21-6) are not inhibitory to the sPLA2-IIA-integrin interaction by themselves alone, clearly inhibition of the interaction between these two proteins appears to require the combination of the pyrazolylthiazole capping molecule and a peptide.
In addition, none of the truncated Cmpd8 analogs are active, meaning that at minimum the combination of the heterocyclic moiety and the AWD*I peptide is required for the inhibition activity of Cmpd8. In contrast, most of truncated analogs of Cmpd21 do demonstrate adhesion inhibitory activity. This opposite trend with Cmpd21 might imply that a specific amino acid sequence is required to fit the space in the integrin-binding pocket of sPLA2-IIA not occupied by the pyrazolylthiazole moiety. The activity of truncated Cmpd21 analogs also indicates that the peptide chain of Cmpd21 can be shortened dramatically. As the cap-G, cap-GR*, cap-GR*G and cap-GR*GD* are all active, the combination of the pyrazolylthiazole and glycine is the minimum required to inhibit the sPLA2-IIA-integrin interaction. Addition of extra amino acids to the cap-G may increase the inhibition activity, as Cmpd21-4 demonstrates, but that depends on what amino acids are attached to the glycine. Cmpd21 contains an Arg-Gly-Asp motif, but Arg and Asp are D-isomers. The classic Arg-Gly-Asp integrin-binding motif is composed of L-amino acids and is present in integrin ligands such as fibronectin. Cmpd21 binds to integrin ligand sPLA2-IIA instead of integrins. Therefore, the Arg-Gly-Asp motif in Cmpd21 is structurally and functionally different from the classic Arg-Gly-Asp motif.
Inhibition of the sPLA2-IIA-integrin interaction by the individual hit compounds in solution not only validates the on-bead screening assays discussed above, it also is consistent with docking simulations we carried out on Cmpd8 and Cmpd21 with sPLA2-IIA. The simulation, which was carried out using AUTODOCK 3,20 indicated binding energies of −16.2 kcal/mol for Cmpd8 and −21.0 kcal/mol for Cmpd21. These simulations predict that Cmpd8 and Cmpd21 both fit into a groove in sPLA2-IIA flanked with the key residues R74 and R100, which are responsible for integrin recognition by the lipase (Figure 6).
Figure 6.
Docking of Cmpd8 and Cmpd21 with sPLA2-IIA. Docking simulation between sPLA-IIA (PDB code 1AYP) and Cmpd8 or Cmpd21 was performed using Autodock3 as described 11. Blue, basic; Red, acidic; yellow, sulfur; white, neural; and pink, hydrophobic.
In conclusion, inhibitors against interaction between sPLA2-IIA and integrin αvβ3 were identified for the first time using heterocycle-capped OBOC libraries coupled to an exquisite differential assay. The two confirmed hit compounds have several structural features in common. First, each has the pyrazolylthiazole moiety. Second, the peptides of these hits also share a similar nonpolar, basic and acidic amino acid “theme”. Structure-activity-relationship study around these hits reveals that the combination of the heterocyclic capping moiety and peptide chain is vital to inhibiting the sPLA2-IIA-integrin interaction. The inhibition effect varies with the length and nature of the residues of the peptide chain. While no truncated Cmpd8 analogs are active, several Cmpd21 truncated analogs remain active in blocking the sPLA2-IIA-integrin interaction. As shown by the ADAM15 control experiment, the blockade of the sPLA2-integrin interaction is rendered through binding to sPLA2 not to the integrin. Cmpd8 and Cmpd21 and their active analogs might offer valuable lead structures for development of novel anti-inflammation agents that target the non-catalytic site of sPLA2-IIA, an unique medical intervention strategy for targeting the pro-inflammatory enzyme.
Supplementary Material
Acknowledgments
This work was supported in part by TRDRP (tobacco-related disease research program; grant 18TX-0169) to YT, the National Science Foundation (CHE-0910870), and the UC–CRCC (University of California–Cancer Research Coordinating Committee).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://####.
References and notes
- 1.Kudo I, Murakami M. Prostaglandins & Other Lipid Mediators. 2002;68-69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 2.Tatulian SA. Biophys. J. 2001;80:789–800. doi: 10.1016/S0006-3495(01)76058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niessen HWM, Krijnen PAJ, Visser CA, Meijer CJLM, Erik Hack C. Cardiovasc. Res. 2003;60:68–77. doi: 10.1016/s0008-6363(03)00324-9. [DOI] [PubMed] [Google Scholar]
- 4.(a) Bryant KJ, Bidgood MJ, Lei P, Taberner M, Salom C;, Kumar V, Lee L, Church WB, Courtenay B, Smart BP, Gelb MH, Cahill MA, Graham GG, McNeil HP, Scott KF. J. Bio. Chem. 2011;286:2492–2503. doi: 10.1074/jbc.M110.123927. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tada K, Murakami M, Kambe T, Kudo I. J. Immunol. 1998;161:5008–5015. [PubMed] [Google Scholar]
- 5.Bradley JD, Dmitrienko AA, Kivitz AJ, Gluck OS, Weaver AL, Wiesenhutter C, Myers SL, Sides GD. J. Rheumatol. 2005;32:417–423. [PubMed] [Google Scholar]
- 6.Bowton DL, Dmitrienko AA, Israel E, Zeiher BG, Sides GD. J. Asthma. 2005;42:65–71. doi: 10.1081/jas-200044748. [DOI] [PubMed] [Google Scholar]
- 7.Ancian P, Lambeau G, Mattéi M-G, Lazdunski M. J. Biol. Chem. 1995;270:8963–8970. doi: 10.1074/jbc.270.15.8963. [DOI] [PubMed] [Google Scholar]
- 8.Lambeau G, Ancian P, Nicolas JP, Beiboer SH, Moinier D, Verheij H, Lazdunski M. J. Biol. Chem. 1995;270:5534–40. doi: 10.1074/jbc.270.10.5534. [DOI] [PubMed] [Google Scholar]
- 9.Murakami M, Kambe T, Shimbara S, Yamamoto S, Kuwata H, Kudo I. J. Biol. Chem. 1999;274:29927–29936. doi: 10.1074/jbc.274.42.29927. [DOI] [PubMed] [Google Scholar]
- 10.Sartipy P, Johansen B, Gasvik K, Hurt-Camejo E. Circ. Res. 2000;86:707–714. doi: 10.1161/01.res.86.6.707. [DOI] [PubMed] [Google Scholar]
- 11.Saegusa J, Akakura N, Wu C-Y, Hoogland C, Ma Z, Lam KS, Liu F-T, Takada YK, Takada Y. J. Biol. Chem. 2008;283:26107–26115. doi: 10.1074/jbc.M804835200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehman A, Gholami S, Hahn M, Lam KS. J. Comb. Chem. 2006;8:562–570. doi: 10.1021/cc0600268. [DOI] [PubMed] [Google Scholar]
- 13.(a) Liu R, Marik J, Lam KS. J. Am. Chem. Soc. 2002;124:7678–7680. doi: 10.1021/ja026421t. [DOI] [PubMed] [Google Scholar]; (b) Edman P. Acta Chem. Scand. 1950;4:283–293. [Google Scholar]
- 14.Aina OH, Liu R, Sutcliffe-Goulden JL, Marik J, Pan C, Lam KS, Lam KS, Lebl M, Krchnak V. Mol. Pharm. Chem. Rev. 2007;1997;497:631–651. doi: 10.1021/mp700073y. [DOI] [PubMed] [Google Scholar]
- 15.Ye L, Knapp JM, Sangwung P, Fettinger JC, Verkman AS, Kurth MJ. J. Med. Chem. 2010;53:3772–3781. doi: 10.1021/jm100235h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang XP, Kamata T;, Yokoyama K;, Puzon-McLaughlin W, Takada Y. J Biol Chem. 1998;273:7345–7350. doi: 10.1074/jbc.273.13.7345. [DOI] [PubMed] [Google Scholar]
- 17.Primakoff P, Myles DG. Trends Genet. 2000;16:83–7. doi: 10.1016/s0168-9525(99)01926-5. [DOI] [PubMed] [Google Scholar]
- 18.Takagi J, Springer TA. Immunol. Rev. 2002;186:141–63. doi: 10.1034/j.1600-065x.2002.18613.x. [DOI] [PubMed] [Google Scholar]
- 19.Ibeas E, Fuentes L, Martín R, Hernández M, Nieto ML. Cardiovasc Res. 2009;81:54–63. doi: 10.1093/cvr/cvn234. [DOI] [PubMed] [Google Scholar]
- 20.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. J. Comput. Chem. 1998;19:1639–1662. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.