Abstract
The epidermal growth factor receptor (EGFR) plays an important role in tumor progression and treatment resistance for many types of malignancies including head and neck, colorectal, and nonsmall cell lung cancer. Several EGFR targeted therapies are efficacious as single agents or in combination with chemotherapy. Given the toxicity associated with chemoradiation and poor outcomes seen in several types of cancers, combinations of EGFR targeted agents with or without chemotherapy have been tested in patients receiving radiation. To date, the only FDA approved use of an anti-EGFR therapy in combination with radiation therapy is for locally advanced head and neck cancer. Given the important role EGFR plays in lung and colorectal cancer and the benefit of EGFR inhibition combined with chemotherapy in these disease sites, it is perplexing why EGFR targeted therapies in combination with radiation or chemoradiation have not been more successful. In this review we summarize the clinical findings of EGFR targeted therapies combined with radiation and chemoradiation regimens. We then discuss the interaction between EGFR and radiation including radiation induced EGFR signaling, the effect of EGFR on DNA damage repair, and potential mechanisms of radiosensitization. Finally, we examine the potential pitfalls with scheduling EGFR targeted therapies with chemoradiation and the use of predictive biomarkers to improve patient selection.
Keywords: Epidermal growth factor receptor, EGFR, chemoradiation, radiation, combined modality therapy, personalized medicine
1. Introduction
The epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase belonging to the ErbB family. EGFR consists of an extracellular domain, a single transmembrane region, and a cytoplasmic kinase domain (Gullick et al., 1985). There are several known ligands for EGFR including EGF, TGFα, HB-EGF, amphiregulin, betacellulin, epigen, and epiregulin (Linggi et al., 2006). Upon ligand binding, EGFR forms a dimer and specific tyrosine residues are phosphorylated promoting signal transduction (Uberall et al., 2008) through many pathways including PI3k/Akt (Hennessy et al., 2005), Ras-MAPK (Nishinaka et al., 2001, Sebolt-Leopold et al., 2004), STAT (Schmidt-Ullrich et al., 1997, Bowman et al., 2000), and PLCγ (Oliva et al., 2005). Activation of these pathways promotes several cellular processes including proliferation, migration and invasion, transformation, differentiation, and angiogenesis (Mendelsohn et al., 2000).
Due to its important role in cell proliferation and other cellular processes, EGFR is an attractive target for cancer therapy. Overexpression or upregulation of EGFR is seen in many types of malignancies including lung (Ciardiello et al., 2001, Herbst et al., 2003), head and neck (Grandis et al., 1993), esophageal (Mukaida et al., 1991), and colorectal cancers (Moroni et al., 2005). Several EGFR targeted drugs are FDA approved for clinical use including the antibodies cetuximab and panitumumab and small molecule inhibitors erlotinib and afatinib. The use of EGFR targeted therapies is standard of care in subsets of patients with metastatic colorectal cancer, metastatic nonsmall cell lung cancer, and locally advanced head and neck cancer.
Concurrent administration of chemotherapy with radiation therapy has been standard practice since the 1980’s. Traditionally, cytotoxic agents such as cisplatin or 5-FU are combined with fractionated radiation therapy in the adjuvant and definitive treatment settings. Combined modality therapy has several potential advantages over radiation alone. These therapies may work synergistically to enhance cell kill through a number of mechanisms. Previous reports have reviewed the potential interactions between radiation and systemic therapy in detail (Steel et al., 1979, Bentzen et al., 2007, Shewach et al., 2007, Morgan et al., 2014, Morris et al., 2014). A consequence of the concurrent administration of chemotherapy with radiation therapy is increased toxicity. For this reason, the use of a systemic radiosensitizing drug targeting a specific pathway more active in cancer cells than normal tissues is an attractive strategy. In this article, we review the completed and ongoing clinical trials that combine EGFR targeted therapies with radiation. We then discuss the interaction between radiation and EGFR signaling and explore potential strategies for optimizing EGFR directed therapies with radiation.
2. Clinical trials with EGFR targeted therapies and radiation
Head and neck cancer
The most successful implementation of an EGFR inhibitor in combination with radiation therapy has been in locally advanced head and neck cancer. Head and neck cancers are frequently driven by EGFR signaling and high expression of EGFR is associated with a poor prognosis (Dassonville et al., 1993, Grandis et al., 1998, Gupta et al., 2002, Ang et al., 2004, Eriksen et al., 2004) and radioresistance (Bonner et al., 1994, Ang et al., 2002, Harari et al., 2002, Liang et al., 2003). In a landmark study by Bonner et al., cetuximab improved local control and survival in patients with locally advanced head and neck cancer receiving definitive radiation therapy (Bonner et al., 2006, Bonner et al., 2010). On subset analysis, the survival benefit was predominately in younger patients with an oropharynx primary treated with an accelerated radiation course (Bonner et al., 2010). Interestingly, patients who experienced a prominent cetuximab-induced acneiform rash had better outcomes than patients not having this reaction.
Although the Bonner study found a benefit with cetuximab in locally advanced head and neck cancer, the results are difficult to interpret because patients on the control arm received radiation therapy alone. Current standard of care for locally advanced head and neck cancer is radiation therapy with concurrent chemotherapy (Pignon et al., 2000). To address this issue, several trials have been performed to study cetuximab in combination with chemoradiation. RTOG 0522 was a phase III study that randomized patients to cisplatin based chemoradiation with or without cetuximab (Ang et al., 2014). This trial found no improvement in progression free survival with the addition of cetuximab. Additionally, treatment with cetuximab plus chemoradiation resulted in more toxicity than chemoradiation alone.
Another question that has been explored is whether or not cetuximab can replace chemotherapy as a radiation sensitizer in a specific subset of patients. GORTEC (TREMPLIN, NCT00169247) published the results from a randomized phase II study where patients with cancer of the larynx or hypopharynx received induction chemotherapy followed by chemotherapy or cetuximab concurrent with radiation. There was no significant difference between the two arms suggesting cetuximab could replace chemotherapy in this setting; however, the outcomes of both arms were not better than that of a previous trial with induction chemotherapy followed by radiation alone in a similar patient population (Lefebvre et al., 2013). A recently completed four arm phase II/III study (2×2 factorial design) from Italy examined the role of induction chemotherapy and concurrent cetuximab or chemotherapy with radiation (NCT01086826). Results presented at ASCO 2012 and 2013 found a similar response rate in the patients who received cisplatin/5-FU or cetuximab with radiation therapy (complete response 36% versus 39%) and no difference in median overall survival (44.7 months in both groups) (Ghi et al., 2013). It should be noted that the primary endpoint for the chemoradiation versus cetuximab-radiation comparison was grade 3–4 in field toxicity and not response rate or survival. Surprisingly, toxicity was not different in patients receiving cetuximab with radiation compared to chemotherapy with radiation (Ghi et al., 2012).
Additional studies comparing chemoradiation to cetuximab with radiation are ongoing. RTOG 1016 is a phase III study that recently completed patient accrual. On this study, patients with locally advanced head and neck cancer are randomized to cetuximab or cisplatin with radiation therapy. This study only includes patients with HPV positive head and neck cancer, which is associated with a better prognosis and is potentially more sensitive to EGFR directed therapies (NCT01302834). Additionally, in Italy there is an ongoing randomized phase II clinical trial studying the use of cisplatin or cetuximab with radiation (NCT01216020) and the Trans-Tasman Radiation Oncology Group is running a phase III study comparing weekly cisplatin to weekly cetuximab with radiation therapy in head and neck cancer patients (TROG 12.01 NCT01855451).
Several other phase III studies with cetuximab in head and neck cancer have recently opened. The ATSCANIII study (NCT01969877) from Sweden is a four arm phase III trial. It examines two different radiation fractionation schemes with concurrent cisplatin or cetuximab. Additionally, a study by the GORTEC (NCT01233843) group in France randomizes patients to concurrent carboplatin with radiation therapy versus induction chemotherapy with docetaxel, cisplatin, and 5-FU followed by concurrent cetuximab and radiation therapy. Additional studies with cetuximab and radiation therapy include RTOG 0920, a phase III study of post-operative radiation with or without cetuximab in locally advanced head and neck cancer patients, and RTOG 1216, (NCT01810913) a three arm phase II/III study of radiation with either docetaxel, docetaxel with cetuximab, or cisplatin in high risk post-operative patients.
In almost all of studies that include cetuximab with radiation therapy, cetuximab is administered with a loading dose of 400 mg/m2 followed by weekly dosing at 250 mg/m2. This dosing schedule is based off PK/PD studies and phase I dose escalation studies which show saturation of EGFR binding at 250 mg/m2 (Robert et al., 2001, Fracasso et al., 2007). Given the complex role EGFR has on the cell cycle, DNA repair, and cell survival/proliferation pathways, it is unclear if this is the optimal dosing schedule when combined with radiation therapy or chemoradiation therapy. This point is discussed in greater detail below.
In addition to cetuximab, results from studies using the fully humanized monoclonal anti-EGFR antibody panitumumab have recently been reported. CONCERT-1 was a randomized phase II study where patients with locally advanced head and neck cancer received chemoradiation therapy with or without panitumumab (Mesia et al., 2015). Local regional control was not significantly different between the two groups (2 year locoregional control 68% without panitumumab versus 61% with panitumumab) and there was higher toxicity with panitumumab (43% vs. 32%). CONCERT-2 was a randomized phase II study where patients with locally advanced head and neck cancer received chemotherapy or panitumumab with radiation (Giralt et al., 2015). The local regional control rate at 2 years was lower but not significantly different with panitumumab (51% panitumumab-RT vs. 61% chemoRT) and the rate of serious toxicity was similar (40% chemoRT vs. 34% panitumumab-RT). Several other trials with panitumumab in head and neck cancer are ongoing including a study by the NCIC that recently finished patient accrual. This phase III study compares cisplatin with standard fractionated radiation therapy and panitumumab with accelerated radiation therapy (NCT00820248).
Nonsmall cell lung cancer
EGFR targeted therapies have become standard of care in a subset of patients with advanced stage nonsmall cell lung cancer. In a landmark study, the small molecule EGFR inhibitor erlotinib improved overall survival in patients with advanced stage NSCLC who had failed chemotherapy (Shepherd et al., 2005). Additionally, the EGFR targeted antibody cetuximab improved survival in patients with metastatic nonsmall cell lung cancer receiving cisplatin and vinorelbine (Pirker et al., 2009). Subset analyses from several studies demonstrate the benefit of small molecule EGFR inhibitors is limited to patients with tumors harboring specific EGFR mutations and wild type KRAS (Lynch et al., 2004, Paez et al., 2004, Pao et al., 2004, Eberhard et al., 2005). Interestingly, specific acquired EGFR mutations (T790M) also lead to erlotinib resistance (Pao et al., 2005).
Given the success of combining cetuximab with radiation in head and neck cancer and the benefit of EGFR targeted therapies in nonsmall cell lung cancer, several clinical trials have been designed to study the effect of adding erlotinib or cetuximab to chemoradiation in patients with locally advanced nonsmall cell lung cancer. The RTOG performed a phase II study (RTOG 0324) where patients with locally advanced nonsmall cell lung cancer received carboplatin, paclitaxel, and cetuximab with radiation. Two year survival was a very encouraging 49% (Blumenschein et al., 2011). The CALGB completed a randomized phase II study in patients with locally advanced nonsmall cell lung cancer receiving carboplatin, pemetrexed, and radiation therapy with or without cetuximab. There was no difference in 18 month overall survival (58% versus 54%) and increased toxicity was seen in the arm containing cetuximab (Govindan et al., 2011). Additionally, van den Heuvel et al. performed a randomized phase II study with cisplatin and radiation with or without cetuximab in 102 patients with locally advanced nonsmall cell lung cancer. Similar to the CALGB trial, use of cetuximab did not improve overall survival (van den Heuvel et al., 2014).
A very important trial with cetuximab and radiation in NSCLC was RTOG 0617. In this phase III study patients were randomized to radiation therapy and concurrent carboplatin and paclitaxel with or without cetuximab. Similar to the phase II studies, there was no survival benefit with cetuximab (median survival 25.0 versus 24.0 months, HR 1.07) and grade 3 or higher toxicity was increased (86% to 70%). However, on subset analysis patients with increased EGFR expression had improved overall survival with the addition of cetuximab (42 months versus 21 months) (Bradley et al., 2015). The RTOG currently has a phase II study open (RTOG 0839, NCT00979212) where patients are randomized to preoperative chemoradiation therapy with or without panitumumab. A second randomization of consolidative chemotherapy with or without cetuximab on RTOG 0839 was closed in 2010.
Many contemporary trials in nonsmall cell lung cancer have focused on patient selection and the use of small molecule inhibitors. Prior studies in locally advanced and metastatic lung cancer have identified subsets of patients that benefit the most from treatment with EGFR small molecule inhibitors. Massachusetts General Hospital is running a phase II study of afatinib followed by concurrent chemoradiation in EGFR mutant nonsmall cell lung cancer (NCT01553942). An ongoing phase II study from Beijing Cancer Hospital (NCT01391260) is studying the combination of gefitinib with radiation alone in patients with specific EGFR activating mutations. Another phase II study from China randomizes patients with exon 19 or 21 EGFR mutations to cisplatin and etoposide or erlotinib with radiation therapy (NCT01714908). With our evolving ability to properly select patients for EGFR targeted therapy in nonsmall cell lung cancer and the findings from RTOG 0617, the use of anti-EGFR therapies with radiation in lung cancer is still promising
Rectal cancer
EGFR plays a very important role in the progression and treatment of colorectal cancer. This protein is overexpressed in approximately 50–80% of colorectal tumors and EGFR targeted antibodies, such as cetuximab and panitumumab, are effective in advanced stage disease (Cunningham et al., 2004, Van Cutsem et al., 2007, Sorbero et al., 2008, Bokemeyer et al., 2009, Van Custem et al., 2009, Douillard et al., 2010). In rectal cancer, EGFR overexpression is associated with a lower pathologic complete response rate and a worse prognosis in patients receiving neoadjuvant chemoradiation (Azria et al., 2005, Giralt et al., 2005, Li et al., 2006, Kim et al., 2006, Bertolini et al., 2007, Zlobec et al., 2008). Interestingly, unlike nonsmall cell lung cancer, in colorectal cancer EGFR overexpression or specific EGFR activating mutations have not been found to be predictive of response to anti-EGFR therapies (Chung et al., 2005, Hebbar et al., 2006). Due to the clear benefit of preoperative chemoradiation in locally advanced rectal cancer, a number of studies have looked at the role of EGFR targeted therapies in combination with radiation therapy for this disease.
The early phase I and II trials using EGFR targeted therapies in combination with radiation therapy for rectal cancer seemed to produce promising results. These studies used pathological complete response rate (pCR) as a surrogate for outcome. A study by Valentini et al. found a 30% pathologic complete response rate with gefitinib and 5-FU based chemoradiation, which was much higher than what has been seen historically (Valentini et al., 2008). Pinto et al. examined the use of panitumumab with 5-FU and oxaliplatin. In this study the pCR rate was an encouraging 21% (Pinto et al., 2011). McCollum et al. also demonstrated a promising pCR rate of 28% in a phase II study of cetuximab and 5-FU based chemoradiation; however, this rate was not significantly different from the pCR rate of patients on the control arm not receiving cetuximab (McCollum et al., 2014).
Prior studies in patients with advanced stage colorectal cancer have demonstrated that anti-EGFR therapies are not as effective in patients with KRAS mutant tumors (Eberhard et al., 2005, Lievre et al., 2006, Amado et al., 2008, De Roock et al., 2008, Bokemeyer et al., 2009, Van Cutsem et al., 2009). It is possible the trials using chemoradiation discussed above found no benefit because they did not select for the patients most likely to respond to EGFR targeted drugs. Dewdney et al. performed a randomized phase IIB study where patients received capecitabine, oxaliplatin, and radiation therapy with or without cetuximab. The subset of patients with KRAS and BRAF wild type tumors had lower response rates to chemoradiation compared to the reports above and there was no statistically significant difference in the pCR rate (11% versus 9%) between the two treatment arms. However, the secondary end points of radiological response rate and overall survival were improved with cetuximab (Dewndey et al., 2012). More recently, a pooled analysis of two phase II studies (IRIX and ERBIRIX trials), which included patients treated with capecitabine, irinotecan, cetuximab and radiation was performed. Interestingly, this analysis found no benefit with the addition of cetuximab to chemoradiation in patients with KRAS wild type tumors (Kim et al., 2013).
Several ongoing studies are exploring the role of EGFR directed therapies in patients receiving preoperative chemoradiation therapy for rectal cancer. The Southwest Oncology Group is running an 80 patient phase II study with preoperative capecitabine, oxaliplatin, cetuximab and radiation (S0713, NCT00686166). Also, the Zhejiang Cancer Hospital is performing a study with nimituzumab, capecitabine, oxaliplatin, and radiation (NCT01899118). Given the importance of patient selection in the advanced stage setting, proper patient selection may be necessary to demonstrate a benefit in patients with locally advanced rectal cancer receiving chemoradiation therapy.
Esophageal cancer
In esophageal cancer, EGFR overexpression is common and associated with a poor prognosis (Chen et al., 1991, Mukaida et al., 1991). Therefore, EGFR has been identified as a potential molecular target in this disease. A 65 patient phase II study from Brown University and the University of Maryland concluded that treatment with carboplatin, paclitaxel, and cetuximab was well tolerated. This study had a clinical complete response rate of 70%, which was very encouraging (Safran et al., 2008). ECOG 2205 was a phase II study of cetuximab, oxaliplatin, 5-FU and radiation in patients with resectable esophageal cancer. Unfortunately, in this study 4 of the 22 patients who underwent surgery died from complications (Gibson et al., 2010). The Southwest Oncology Group also completed a phase II study in patients with locally advanced, unresectable esophageal cancer receiving cetuximab, cisplatin, irinotecan, and radiation therapy. Similar to ECOG 2205, this combination was poorly tolerated with a treatment related mortality rate of 10% (Tomblyn et al., 2012). Conflicting with these findings, the SAKK 75/06 study concluded that adding cetuximab to cisplatin/docetaxel with radiation was not associated with increased postoperative mortality (Ruhstaller et al., 2011). Given the high rate of toxicity in many of these trials, less intensive regimens incorporating EGFR inhibitors need to be studied. The Hoosier Oncology Group completed a phase II study of cetuximab and radiation (without chemotherapy) in patients with resectable esophageal cancer. Pathological CR rates were promising at 37% and treatment was well tolerated (Becerra et al., 2013).
More recently, two randomized phase II studies with cetuximab and chemoradiation therapy for esophageal cancer have been reported. RTOG 0436 was a phase II study that evaluated the addition of cetuximab to cisplatin and paclitaxel in patients with locally advanced esophageal cancer treated without surgery (Suntharalingam et al., 2014). The addition of cetuximab did not improve overall survival at 2 years (44% versus 42%). Similarly, the SCOPE1 trial was a randomized phase II study were patients received definitive chemoradiation with 5-FU/cisplatin with or without cetuximab. Use of cetuximab was associated with increased treatment failure, decreased survival, and worse toxicity (Crosby et al., 2013).
Several other trials of EGFR inhibitors and radiation therapy in esophageal cancer are ongoing. A phase II study from Germany randomizes patients receiving chemoradiation to cisplatin and 5-FU with or without cetuximab (NCT01787006). The Swiss Group for Clinical Cancer Research has a phase III study open where patients receive cisplatin and docetaxel with or without cetuximab followed by surgery (NCT01107639). There is also an open phase III study in China where patients with esophageal cancer are randomized to cisplatin, paclitaxel, and radiation with or without erlotinib (NCT00686114). In summary, the role of anti-EGFR targeted therapies in combination with chemoradiation for esophageal cancer is not well defined at this time. The phase II studies reported so far are concerning for high toxicity and a lack of efficacy.
Other disease sites
In addition to the disease sites discussed above, EGFR targeted therapies and radiation have been studied in cancers originating from the anal canal and cervix. Similar to head and neck cancer, in anal cancer overexpression of EGFR is common and KRAS mutations are rare (Paliga et al., 2012). Additionally, HPV is responsible for up to 90% of anal cancers (www.cdc.gov/cancer/hpv/statistics). Since HPV positive head and neck cancer patients may benefit from cetuximab, its use in anal cancer is promising. Several studies are ongoing in this disease site. The AIDS Malignancy Clinical Trials Consortium is running a phase II study with cisplatin, 5-FU, cetuximab, and radiation therapy in patients with HIV and anal cancer (NCT00324415). ECOG currently has a phase II study open with cetuximab and chemoradiation in patients with stage I–III anal cancer (NCT00316888). The FFCD also has an open phase I/II trial in anal cancer where patients receive 5-FU, mitomycin C, and panitumumab (NCT01581840). Another study from Switzerland is examining the use of capecitabine, 5-FU, and panitumumab in this patient population (NCT01843452). Given the role EGFR plays in anal cancer and the toxicities associated with mitomycin C and cisplatin, the use of EGFR targeted agents in this disease is promising.
Cervical cancer is another potential disease for the addition of EGFR targeted therapies to chemoradiation. Similar to head and neck cancer, in cervical cancer EGFR is commonly overexpressed and overexpression is associated with a worse prognosis (Soonthornthum et al., 2011). Additionally, HPV plays a very important role in this disease and chemoradiation is the primary treatment modality for many patients. The GOG recently completed a 64 patient phase I study with cetuximab, cisplatin, and radiation in stage I–III cervical cancer (GOG-9918, NCT00104910). Results from this study have not been reported. To date, the use of EGFR targeted therapies for cervical cancer remains an area of active research.
Multiple phase I studies and phase II studies with radiation therapy and an EGFR targeted agent have been completed or are underway in several other disease sites. A full summary of each of these trials and disease sites is beyond the scope of this review.
3. Interaction of EGFR with Radiation and Chemotherapy
Interaction of EGFR with radiation therapy
The interaction between EGFR signaling and radiation was first described over 20 years ago. Early studies demonstrated that prolonged exposure to EGF increases the cytotoxic effects of radiation (Kwok et al., 1989, Bonner et al., 1994). Additionally, the expression of EGFR increases after radiation therapy (Peter et al., 1993) and the cytotoxic effect of radiation is inversely correlated with EGFR expression (Sheridan et al., 1997, Akimoto et al., 1999, Lammering et al., 2001, Milas et al., 2003, Milas et al., 2004). Translational studies performed in the 1990’s further demonstrated that EGFR overexpression is associated with radioresistance in patients (Miyaguchi et al., 1991, Zhu et al., 1996). These pioneering studies paved the way for the use of EGFR inhibitors with radiation therapy in the clinic.
The mechanism of radiosensitization with EGFR inhibitors is complex. Three distinct phases of EGFR’s role in the radiation response have been elucidated. These phases include: EGFR’s role in DNA repair, the activation of pro-survival pathways, and enhanced cell proliferation (Huang et al., 2000, Nyati et al., 2006, Chen et al., 2007). Given the observation that treatment with EGFR directed therapies leads to G1 arrest in most cell types (Peng et al., 1996, Di Gennaro et al., 2003), one explanation of radiosensitization is that the cytostatic effect of EGFR inhibition limits tumor repopulation during a course of fractionated radiation therapy. Other studies show the role behind radiosensitization may be more complex than the induction of cell cycle arrest. For example, studies by Huang and Harari found that cetuximab promoted radiation induced apoptosis (Huang et al., 1999, Harari et al., 2002), impaired sublethal DNA damage repair, and affected the nuclear translocation of DNA-PK (Huang et al., 2000). Interestingly, the effect of radiation on EGFR activation appears to be most pronounced in confluent or serum starved cells (Schmidt-Ullrich et al., 1997). More recent studies by Ahsan et al. found that quiescent and proliferating cell lines differ in their radiosensitivity and response to EGFR inhibition. Specifically, in quiescent cells radiation induces a brief period of EGFR activation leading to S phase progression, impaired DNA repair, and enhanced cell death (Ahsan et al., 2009). Thus, EGFR inhibition during the first few hours after radiation may actually protect cells, whereas 24 hours after inhibition, the combined effects of G1 arrest and inhibition of DNA repair might produce sensitization.
The majority of kinases have functions beyond their kinase activity (Rauch et al., 2011). Given the complex role EGFR plays in several cellular locations, inhibition alone may not be the optimal means to target this protein. In addition to forming a homodimer with itself, EGFR can dimerize with other receptor tyrosine kinases including Erb2, Erb3, and c-Met leading to downstream signal transduction (Mueller et al., 2010, Ahsan et al., 2014). Additionally, EGFR binds to the chaperone protein hsp90 promoting stability of the inactivated receptor (Ahsan et al., 2013). Recent studies suggest novel drugs that degrade EGFR are more effective than drugs that inhibit EGFR in preclinical models (Haglund et al., 2003, Zhuang et al., 2003, Feng et al., 2007, Kirisits et al., 2007, Ahsan et al., 2009, Ahsan et al., 2010). Due to the multiple functions of EGFR within a cell, blocking activation with antibodies or small molecules may not be the optimal means to target this protein.
Nuclear EGFR
Although EGFR is typically depicted as a cell surface receptor, this protein is involved in several nuclear processes. Nuclear EGFR is prognostic in several types of cancer including head and neck (Psyrri et al., 2005, Psyrri et al., 2008), breast (Lo et al., 2005, Hadzisejdic et al., 2010), esophageal (Hoshino et al., 2007), and ovarian cancer (Xia et al., 2009). Additionally, resistance to radiation therapy is related to nuclear EGFR expression (Chen et al., 2007). Nuclear EGFR signaling plays an important role in gene expression (Wang et al., 2009, Hadzisejdic et al., 2010, Huo et al., 2010). EGFR is a transcriptional co-activator for many cancer related genes including cyclin D1 (Lin et al., 2001), COX2 (Lo et al., 2010), c-Myc (Jaganathan et al., 2011), aurora kinase A (Huang et al., 2008), and nitric oxide synthase (Lo et al., 2005).
In addition to gene regulation, nuclear EGFR plays an important role in DNA repair (Bandyopadhyay et al., 1998, Dittmann et al., 2005). Radiation promotes the internalization and transport of EGFR by caveolin-1 leading to the activation of DNA-PK in response to DNA damage (Dittmann et al., 2008, Dittmann et al., 2010, Liccardi et al., 2011). DNA-PK is a vital kinase for nonhomologous end joining repair. Interestingly, inhibition of EGFR with cetuximab attenuates EGFR nuclear import and suppresses DNA-PK activity (Dittmann et al., 2005). EGFR also affects the DNA repair proteins XRCC1 and ATM (Debucquoy et al., 2010) and interacts with the ribonuclease PNPase through DNA-PK mediated phosphorylation leading to increased radioresistance (Yu et al., 2012). Finally, nuclear EGFR phosphorylates PCNA which plays an important role in recruiting DNA polymerase, DNA ligase, and other DNA repair proteins (Wang et al., 2006, Stoimenov et al., 2009).
Interestingly, nuclear EGFR also plays a role in resistance to EGFR targeted therapies. Cell lines that develop cetuximab resistance after prolonged exposure are characterized by high levels of nuclear EGFR (Wheeler et al., 2008, Li et al., 2009). Additionally, nuclear EGFR is associated with resistance to gefitinib (Kwak et al., 2005, Nishimura et al., 2008) and this effect can be attenuated by AKT inhibition (Huang et al., 2011). Cells resistant to gefitinib also express high levels of BCRP and other ATP-binding cassette transporters associated with drug resistance (Nakamura et al., 2006, Shi et al., 2009)
Interaction of EGFR with chemotherapy
Extensive effort has been directed towards combining EGFR targeted therapies with chemotherapy. The interaction between chemotherapy and EGFR inhibitors involves several potential mechanisms including attenuation of EGFR signaling pathways, induction of cell cycle arrest, and impairment of DNA repair. Given that the focus of this article is on EGFR targeted agents with radiation and chemoradiation, we will briefly discuss the interaction between EGFR inhibitors and chemotherapy.
Similar to radiation therapy, EGFR signaling is activated in response to chemotherapeutic agents including 5-FU, gemcitabine (Chun et al., 2006), cisplatin (Benhar et al., 2002), and taxanes (Sumitomo et al., 2004). The mechanism behind chemotherapy induced EGFR phosphorylation is not fully understood and is possibly different for each drug class. For example, cisplatin induces nuclear translocation of EGFR (Dittmann et al., 2005). It is postulated that this action leads to chemoresistance through a DNA-PK mediated mechanism (Liccardi et al., 2011). Other reports have demonstrated that cisplatin induced EGFR activation is dependent on Src (Benhar et al., 2002). Also, gefitinib attenuates the repair of cisplatin induced DNA crosslinks (Friedmann et al., 2004, Friedmann et al., 2006) and reduces DNA-PK activity and expression (Magne et al., 2003, Friedmann et al., 2006).
4. Scheduling of EGFR inhibitors with radiation and patient selection
Importance of treatment schedule with EGFR targeted therapies
The optimal dosing schedules of EGFR targeted therapies with chemoradiation or radiation therapy are yet to be determined. The most successful implementation of an EGFR targeted drug and radiation has been in head and neck cancer. In the Bonner trial, cetuximab added to radiation alone improved survival (Bonner et al., 2010). Additionally, multiple studies have demonstrated a benefit when cetuximab or other EGFR targeted therapies are added to chemotherapy. Given these results, it is perplexing why the combination of EGFR inhibitors with chemoradiation has been a failure so far.
Similar to radiation, the effect of EGFR inhibition on the cell cycle plays an important role in the interaction between EGFR directed drugs and chemotherapy. Many chemotherapy agents are more effective in cells that are actively dividing. Given the G1 arrest seen after EGFR inhibition, it is possible EGFR inhibitors are antagonizing the effects of chemotherapy when administered simultaneously. Supporting this point are studies showing that treatment with gemcitabine followed by gefitinib is more cytotoxic than the reverse sequence (Chun et al., 2006). The sequence dependence of chemotherapy and EGFR inhibition has also been demonstrated with other chemotherapy agents including cisplatin and oxaliplatin (Xu et al., 2003, Azzariti et al., 2004, Morelli et al., 2005). Given the lack of efficacy in most of the clinical trials discussed above and the clear sequence dependence seen in preclinical studies, pilot studies using alternative schedules of chemoradiation therapy with EGFR directed therapies are greatly needed prior to investing large amounts of resources into phase II/III clinical trials.
To improve the effectiveness of EGFR directed therapies with chemoradiation both proper patient selection and proper drug scheduling are needed. As discussed above, preclinical studies demonstrate that the sequencing of EGFR inhibitors with chemotherapy and with radiation therapy affect sensitivity and synergy. Given the G1 arrest associated with EGFR inhibition, it would theoretically be more effective if EGFR targeted agents are sequenced after chemotherapy versus before (Morelli et al., 2005). There are several issues with proper scheduling of EGFR inhibitors. First off, the most commonly used drug with radiation, cetuximab, has a long half-life (approximately 97–114 hours). Drugs with shorter half-lives may be needed for proper sequencing with daily radiation treatments. Additionally, it is unclear if the standard dosing of cetuximab is appropriate when combined with concurrent chemoradiation therapy and if this dosing is optimal for each individual due to patient heterogeneity.
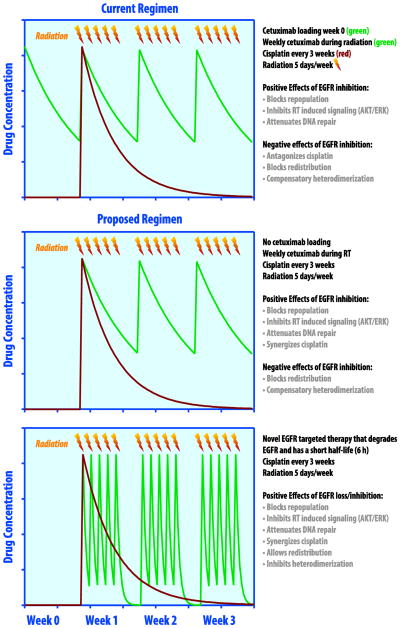
An effective biomarker of EGFR activity would be helpful to confirm appropriate target inhibition. In the Bonner study the development of an acneiform rash was associated with a more favorable response (Bonner et al., 2010). This finding suggests that variable patient factors alter cetuximab’s pharmacokinetics and pharmacodynamics. It is likely that some patients require a higher dose than others to achieve the optimal clinical effect. Potential variations on chemoradiation therapy regimens incorporating an EGFR inhibitor are described in figure 1.
Figure 1.
Scheduling of EGFR targeted therapies with chemoradiation. EGFR inhibitors interact with radiation therapy through attenuation of DNA repair, inhibition of radiation induced pro-survival signaling pathways such as PI3k/AKT and MAPK/ERK, and by blocking tumor repopulation in between radiation fractions (Nyati et al., 2006, Chen et al., 2007, Huang et al., 2000). Top panel, treatment schedule used in RTOG 0522 (Ang et al., 2014). Shown are the concentrations of cetuximab (green) and cisplatin (red) as a function of time over the first 4 weeks of treatment. The loading dose of cetuximab theoretically antagonizes the effect of cisplatin (Morelli et al., 2005) and the long half-life of cetuximab potentially blocks redistribution of tumor cells to a more radiosensitive phase of the cell cycle (Peng et al., 1996, Di Gennaro et al., 2003). Additionally, compensatory heterodimerization of EGFR with another tyrosine kinase (e.g. c-Met, her2) can occur. Middle panel, by eliminating the loading dose of cetuximab the antagonistic effect of EGFR inhibition preceding cisplatin is mitigated. Bottom panel, by using a novel EGFR targeted agent that degrades rather than inhibits EGFR with a short half-life (6 hours) dosed daily after radiation, the negative effects of EGFR inhibition on cell cycle distribution can further be diminished while preserving the beneficial effects of these drugs.
Patient selection for EGFR targeted therapies in combination with chemoradiation
The Bonner Study, RTOG 0617, and the EXPERT-C study demonstrate the importance of patient selection when using EGFR targeted therapies with radiation. In the Bonner Study the benefit of cetuximab was limited to a subset of patients with an oropharynx primary (Bonner et al., 2010). In RTOG 0617 a benefit from cetuximab was only seen in the subset of patients expressing high levels of EGFR (Bradley et al., 2015). Additionally, in the EXPERT-C study patients with KRAS/BRAF wild type rectal cancers benefited from the addition of cetuximab to chemoradiation (Dewdney et al., 2012)
In the advanced stage setting, several predictive biomarkers related to EGFR inhibitors have been discovered. Interestingly, the criteria for predicting response is different for each disease site. In lung cancer, specific EGFR mutations determine sensitivity and resistance to small molecule inhibitors (Pao et al., 2004, Lynch et al., 2004, Paez et al., 2004, Pao et al., 2005). In colorectal cancer, increased EGFR gene copy number may be predictive of response to cetuximab (Moroni et al., 2005). Interestingly, increased gene copy number does not correlate with overexpression (Moroni et al., 2005, Cappuzzo et al., 2008) and neither EGFR mutations nor EGFR overexpression are predictive of response. In colorectal cancer and other malignancies, alterations of several downstream proteins are predictive of resistance to EGFR directed therapies. The most robust is KRAS mutational status (Eberhard et al., 2005, Lievre et al., 2006, De Roock et al., 2008, Amado et al., 2008); however, BRAF, PI3K, or PTEN mutations and c-Met or Her2 overexpression are also associated with anti-EGFR therapy resistance (reviewed in Misale et al., 2014). Almost all of the findings above are from trials with EGFR directed therapies in combination with chemotherapy. It is not clear if the same predictors of response apply in the radiation and chemoradiation settings. The table included in this report lists several of the known patient and tumor related factors associated with sensitivity and resistance to EGFR directed therapies.
Another challenge with EGFR inhibitors is that prolonged treatment with these drugs leads to resistance through several of the mechanisms described above. In nonsmall cell lung cancer, treatment with erlotinib is associated with the development of/selection for cells with a resistant T790M EGFR mutation (Pao et al., 2005). Recently, drugs have been developed to target cells harboring a T790M EGFR mutation. AZD9291 is an irreversible tyrosine kinase inhibitor with selectivity against mutant forms of EGFR (Cross et al., 2014, Finlay et al., 2014). This drug was found to be effective in patients with nonsmall cell lung cancer harboring the T790M EGFR mutation who had progressed on treatment with EGFR small molecule inhibitors (Janne et al., 2015). Interestingly, resistance to AZD9291 develops through another acquired EGFR mutation (C797S), illustrating the complexity and challenges associated with anti-EGFR therapy (Thress et al., 2015).
For EGFR targeted therapies to be successful, both proper patient selection and monitoring for early resistance are needed to optimize efficacy. The development of novel agents which target EGFR by different mechanisms such as receptor degradation and inhibition of dimerization may also be necessary to overcome resistance. Additionally, combinations of EGFR targeted therapies with other novel agents, such as drugs targeting hsp90, may improve the efficacy of these therapies (Ahsan et al. 2013).
Conclusions
Given the important role EGFR plays in several types of cancer and the well-defined role of EGFR in the response to radiation therapy, this receptor remains an important target for radio- and chemoradiosensitization. Next generation EGFR targeted therapies may allow for more control over proper sequencing with chemoradiation therapy. Additionally, agents that target EGFR by novel mechanisms may improve the effectiveness of these approaches. Lastly, discovery of predictive biomarkers related to EGFR targeted radiosensitization will allow for the selection of patients most likely to benefit from this strategy.
Table 1.
| Study | Disease | Treatment arms | N | Results (red indicates statistically significant result) | Toxicity/Notes |
|---|---|---|---|---|---|
| Alabama-Birmingham Bonner et al., 2006, 2010 Phase III |
Head and neck cancer All sites |
|
424 | 3 y locoregional control: 34% RT vs. 47% RT + cetuximab Median OS: 2.4 y RT vs. 4.1 y RT + cetuximab 5 y OS: 36% RT vs. 46% RT + cetuximab |
Grade 2+ rash: 61% with cetuximab No difference in QOL |
| RTOG 0522 Ang et al., 2014 Phase III |
Head and neck cancer All sites |
|
895 | 3 y OS: 73% chemoRT vs. 76% chemoRT + cetuximab 3 y PFS: 61% chemoRT vs. 59% chemoRT + cetuximab 3 y DM: 13% chemoRT vs. 10% chemoRT + cetuximab |
Grade 3–4 mucositis higher with cetuximab More skin toxicity with cetuximab |
| TREMPLIN Lefebvre et al., 2013 Randomized Phase II |
Head and neck cancer Larynx Hypopharynx |
Induction docetaxel/cisplatin, if response:
|
116 | 3 mo larynx preservation: 95% RT + cisplatin vs. 93% RT+ cetuximab 18 mo OS: 92% RT + cisplatin vs. 89% RT+ cetuximab |
Treatment compliance higher in cetuximab arm Similar results with induction chemo followed by RT alone |
| Italian Study Group Ghi et al. 2012, 2013 Randomized Phase II |
Head and neck cancer All sites |
2×2 factorial design
|
421 | Complete response: 36% chemoRT vs. 39% cetuximab-RT Median PFS: 21.6 mochemoRT vs. 20.7 mo cetuximab-RT Median OS: 44.7 mo chemoRT vs. 44.7 mo cetuximab-RT |
Primary endpoint was in field grade 3–4 toxicity: Mucositis 29% chemoRT vs. 23% cetuximab-RT Skin reaction: 11% chemoRT vs. 14% cetuximab-RT |
| CONCERT-1 Mesia et al., 2015 Randomized phase II |
Head and neck cancer All sites |
|
153 | 2 y locoregional control; 68% chemoRT vs. 61% chemoRT+ panitumumab |
Serious toxicity rate: 32% chemoRT vs. 43% chemoRT + panitumumab |
| CONCERT-2 Giralt et al., 2015 Randomized phase II |
Head and neck cancer All sites |
|
152 | 2 y locoregional control: 61% chemoRT vs. 51% panitumumab-RT | Serious toxicity rate: 40% chemoRT vs. 34% panitumumab-RT |
| RTOG 0324 Blumenschein et al., 2011 Phase II |
Nonsmall cell lung cancer | Radiation with carboplatin/paclitaxel + cetuximab | 93 | Response rate: 62% Median OS: 22.7 months 2 y OS: 49% |
Grade 4 hematological toxicity: 22% Grade 3 esophagitis :8%; G3-4 pneumonitis: 7% 5 treatment related deaths |
| CALGB 30407 Govindan et al., 2011 Randomized Phase II |
Nonsmall cell lung cancer |
|
101 | 18 mo OS: 58% chemoRT vs. 54% chemoRT + cetuximab | Grade 3+toxicity: 76% ChemoRT vs. 85% ChemoRT + cetuximab |
| Netherlands Van den Heuvel et al., 2014 Randomized Phase II |
Nonsmall cell lung cancer |
|
102 | Local control: 84% chemoRT vs. 92% chemoRT + cetuximab 1 y OS:82% chemoRT vs. 71% chemoRT + cetuximab |
Toxicity similar between groups |
| RTOG 0617 Bradley et al., 2015 Phase III |
Nonsmall cell lung cancer |
|
544 |
|
Grade 3+toxicity increased with cetuximab: 86% vs. 70% |
| StarPan/STAR-02 Pinto et al., 2011 Phase II |
Rectal cancer | Preoperative radiation with 5-FU/oxaliplatin + panitumumab | 55 | Pathological CR: 21% Pathological downstaging: 58% |
Grade 3–4 diarrhea: 39% |
| US Oncology McCollum et al., 2014 Randomized Phase II |
Rectal cancer |
|
139 | Pathologic CR: 28% chemoRT vs. 27% chemoRT + cetuximab 5 y RFS: 61% chemoRT vs. 64% chemoRT + cetuximab |
Higher grade 3–4 diarrhea with cetuximab: 16% vs. 22% |
| EXPERT-C Dewdney et al., 2012 Randomized Phase II |
Rectal cancer KRAS and BRAF wild-type |
Preoperative chemotherapy followed by:
|
90 |
|
In the entire study population of 160 patients there were no significant differences in all endpoints. Benefit was only seen in KRAS/BRAF wild type tumors. |
| ECOG 2205 Gibson et al., 2010 Phase II |
Esophageal cancer | Preoperative radiation with 5-FU/oxaliplatin + cetuximab followed by postoperative docetaxel and cetuximab | 22 | Pathologic CR: 32% | Four post-operative deaths |
| SWOG 0414 Tomblyn et al., 2012 Phase II |
Esophageal cancer | Definitive radiation with cisplatin/irinotecan + cetuximab | 21 | 2 y OS: 33% 2 y PFS: 24% Response rate: 18% |
Grade 3 toxicity: 48%; Grade 4 toxicity: 29% Two treatment related deaths Non-operative patients |
| HOG G05-92 Becerra et al., 2013 Phase II |
Esophageal cancer | Preoperative radiation + cetuximab | 39 | Pathological CR: 37% | Treatment well tolerated No chemotherapy |
| SCOPE1 Crosby et al., 2013 Randomized Phase II/III |
Esophageal cancer |
|
258 | Failure free at 24 weeks: 77% chemoRT vs. 64% chemoRT + cetuximab Median OS: 25 months chemoRT vs. 21 months chemoRT + cetuximab |
Increased grade 3–4 toxicity with cetuximab: 79% vs. 63% |
| RTOG 0436 Suntharalingam et al., 2014 Phase III |
Esophageal cancer |
|
344 | Clinical CR: 59% chemoRT vs. 56% chemoRT + cetuximab 2 y OS: 42% chemoRT vs. 44% chemoRT + cetuximab |
Grade 4+ toxicity higher with cetuximab: 26% vs. 18% |
Table 2.
| Disease | Factors Associated with Sensitivity | Factors Associated with Resistance |
|---|---|---|
| Head and neck cancer | Accelerated radiation fractionation Acneiform rash (cetuximab) Oropharynx primary |
HPV negative tumor Smoker Non-oropharynx primary |
| Nonsmall cell lung cancer | EGFR mutation: exon 19 and 12 (L858R, L861), exon 18 (G719X, G719), exon 20 (S7681) KRAS wild type EGFR overexpression Non-squamous cell histology Non-smoker Asian heritage Female sex |
EGFR T790M mutation EGFR exon 20 insertion KRAS mutation Squamous cell carcinoma MET amplification/overexpression Epithelial to mesenchymal transition |
| Colorectal cancer | RAS wild type BRAF wild type Increased EGFR gene copy number |
KRAS mutation NRAS mutation BRAF mutation MET amplification/overexpression HER2 amplification/overexpression EGFR mutation in cetuximab binding domain (rare) |
Acknowledgments
This project was partially supported by the University of Michigan GI SPORE Career Development Award.
Abbreviations
- DM
distant metastasis
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- OS
overall survival
- pCR
pathologic complete response
- PFS
progression free survival
Footnotes
Conflicts of Interest Statement
Drs. Lawrence and Nyati hold a patent (application #20120190622) for “Inhbitors of the Epidermal Growth Factor Receptor-Heat Shock 90 Protein Interactions”. They also have a company named Pi Squared Therapeutics related to this patent. The authors have no other conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahsan A, Hiniker SM, Davis MA, Lawrence TS, Nyati MK. Role of cell cycle in epidermal growth factor receptor inhibitor-mediated radiosensitization. Cancer Res. 2009;69:5108–5114. doi: 10.1158/0008-5472.CAN-09-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahsan A, Hiniker SM, Ramanand SG, Nyati S, Hegde A, Helman A, Menawat R, Bhojani MS, Lawrence TS, Nyati MK. Role of epidermal growth factor receptor degradation in cisplatin-induced cytotoxicity in head and neck cancer. Cancer Res. 2010;70:2862–2869. doi: 10.1158/0008-5472.CAN-09-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahsan A, Ray D, Ramanand SG, Hegde A, Whitehead C, Rehemtulla A, Morishima Y, Pratt WB, Osawa Y, Lawrence TS, Nyati MK. Destabilization of the epidermal growth factor receptor (EGFR) by a peptide that inhibits EGFR binding to heat shock protein 90 and receptor dimerization. J Biol Chem. 2013;288:26879–26886. doi: 10.1074/jbc.M113.492280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahsan A, Ramanand SG, Bergin IL, Zhao L, Whitehead CE, Rehemtulla A, Ray D, Pratt WB, Lawrence TS, Nyati MK. Efficacy of an EGFR-specific peptide against EGFR-dependent cancer cell lines and tumor xenografts. Neoplasia. 2014;16:105–114. doi: 10.1593/neo.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akimoto T, Hunter NR, Buchmiller L, Mason K, Ang KK, Milas L. Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin Cancer Res. 1999;5:2884–2890. [PubMed] [Google Scholar]
- 6.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 7.Ang KK, Andratschke NH, Milas L. Epidermal growth factor receptor and response of head-and-neck carcinoma to therapy. Int J Radiat Oncol Biol Phys. 2004;58:959–965. doi: 10.1016/j.ijrobp.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, Fu KK, Milas L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 9.Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, El-Naggar AK, Gillison ML, Jordan RC, Konski AA, Thorstad WL, Trotti A, Beitler JJ, Garden AS, Spanos WJ, Yom SS, Axelrod RS. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azria D, Bibeau F, Barbier N, Zouhair A, Lemanski C, Rouanet P, Ychou M, Senesse P, Ozsahin M, Pelegrin A, Dubois JB, Thezenas S. Prognostic impact of epidermal growth factor receptor (EGFR) expression on loco-regional recurrence after preoperative radiotherapy in rectal cancer. BMC Cancer. 2005;5:62. doi: 10.1186/1471-2407-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azzariti A, Xu JM, Porcelli L, Paradiso A. The schedule-dependent enhanced cytotoxic activity of 7-ethyl-10-hydroxy-camptothecin (SN-38) in combination with Gefitinib (Iressa, ZD1839) Biochem Pharmacol. 2004;68:135–144. doi: 10.1016/j.bcp.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Bandyopadhyay D, Mandal M, Adam L, Mendelsohn J, Kumar R. Physical interaction between epidermal growth factor receptor and DNA-dependent protein kinase in mammalian cells. J Biol Chem. 1998;273:1568–1573. doi: 10.1074/jbc.273.3.1568. [DOI] [PubMed] [Google Scholar]
- 13.Becerra CR, Hanna N, McCollum AD, Becharm N, Timmerman RD, DiMaio M, Kesler KA, Yu M, Yan T, Choy H. A phase II study with cetuximab and radiation therapy for patients with surgically resectable esophageal and GE junction carcinomas: Hoosier Oncology Group G05-92. J Thorac Oncol. 2013;8:1425–1429. doi: 10.1097/JTO.0b013e3182a46c3b. [DOI] [PubMed] [Google Scholar]
- 14.Benhar M, Engelberg D, Levitzki A. Cisplatin-induced activation of the EGF receptor. Oncogene. 2002;21:8723–8731. doi: 10.1038/sj.onc.1205980. [DOI] [PubMed] [Google Scholar]
- 15.Bentzen SM, Harari PM, Bernier J. Exploitable mechanisms for combining drugs with radiation: concepts, achievements and future directions. Nat Clin Pract Oncol. 2007;4:172–180. doi: 10.1038/ncponc0744. [DOI] [PubMed] [Google Scholar]
- 16.Bertolini F, Bengala C, Losi L, Pagano M, Iachetta F, Dealis C, Jovic G, Depenni R, Zironi S, Falchi AM, Luppi G, Conte PF. Prognostic and predictive value of baseline and posttreatment molecular marker expression in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1455–1461. doi: 10.1016/j.ijrobp.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Blumenschein GR, Jr, Paulus R, Curran WJ, Robert F, Fossella F, Werner-Wasik M, Herbst RS, Doescher PO, Choy H, Komaki R. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J Clin Oncol. 2011;29:2312–2318. doi: 10.1200/JCO.2010.31.7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 19.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 20.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, Youssoufian H, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 21.Bonner JA, Maihle NJ, Folven BR, Christianson TJ, Spain K. The interaction of epidermal growth factor and radiation in human head and neck squamous cell carcinoma cell lines with vastly different radiosensitivities. Int J Radiat Oncol Biol Phys. 1994;29:243–247. doi: 10.1016/0360-3016(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 22.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 23.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A, Kavadi V, Garces YI, Narayan S, Iyengar P, Robinson C, Wynn RB, Koprowski C, Meng J, Beitler J, Gaur R, Curran W, Jr, Choy H. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015 doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cappuzzo F, Finocchiaro G, Rossi E, Janne PA, Carnaghi C, Calandri C, Bencardino K, Ligorio C, Ciardiello F, Pressiani T, Destro A, Roncalli M, Crino L, Franklin WA, Santoro A, Varella-Garcia M. EGFR FISH assay predicts for response to cetuximab in chemotherapy refractory colorectal cancer patients. Ann Oncol. 2008;19:717–723. doi: 10.1093/annonc/mdm492. [DOI] [PubMed] [Google Scholar]
- 25.Chen DJ, Nirodi CS. The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage. Clin Cancer Res. 2007;13:6555–6560. doi: 10.1158/1078-0432.CCR-07-1610. [DOI] [PubMed] [Google Scholar]
- 26.Chen SC, Chou CK, Wong FH, Chang CM, Hu CP. Overexpression of epidermal growth factor and insulin-like growth factor-I receptors and autocrine stimulation in human esophageal carcinoma cells. Cancer Res. 1991;51:1898–1903. [PubMed] [Google Scholar]
- 27.Chun PY, Feng FY, Scheurer AM, Davis MA, Lawrence TS, Nyati MK. Synergistic effects of gemcitabine and gefitinib in the treatment of head and neck carcinoma. Cancer Res. 2006;66:981–988. doi: 10.1158/0008-5472.CAN-05-2665. [DOI] [PubMed] [Google Scholar]
- 28.Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, Klimstra DS, Fridman D, Kelsen DP, Saltz LB. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 29.Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958–2970. [PubMed] [Google Scholar]
- 30.Crosby T, Hurt CN, Falk S, Gollins S, Mukherjee S, Staffurth J, Ray R, Bashir N, Bridgewater JA, Geh JI, Cunningham D, Blazeby J, Roy R, Maughan T, Griffiths G. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol. 2013;14:627–637. doi: 10.1016/S1470-2045(13)70136-0. [DOI] [PubMed] [Google Scholar]
- 31.Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ, Hughes G, Rahi A, Jacobs VN, Red Brewer M, Ichihara E, Sun J, Jin H, Ballard P, Al-Kadhimi K, Rowlinson R, Klinowska T, Richmond GH, Cantarini M, Kim DW, Ranson MR, Pao W. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 33.Dassonville O, Formento JL, Francoual M, Ramaioli A, Santini J, Schneider M, Demard F, Milano G. Expression of epidermal growth factor receptor and survival in upper aerodigestive tract cancer. J Clin Oncol. 1993;11:1873–1878. doi: 10.1200/JCO.1993.11.10.1873. [DOI] [PubMed] [Google Scholar]
- 34.De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, Biesmans B, Van Laethem JL, Peeters M, Humblet Y, Van Cutsem E, Tejpar S. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–515. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 35.Debucquoy A, Machiels JP, McBride WH, Haustermans K. Integration of epidermal growth factor receptor inhibitors with preoperative chemoradiation. Clin Cancer Res. 2010;16:2709–2714. doi: 10.1158/1078-0432.CCR-09-1622. [DOI] [PubMed] [Google Scholar]
- 36.Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A, Tait D, Brown G, Wotherspoon A, Gonzalez de Castro D, Chua YJ, Wong R, Barbachano Y, Oates J, Chau I. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) J Clin Oncol. 2012;30:1620–1627. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 37.Di Gennaro E, Barbarino M, Bruzzese F, De Lorenzo S, Caraglia M, Abbruzzese A, Avallone A, Comella P, Caponigro F, Pepe S, Budillon A. Critical role of both p27KIP1 and p21CIP1/WAF1 in the antiproliferative effect of ZD1839 (‘Iressa’), an epidermal growth factor receptor tyrosine kinase inhibitor, in head and neck squamous carcinoma cells. J Cell Physiol. 2003;195:139–150. doi: 10.1002/jcp.10239. [DOI] [PubMed] [Google Scholar]
- 38.Dittmann K, Mayer C, Fehrenbacher B, Schaller M, Kehlbach R, Rodemann HP. Nuclear EGFR shuttling induced by ionizing radiation is regulated by phosphorylation at residue Thr654. FEBS Lett. 2010;584:3878–3884. doi: 10.1016/j.febslet.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Dittmann K, Mayer C, Fehrenbacher B, Schaller M, Raju U, Milas L, Chen DJ, Kehlbach R, Rodemann HP. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005;280:31182–31189. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- 40.Dittmann K, Mayer C, Kehlbach R, Rodemann HP. Radiation-induced caveolin-1 associated EGFR internalization is linked with nuclear EGFR transport and activation of DNA-PK. Mol Cancer. 2008;7:69. doi: 10.1186/1476-4598-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocakova I, Ruff P, Blasinska-Morawiec M, Smakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 42.Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, Ince WL, Janne PA, Januario T, Johnson DH, Klein P, Miller VA, Ostland MA, Ramies DA, Sebisanovic D, Stinson JA, Zhang YR, Seshagiri S, Hillan KJ. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 43.Eriksen JG, Steiniche T, Askaa J, Alsner J, Overgaard J. The prognostic value of epidermal growth factor receptor is related to tumor differentiation and the overall treatment time of radiotherapy in squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys. 2004;58:561–566. doi: 10.1016/j.ijrobp.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 44.Feng FY, Lopez CA, Normolle DP, Varambally S, Li X, Chun PY, Davis MA, Lawrence TS, Nyati MK. Effect of epidermal growth factor receptor inhibitor class in the treatment of head and neck cancer with concurrent radiochemotherapy in vivo. Clin Cancer Res. 2007;13:2512–2518. doi: 10.1158/1078-0432.CCR-06-2582. [DOI] [PubMed] [Google Scholar]
- 45.Finlay MR, Anderton M, Ashton S, Ballard P, Bethel PA, Box MR, Bradbury RH, Brown SJ, Butterworth S, Campbell A, Chorley C, Colclough N, Cross DA, Currie GS, Grist M, Hassall L, Hill GB, James D, James M, Kemmitt P, Klinowska T, Lamont G, Lamont SG, Martin N, McFarland HL, Mellor MJ, Orme JP, Perkins D, Perkins P, Richmond G, Smith P, Ward RA, Waring MJ, Whittaker D, Wells S, Wrigley GL. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistance mutations that spares the wild type form of the receptor. J Med Chem. 2014;57:8249–8267. doi: 10.1021/jm500973a. [DOI] [PubMed] [Google Scholar]
- 46.Fracasso PM, Burris H, 3rd, Arquette MA, Govindan R, Gao F, Wright LP, Goodner SA, Greco FA, Jones SF, Willcut N, Chodkiewicz C, Pathak A, Springett GM, Simon GR, Sullivan DM, Marcelpoil R, Mayfield SD, Mauro D, Garrett CR. A phase 1 escalating single-dose and weekly fixed-dose study of cetuximab: pharmacokinetic and pharmacodynamic rationale for dosing. Clin Cancer Res. 2007;13:986–993. doi: 10.1158/1078-0432.CCR-06-1542. [DOI] [PubMed] [Google Scholar]
- 47.Friedmann B, Caplin M, Hartley JA, Hochhauser D. Modulation of DNA repair in vitro after treatment with chemotherapeutic agents by the epidermal growth factor receptor inhibitor gefitinib (ZD1839) Clin Cancer Res. 2004;10:6476–6486. doi: 10.1158/1078-0432.CCR-04-0586. [DOI] [PubMed] [Google Scholar]
- 48.Friedmann BJ, Caplin M, Savic B, Shah T, Lord CJ, Ashworth A, Hartley JA, Hochhauser D. Interaction of the epidermal growth factor receptor and the DNA-dependent protein kinase pathway following gefitinib treatment. Mol Cancer Ther. 2006;5:209–218. doi: 10.1158/1535-7163.MCT-05-0239. [DOI] [PubMed] [Google Scholar]
- 49.Ghi MG, Paccagnella A, Ferrari D, Foa P, Nole F, Morelli F, Azzarello G, D’Ambrosio C, Casanova C, Guaraldi M, Mantovani G, Rossetto C, Bonetti A, Siena S, Crino L, Buffoli A, Koussis H, Pieri G, Gava A, Floriani I. Cetuximab/radiotherapy (CET+RT) versus concomitant chemoradiotherapy (cCHT+RT) with or without induction docetaxel/cisplatin/5-fluorouracil (TPF) in locally advanced head and neck squamous cell carcinoma (LASCCHN): Preliminary results on toxicity of a randomized, 2×2 factorial, phase II–III study ( NCT01086826) J Clin Oncol. 2012;30(suppl):abstr 5513. [Google Scholar]
- 50.Ghi MG, Paccagnella A, Ferrari D, Foa P, Rocca MC, Verri E, Maiello E, Azzarello G, D’Ambrosio C, Casanova C, Guaraldi M, Mantovani G, Rossetto C, Bonetti A, Cipani T, Crino L, Koussis H, Pieri G, Gava A, Floriani I. A phase II–III study comparing concomitant chemoradiotherapy (CRT) versus cetuximab/RT (CET/RT) with or without induction docetaxel/cisplatin/5-fluorouracil (TPF) in locally advanced head and neck squamous cell carcinoma (LASCCHN): Efficacy results ( NCT01086826) J Clin Oncol. 2013;31(suppl):abstr 6003. [Google Scholar]
- 51.Gibson MK, Catalano PJ, Kleinberg L, et al. E2205: A phase II study to measure response rate and toxicity of neoadjuvant chemoradiotherapy (CRT) with oxaliplatin (OX) and infusional 5-fluorouracil (5-FU) plus cetuximab (C) followed by postoperative docetaxel (DT) and C in patients with operable adenocarcinoma of the esophagus. J Clin Oncol. 2010;28(suppl):abstr 4064316s. doi: 10.1634/theoncologist.2018-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giralt J, de las Heras M, Cerezo L, Eraso A, Hermosilla E, Velez D, Lujan J, Espin E, Rosello J, Majo J, Benavente S, Armengol M, de Torres I Grupo Espanol de Investigacion Clinica en Oncologia R. The expression of epidermal growth factor receptor results in a worse prognosis for patients with rectal cancer treated with preoperative radiotherapy: a multicenter, retrospective analysis. Radiother Oncol. 2005;74:101–108. doi: 10.1016/j.radonc.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 53.Giralt J, Trigo J, Nuyts S, Ozsahin M, Skladowski K, Hatoum G, Daisne JF, Yunes Ancona AC, Cmelak A, Mesia R, Zhang A, Oliner KS, VanderWalde A. Panitumumab plus radiotherapy versus chemoradiotherapy in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-2): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015;16:221–232. doi: 10.1016/S1470-2045(14)71200-8. [DOI] [PubMed] [Google Scholar]
- 54.Govindan R, Bogart J, Stinchcombe T, Wang X, Hodgson L, Kratzke R, Garst J, Brotherton T, Vokes EE. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J Clin Oncol. 2011;29:3120–3125. doi: 10.1200/JCO.2010.33.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579–3584. [PubMed] [Google Scholar]
- 56.Grandis JR, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, Drenning SD, Tweardy DJ. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 57.Gullick WJ, Downward J, Parker PJ, Whittle N, Kris R, Schlessinger J, Ullrich A, Waterfield MD. The structure and function of the epidermal growth factor receptor studied by using antisynthetic peptide antibodies. Proc R Soc Lond B Biol Sci. 1985;226:127–134. doi: 10.1098/rspb.1985.0087. [DOI] [PubMed] [Google Scholar]
- 58.Gupta AK, McKenna WG, Weber CN, Feldman MD, Goldsmith JD, Mick R, Machtay M, Rosenthal DI, Bakanauskas VJ, Cerniglia GJ, Bernhard EJ, Weber RS, Muschel RJ. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin Cancer Res. 2002;8:885–892. [PubMed] [Google Scholar]
- 59.Hadzisejdic I, Mustac E, Jonjic N, Petkovic M, Grahovac B. Nuclear EGFR in ductal invasive breast cancer: correlation with cyclin-D1 and prognosis. Mod Pathol. 2010;23:392–403. doi: 10.1038/modpathol.2009.166. [DOI] [PubMed] [Google Scholar]
- 60.Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- 61.Harari PM, Huang SM. Epidermal growth factor receptor modulation of radiation response: preclinical and clinical development. Semin Radiat Oncol. 2002;12:21–26. doi: 10.1053/srao.2002.34865. [DOI] [PubMed] [Google Scholar]
- 62.Hebbar M, Wacrenier A, Desauw C, Romano O, Cattan S, Triboulet JP, Pruvot FR. Lack of usefulness of epidermal growth factor receptor expression determination for cetuximab therapy in patients with colorectal cancer. Anticancer Drugs. 2006;17:855–857. doi: 10.1097/01.cad.0000217425.44584.9f. [DOI] [PubMed] [Google Scholar]
- 63.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 64.Herbst RS, Bunn PA., Jr Targeting the epidermal growth factor receptor in non-small cell lung cancer. Clin Cancer Res. 2003;9:5813–5824. [PubMed] [Google Scholar]
- 65.Hoshino M, Fukui H, Ono Y, Sekikawa A, Ichikawa K, Tomita S, Imai Y, Imura J, Hiraishi H, Fujimori T. Nuclear expression of phosphorylated EGFR is associated with poor prognosis of patients with esophageal squamous cell carcinoma. Pathobiology. 2007;74:15–21. doi: 10.1159/000101047. [DOI] [PubMed] [Google Scholar]
- 66.Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–1940. [PubMed] [Google Scholar]
- 67.Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000;6:2166–2174. [PubMed] [Google Scholar]
- 68.Huang WC, Chen YJ, Li LY, Wei YL, Hsu SC, Tsai SL, Chiu PC, Huang WP, Wang YN, Chen CH, Chang WC, Chang WC, Chen AJ, Tsai CH, Hung MC. Nuclear translocation of epidermal growth factor receptor by Akt-dependent phosphorylation enhances breast cancer-resistant protein expression in gefitinib-resistant cells. J Biol Chem. 2011;286:20558–20568. doi: 10.1074/jbc.M111.240796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hung LY, Tseng JT, Lee YC, Xia W, Wang YN, Wu ML, Chuang YH, Lai CH, Chang WC. Nuclear epidermal growth factor receptor (EGFR) interacts with signal transducer and activator of transcription 5 (STAT5) in activating Aurora-A gene expression. Nucleic Acids Res. 2008;36:4337–4351. doi: 10.1093/nar/gkn417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huo L, Wang YN, Xia W, Hsu SC, Lai CC, Li LY, Chang WC, Wang Y, Hsu MC, Yu YL, Huang TH, Ding Q, Chen CH, Tsai CH, Hung MC. RNA helicase A is a DNA-binding partner for EGFR-mediated transcriptional activation in the nucleus. Proc Natl Acad Sci U S A. 2010;107:16125–16130. doi: 10.1073/pnas.1000743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jaganathan S, Yue P, Paladino DC, Bogdanovic J, Huo Q, Turkson J. A functional nuclear epidermal growth factor receptor, SRC and Stat3 heteromeric complex in pancreatic cancer cells. PLoS One. 2011;6:e19605. doi: 10.1371/journal.pone.0019605. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, Haggstrom D, Felip E, Kim JH, Frewer P, Cantarini M, Brown KH, Dickinson PA, Ghiorghiu S, Ranson M. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 73.Kim JS, Kim JM, Li S, Yoon WH, Song KS, Kim KH, Yeo SG, Nam JS, Cho MJ. Epidermal growth factor receptor as a predictor of tumor downstaging in locally advanced rectal cancer patients treated with preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:195–200. doi: 10.1016/j.ijrobp.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 74.Kim SY, Shim EK, Yeo HY, Baek JY, Hong YS, Kim DY, Kim TW, Kim JH, Im SA, Jung KH, Chang HJ. KRAS mutation status and clinical outcome of preoperative chemoradiation with cetuximab in locally advanced rectal cancer: a pooled analysis of 2 phase II trials. Int J Radiat Oncol Biol Phys. 2013;85:201–207. doi: 10.1016/j.ijrobp.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 75.Kirisits A, Pils D, Krainer M. Epidermal growth factor receptor degradation: an alternative view of oncogenic pathways. Int J Biochem Cell Biol. 2007;39:2173–2182. doi: 10.1016/j.biocel.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 76.Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, Brannigan BW, Harris PL, Driscoll DR, Fidias P, Lynch TJ, Rabindran SK, McGinnis JP, Wissner A, Sharma SV, Isselbacher KJ, Settleman J, Haber DA. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwok TT, Sutherland RM. Enhancement of sensitivity of human squamous carcinoma cells to radiation by epidermal growth factor. J Natl Cancer Inst. 1989;81:1020–1024. doi: 10.1093/jnci/81.13.1020. [DOI] [PubMed] [Google Scholar]
- 78.Lammering G, Valerie K, Lin PS, Mikkelsen RB, Contessa JN, Feden JP, Farnsworth J, Dent P, Schmidt-Ullrich RK. Radiosensitization of malignant glioma cells through overexpression of dominant-negative epidermal growth factor receptor. Clin Cancer Res. 2001;7:682–690. [PubMed] [Google Scholar]
- 79.Lefebvre JL, Pointreau Y, Rolland F, Alfonsi M, Baudoux A, Sire C, de Raucourt D, Malard O, Degardin M, Tuchais C, Blot E, Rives M, Reyt E, Tourani JM, Geoffrois L, Peyrade F, Guichard F, Chevalier D, Babin E, Lang P, Janot F, Calais G, Garaud P, Bardet E. Induction chemotherapy followed by either chemoradiotherapy or bioradiotherapy for larynx preservation: the TREMPLIN randomized phase II study. J Clin Oncol. 2013;31:853–859. doi: 10.1200/JCO.2012.42.3988. [DOI] [PubMed] [Google Scholar]
- 80.Li C, Iida M, Dunn EF, Ghia AJ, Wheeler DL. Nuclear EGFR contributes to acquired resistance to cetuximab. Oncogene. 2009;28:3801–3813. doi: 10.1038/onc.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li S, Kim JS, Kim JM, Cho MJ, Yoon WH, Song KS, Yeo SG, Kim JS. Epidermal growth factor receptor as a prognostic factor in locally advanced rectal-cancer patients treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys. 2006;65:705–712. doi: 10.1016/j.ijrobp.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 82.Liang K, Ang KK, Milas L, Hunter N, Fan Z. The epidermal growth factor receptor mediates radioresistance. Int J Radiat Oncol Biol Phys. 2003;57:246–254. doi: 10.1016/s0360-3016(03)00511-x. [DOI] [PubMed] [Google Scholar]
- 83.Liccardi G, Hartley JA, Hochhauser D. EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treatment. Cancer Res. 2011;71:1103–1114. doi: 10.1158/0008-5472.CAN-10-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M, Rougier P, Penault-Llorca F, Laurent-Puig P. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 85.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 86.Linggi B, Carpenter G. ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol. 2006;16:649–656. doi: 10.1016/j.tcb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 87.Lo HW, Cao X, Zhu H, Ali-Osman F. Cyclooxygenase-2 is a novel transcriptional target of the nuclear EGFR-STAT3 and EGFRvIII-STAT3 signaling axes. Mol Cancer Res. 2010;8:232–245. doi: 10.1158/1541-7786.MCR-09-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia W, Wei Y, Bartholomeusz G, Shih JY, Hung MC. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7:575–589. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 89.Lo HW, Xia W, Wei Y, Ali-Seyed M, Huang SF, Hung MC. Novel prognostic value of nuclear epidermal growth factor receptor in breast cancer. Cancer Res. 2005;65:338–348. [PubMed] [Google Scholar]
- 90.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 91.Magne N, Fischel JL, Tiffon C, Formento P, Dubreuil A, Renee N, Formento JL, Francoual M, Ciccolini J, Etienne MC, Milano G. Molecular mechanisms underlying the interaction between ZD1839 (‘Iressa’) and cisplatin/5-fluorouracil. Br J Cancer. 2003;89:585–592. doi: 10.1038/sj.bjc.6601131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCollum AD, Kocs DM, Chadha P, Monticelli MA, Boyd TE, Fain JD, Kasper M, Sanchez J, Simon M, Singh P, Thummala A, Vukelja SJ, Wang Y, Asmar L, Richards DA. Randomized phase II trial of preoperative chemoradiotherapy with or without cetuximab in locally advanced rectal adenocarcinoma. J Clin Oncol. 2014;32(suppl 3):abstr 537. [Google Scholar]
- 93.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 94.Mesia R, Henke M, Fortin A, Minn H, Yunes Ancona AC, Cmelak A, Markowitz AB, Hotte SJ, Singh S, Chan AT, Merlano MC, Skladowski K, Zhang A, Oliner KS, VanderWalde A, Giralt J. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015;16:208–220. doi: 10.1016/S1470-2045(14)71198-2. [DOI] [PubMed] [Google Scholar]
- 95.Milas L, Fan Z, Andratschke NH, Ang KK. Epidermal growth factor receptor and tumor response to radiation: in vivo preclinical studies. Int J Radiat Oncol Biol Phys. 2004;58:966–971. doi: 10.1016/j.ijrobp.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 96.Milas L, Mason KA, Ang KK. Epidermal growth factor receptor and its inhibition in radiotherapy: in vivo findings. Int J Radiat Biol. 2003;79:539–545. doi: 10.1080/0955300031000114747. [DOI] [PubMed] [Google Scholar]
- 97.Misale S, Di Nicolantonio F, Sartore-Bianchi A, Siena S, Bardelli A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov. 2014;4:1269–1280. doi: 10.1158/2159-8290.CD-14-0462. [DOI] [PubMed] [Google Scholar]
- 98.Miyaguchi M, Olofsson J, Hellquist HB. Expression of epidermal growth factor receptor in glottic carcinoma and its relation to recurrence after radiotherapy. Clin Otolaryngol Allied Sci. 1991;16:466–469. doi: 10.1111/j.1365-2273.1991.tb01041.x. [DOI] [PubMed] [Google Scholar]
- 99.Morelli MP, Cascone T, Troiani T, De Vita F, Orditura M, Laus G, Eckhardt SG, Pepe S, Tortora G, Ciardiello F. Sequence-dependent antiproliferative effects of cytotoxic drugs and epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16(Suppl 4):iv61–68. doi: 10.1093/annonc/mdi910. [DOI] [PubMed] [Google Scholar]
- 100.Morgan MA, Parsels LA, Maybaum J, Lawrence TS. Improving the efficacy of chemoradiation with targeted agents. Cancer Discov. 2014;4:280–291. doi: 10.1158/2159-8290.CD-13-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moroni M, Veronese S, Benvenuti S, Marrapese G, Sartore-Bianchi A, Di Nicolantonio F, Gambacorta M, Siena S, Bardelli A. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol. 2005;6:279–286. doi: 10.1016/S1470-2045(05)70102-9. [DOI] [PubMed] [Google Scholar]
- 102.Morris ZS, Harari PM. Interaction of radiation therapy with molecular targeted agents. J Clin Oncol. 2014;32:2886–2893. doi: 10.1200/JCO.2014.55.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mueller KL, Yang ZQ, Haddad R, Ethier SP, Boerner JL. EGFR/Met association regulates EGFR TKI resistance in breast cancer. J Mol Signal. 2010;5:8. doi: 10.1186/1750-2187-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mukaida H, Toi M, Hirai T, Yamashita Y, Toge T. Clinical significance of the expression of epidermal growth factor and its receptor in esophageal cancer. Cancer. 1991;68:142–148. doi: 10.1002/1097-0142(19910701)68:1<142::aid-cncr2820680126>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 105.Nakamura Y, Oka M, Soda H, Shiozawa K, Yoshikawa M, Itoh A, Ikegami Y, Tsurutani J, Nakatomi K, Kitazaki T, Doi S, Yoshida H, Kohno S. Gefitinib (“Iressa”, ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, reverses breast cancer resistance protein/ABCG2-mediated drug resistance. Cancer Res. 2005;65:1541–1546. doi: 10.1158/0008-5472.CAN-03-2417. [DOI] [PubMed] [Google Scholar]
- 106.Nishimura Y, Yoshioka K, Bereczky B, Itoh K. Evidence for efficient phosphorylation of EGFR and rapid endocytosis of phosphorylated EGFR via the early/late endocytic pathway in a gefitinib-sensitive non-small cell lung cancer cell line. Mol Cancer. 2008;7:42. doi: 10.1186/1476-4598-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nishinaka T, Yabe-Nishimura C. EGF receptor-ERK pathway is the major signaling pathway that mediates upregulation of aldose reductase expression under oxidative stress. Free Radic Biol Med. 2001;31:205–216. doi: 10.1016/s0891-5849(01)00571-8. [DOI] [PubMed] [Google Scholar]
- 108.Nyati MK, Morgan MA, Feng FY, Lawrence TS. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer. 2006;6:876–885. doi: 10.1038/nrc1953. [DOI] [PubMed] [Google Scholar]
- 109.Oliva JL, Griner EM, Kazanietz MG. PKC isozymes and diacylglycerol-regulated proteins as effectors of growth factor receptors. Growth Factors. 2005;23:245–252. doi: 10.1080/08977190500366043. [DOI] [PubMed] [Google Scholar]
- 110.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 111.Paliga A, Onerheim R, Gologan A, Chong G, Spatz A, Niazi T, Garant A, Macheto D, Alcindor T, Vuong T. EGFR and K-ras gene mutation status in squamous cell anal carcinoma: a role for concurrent radiation and EGFR inhibitors? Br J Cancer. 2012;107:1864–1868. doi: 10.1038/bjc.2012.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peng D, Fan Z, Lu Y, DeBlasio T, Scher H, Mendelsohn J. Anti-epidermal growth factor receptor monoclonal antibody 225 up-regulates p27KIP1 and induces G1 arrest in prostatic cancer cell line DU145. Cancer Res. 1996;56:3666–3669. [PubMed] [Google Scholar]
- 115.Peter RU, Beetz A, Ried C, Michel G, van Beuningen D, Ruzicka T. Increased expression of the epidermal growth factor receptor in human epidermal keratinocytes after exposure to ionizing radiation. Radiat Res. 1993;136:65–70. [PubMed] [Google Scholar]
- 116.Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 117.Pinto C, Di Fabio F, Maiello E, Pini S, Latiano T, Aschele C, Garufi C, Bochicchio A, Rosati G, Aprile G, Giaquinta S, Torri V, Bardelli A, Gion M, Martoni A. Phase II study of panitumumab, oxaliplatin, 5-fluorouracil, and concurrent radiotherapy as preoperative treatment in high-risk locally advanced rectal cancer patients (StarPan/STAR-02 Study) Ann Oncol. 2011;22:2424–2430. doi: 10.1093/annonc/mdq782. [DOI] [PubMed] [Google Scholar]
- 118.Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, Vynnychenko I, Park K, Yu CT, Ganul V, Roh JK, Bajetta E, O’Byrne K, de Marinis F, Eberhardt W, Goddemeier T, Emig M, Gatzemeier U, Team FS. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 119.Psyrri A, Egleston B, Weinberger P, Yu Z, Kowalski D, Sasaki C, Haffty B, Rimm D, Burtness B. Correlates and determinants of nuclear epidermal growth factor receptor content in an oropharyngeal cancer tissue microarray. Cancer Epidemiol Biomarkers Prev. 2008;17:1486–1492. doi: 10.1158/1055-9965.EPI-07-2684. [DOI] [PubMed] [Google Scholar]
- 120.Psyrri A, Yu Z, Weinberger PM, Sasaki C, Haffty B, Camp R, Rimm D, Burtness BA. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin Cancer Res. 2005;11:5856–5862. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- 121.Rauch J, Volinsky N, Romano D, Kolch W. The secret life of kinases: functions beyond catalysis. Cell Commun Signal. 2011;9:23. doi: 10.1186/1478-811X-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Robert F, Ezekiel MP, Spencer SA, Meredith RF, Bonner JA, Khazaeli MB, Saleh MN, Carey D, LoBuglio AF, Wheeler RH, Cooper MR, Waksal HW. Phase I study of anti--epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:3234–3243. doi: 10.1200/JCO.2001.19.13.3234. [DOI] [PubMed] [Google Scholar]
- 123.Ruhstaller T, Pless M, Dietrich D, Kranzbuehler H, von Moos R, Moosmann P, Montemurro M, Schneider PM, Rauch D, Gautschi O, Mingrone W, Widmer L, Inauen R, Brauchli P, Hess V. Cetuximab in combination with chemoradiotherapy before surgery in patients with resectable, locally advanced esophageal carcinoma: a prospective, multicenter phase IB/II Trial (SAKK 75/06) J Clin Oncol. 2011;29:626–631. doi: 10.1200/JCO.2010.31.9715. [DOI] [PubMed] [Google Scholar]
- 124.Safran H, Suntharalingam M, Dipetrillo T, Ng T, Doyle LA, Krasna M, Plette A, Evans D, Wanebo H, Akerman P, Spector J, Kennedy N, Kennedy T. Cetuximab with concurrent chemoradiation for esophagogastric cancer: assessment of toxicity. Int J Radiat Oncol Biol Phys. 2008;70:391–395. doi: 10.1016/j.ijrobp.2007.07.2325. [DOI] [PubMed] [Google Scholar]
- 125.Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanagh BD, Contessa JN, Rorrer WK, Chen PB. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–1197. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- 126.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 127.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L National Cancer Institute of Canada Clinical Trials G. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 128.Sheridan MT, O’Dwyer T, Seymour CB, Mothersill CE. Potential indicators of radiosensitivity in squamous cell carcinoma of the head and neck. Radiat Oncol Investig. 1997;5:180–186. doi: 10.1002/(SICI)1520-6823(1997)5:4<180::AID-ROI3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 129.Shewach DS, Lawrence TS. Antimetabolite radiosensitizers. J Clin Oncol. 2007;25:4043–4050. doi: 10.1200/JCO.2007.11.5287. [DOI] [PubMed] [Google Scholar]
- 130.Shi Z, Parmar S, Peng XX, Shen T, Robey RW, Bates SE, Fu LW, Shao Y, Chen YM, Zang F, Chen ZS. The epidermal growth factor tyrosine kinase inhibitor AG1478 and erlotinib reverse ABCG2-mediated drug resistance. Oncol Rep. 2009;21:483–489. [PMC free article] [PubMed] [Google Scholar]
- 131.Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, Lenz HJ, Borg C, Middleton G, Kroning H, Luppi G, Kisker O, Zubel A, Langer C, Kopit J, Burris HA., 3rd EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–2319. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 132.Soonthornthum T, Arias-Pulido H, Joste N, Lomo L, Muller C, Rutledge T, Verschraegen C. Epidermal growth factor receptor as a biomarker for cervical cancer. Ann Oncol. 2011;22:2166–2178. doi: 10.1093/annonc/mdq723. [DOI] [PubMed] [Google Scholar]
- 133.Steel GG. Terminology in the description of drug-radiation interactions. Int J Radiat Oncol Biol Phys. 1979;5:1145–1150. doi: 10.1016/0360-3016(79)90634-5. [DOI] [PubMed] [Google Scholar]
- 134.Stoimenov I, Helleday T. PCNA on the crossroad of cancer. Biochem Soc Trans. 2009;37:605–613. doi: 10.1042/BST0370605. [DOI] [PubMed] [Google Scholar]
- 135.Sumitomo M, Asano T, Asakuma J, Asano T, Horiguchi A, Hayakawa M. ZD1839 modulates paclitaxel response in renal cancer by blocking paclitaxel-induced activation of the epidermal growth factor receptor-extracellular signal-regulated kinase pathway. Clin Cancer Res. 2004;10:794–801. doi: 10.1158/1078-0432.ccr-0948-03. [DOI] [PubMed] [Google Scholar]
- 136.Suntharalingam M, Winter K, Ilson DH, Dicker A, Kachnic LA, Konski AA, Chakravarthy B, Gaffney DK, Thakrar HV, Horiba MN, Deutsch M, Kavadi V, Raben A, Roof KS, Videtic GMM, Pollock J, Safran H, Crane CH. The initial report of RTOG 0436: A phase III trial evaluating the addition of cetuximab to paclitaxel, cisplatin, and radiation for patients with esophageal cancer treated without surgery. J Clin Oncol. 2014;32(suppl 3):abstr LBA6. [Google Scholar]
- 137.Thress KS, Paweletz CP, Felip E, Cho BC, Stetson D, Dougherty B, Lai Z, Markovets A, Vivancos A, Kuang Y, Ercan D, Matthews SE, Cantarini M, Barrett JC, Janne PA, Oxnard GR. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med. 2015;21:560–562. doi: 10.1038/nm.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tomblyn MB, Goldman BH, Thomas CR, Jr, Benedetti JK, Lenz HJ, Mehta V, Beeker T, Gold PJ, Abbruzzese JL, Blanke CD, Committee SG. Cetuximab plus cisplatin, irinotecan, and thoracic radiotherapy as definitive treatment for locally advanced, unresectable esophageal cancer: a phase-II study of the SWOG (S0414) J Thorac Oncol. 2012;7:906–912. doi: 10.1097/JTO.0b013e31824c7bed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Uberall I, Kolar Z, Trojanec R, Berkovcova J, Hajduch M. The status and role of ErbB receptors in human cancer. Exp Mol Pathol. 2008;84:79–89. doi: 10.1016/j.yexmp.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 140.Valentini V, De Paoli A, Gambacorta MA, Mantini G, Ratto C, Vecchio FM, Barbaro B, Innocente R, Rossi C, Boz G, Barba MC, Frattegiani A, Lupattelli M, Doglietto GB. Infusional 5-fluorouracil and ZD1839 (Gefitinib-Iressa) in combination with preoperative radiotherapy in patients with locally advanced rectal cancer: a phase I and II Trial (1839IL/0092) Int J Radiat Oncol Biol Phys. 2008;72:644–649. doi: 10.1016/j.ijrobp.2008.01.046. [DOI] [PubMed] [Google Scholar]
- 141.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 142.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 143.van den Heuvel MM, Uyterlinde W, Vincent AD, de Jong J, Aerts J, Koppe F, Knegjens J, Codrington H, Kunst PW, Dieleman E, Verheij M, Belderbos J. Additional weekly Cetuximab to concurrent chemoradiotherapy in locally advanced non-small cell lung carcinoma: efficacy and safety outcomes of a randomized, multi-center phase II study investigating. Radiother Oncol. 2014;110:126–131. doi: 10.1016/j.radonc.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 144.Wang SC, Hung MC. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin Cancer Res. 2009;15:6484–6489. doi: 10.1158/1078-0432.CCR-08-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang SC, Nakajima Y, Yu YL, Xia W, Chen CT, Yang CC, McIntush EW, Li LY, Hawke DH, Kobayashi R, Hung MC. Tyrosine phosphorylation controls PCNA function through protein stability. Nat Cell Biol. 2006;8:1359–1368. doi: 10.1038/ncb1501. [DOI] [PubMed] [Google Scholar]
- 146.Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, Gondi V, Hsu KT, Harari PM. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–3956. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xia W, Wei Y, Du Y, Liu J, Chang B, Yu YL, Huo LF, Miller S, Hung MC. Nuclear expression of epidermal growth factor receptor is a novel prognostic value in patients with ovarian cancer. Mol Carcinog. 2009;48:610–617. doi: 10.1002/mc.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu JM, Azzariti A, Severino M, Lu B, Colucci G, Paradiso A. Characterization of sequence-dependent synergy between ZD1839 (“Iressa”) and oxaliplatin. Biochem Pharmacol. 2003;66:551–563. doi: 10.1016/s0006-2952(03)00291-0. [DOI] [PubMed] [Google Scholar]
- 149.Yu YL, Chou RH, Wu CH, Wang YN, Chang WJ, Tseng YJ, Chang WC, Lai CC, Lee HJ, Huo L, Chen CH, Hung MC. Nuclear EGFR suppresses ribonuclease activity of polynucleotide phosphorylase through DNAPK-mediated phosphorylation at serine 776. J Biol Chem. 2012;287:31015–31026. doi: 10.1074/jbc.M112.358077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhu A, Shaeffer J, Leslie S, Kolm P, El-Mahdi AM. Epidermal growth factor receptor: an independent predictor of survival in astrocytic tumors given definitive irradiation. Int J Radiat Oncol Biol Phys. 1996;34:809–815. doi: 10.1016/0360-3016(95)02184-1. [DOI] [PubMed] [Google Scholar]
- 151.Zhuang S, Ouedraogo GD, Kochevar IE. Downregulation of epidermal growth factor receptor signaling by singlet oxygen through activation of caspase-3 and protein phosphatases. Oncogene. 2003;22:4413–4424. doi: 10.1038/sj.onc.1206604. [DOI] [PubMed] [Google Scholar]
- 152.Zlobec I, Vuong T, Compton CC, Lugli A, Michel RP, Hayashi S, Jass JR. Combined analysis of VEGF and EGFR predicts complete tumour response in rectal cancer treated with preoperative radiotherapy. Br J Cancer. 2008;98:450–456. doi: 10.1038/sj.bjc.6604172. [DOI] [PMC free article] [PubMed] [Google Scholar]