Summary
Increased activity of T follicular helper (Tfh) cells plays a major pathogenic role in systemic lupus erythematosus (SLE). However, the mechanisms that cause aberrant Tfh cell responses in SLE remain elusive. Here we showed the OX40 ligand (OX40L)-OX40 axis contributes to the aberrant Tfh response in SLE. OX40L was expressed by myeloid antigen-presenting cells (APCs), but not B cells, in blood and in inflamed tissues in adult and pediatric SLE patients. The frequency of circulating OX40L-expressing myeloid APCs positively correlated with disease activity and the frequency of ICOS+ blood Tfh cells in SLE. OX40 signals promoted naïve and memory CD4+ T cells to express multiple Tfh cell molecules, and were sufficient to induce them to become functional B cell helpers. Immune complexes containing RNA induced OX40L expression on myeloid APCs via TLR7 activation. Our study provides a rationale to target the OX40L-OX40 axis as a therapeutic modality for SLE.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic systemic inflammatory autoimmune disease characterized by a breakdown of tolerance to nuclear antigens (Tsokos, 2011). A more comprehensive understanding of SLE pathogenesis is long overdue; in the past 50 years, only one new drug has been approved for SLE treatment (Murphy et al., 2013; Stohl et al., 2012). Genome-wide association studies (GWAS) have identified many susceptibility loci for SLE, confirming that SLE patients display predisposing genetic factors (Cunninghame Graham et al., 2008; Delgado-Vega et al., 2009). Such predisposing genetic factors affect the immune system in particular when challenged with environmental factors, and alter the functions of antigen presenting cells (APCs) and lymphocytes in SLE patients. APCs including dendritic cells (DCs) are aberrantly activated in SLE patients, and promote the activation of autoreactive T and B cells (Blanco et al., 2001; Blanco et al., 2008). The developed autoreactive plasma cells produce pathogenic autoantibodies directed against nuclear components and cause tissue injury.
Studies with murine models have demonstrated that T follicular helper cells (Tfh), a CD4+ helper T (Th) cell subset specialized for provision of help to B cells, play a major pathogenic role in lupus (Crotty, 2014; Ueno et al., 2015). Tfh cells are essential for the formation of germinal centers (GCs), the site for the selection of high-affinity B cells and for the development of B cell memory (Vinuesa and Cyster, 2011). Tfh cells are equipped with multiple features required for B cell help. IL-21 secreted by Tfh cells and their precursors (Bentebibel et al., 2011; Bryant et al., 2007) potently promotes the growth, differentiation, and class-switching of B cells (Tangye et al., 2013). Inducible co stimulator (ICOS) is highly expressed by GC Tfh cells and mediates the interaction with B cells (Crotty, 2014). CD40 ligand (CD40L) expressed by Tfh cells provides signals to B cells through CD40 for their differentiation and class-switching (Ueno et al., 2015). The importance of these Tfh molecules in lupus pathogenesis is underscored by the observations in lupus mouse models where inhibition of the function of CD40L (Boumpas et al., 2003; Kalled et al., 1998), ICOS (Odegard et al., 2008), IL-21 and/or IL-21 receptor (Bubier et al., 2009; Herber et al., 2007) delays the disease course and/or improves the clinical symptoms. Furthermore, inhibition of the generation of Tfh cells in lupus prone sanroque mice by deleting SAP molecule abrogates the development of renal pathology (Linterman et al., 2009). These studies provide a strong rationale that inhibition of the generation and/or activity of Tfh cells is beneficial for the prevention of lupus disease from subjects with susceptible loci and/or for the treatment of lupus patients.
In human SLE, a majority of IgG class autoantibody-producing B cells are somatically mutated (Tiller et al., 2007), suggesting that they are derived from GCs through interactions with Tfh cells. The frequency of blood Tfh cells with active phenotype is increased in active SLE patients (He et al., 2013; Simpson et al., 2010). Furthermore, Tfh cells are also found in T-cell and B-cell aggregates and ectopic germinal centers in kidneys of patients with lupus nephritis (Chang et al., 2011; Liarski et al., 2014). These observations support the pathogenic role of Tfh cells in human SLE. However, the mechanisms involved in increased Tfh response in SLE patients remains unknown.
Here we show that the OX40 ligand (OX40L)-OX40 axis contributes to the aberrant Tfh cell response in SLE. OX40L was expressed by myeloid APCs, but not by B cells, in blood of adult and pediatric active SLE patients. In inflamed tissues of SLE patients, OX40L was expressed by various types of cells including myeloid APCs, but not B cells. OX40L stimulation induced human Th cells to express Tfh cell-associated molecules, and was sufficient to induce them to become functional B cell helpers. Finally, we show that immune complexes (ICs) containing ribonucleoprotein (RNP) present in lupus sera induce OX40L expression by myeloid APCs through activation of TLR7. Thus, our study shows that the RNP IC-OX40L axis likely provides an amplification loop of the generation of autoantibodies in SLE.
RESULTS
OX40L is abundantly expressed in inflamed tonsils
We previously demonstrated that dermal CD14+ DCs preferentially induce the generation of Tfh-like cells in vitro (Klechevsky et al., 2008). CD206+ DCs in the lymph nodes, a proposed counterpart of migrating dermal CD14+ DCs, also appear to share this property (Segura et al., 2012). While these observations suggest the involvement of dermal CD14+ DCs in the generation of Tfh cells in draining lymph nodes of skin, the phenotype of APCs associated with Tfh responses in inflammatory lymphoid organs such as tonsils has not been determined.
Previous studies in mouse models demonstrated the importance of ICOS ligand (ICOSL) expressed by DCs for the differentiation of Tfh cells (Choi et al., 2011). We analyzed whether myeloid APCs (CD11c+HLA-DR+) express ICOSL in pediatric tonsils that are enriched with mature Tfh cells along with GCs (Bentebibel et al., 2011). Consistent with a previous report (Aicher et al., 2000), ICOSL was not expressed at detectable levels on either CD11c+ APCs or B cells (Fig. 1A, B, and not shown). Staining of tonsil tissue sections with three different anti-ICOSL clones or with an ICOSIg chimera protein also failed to detect ICOSL+ cells (not shown). As low ICOSL expression might be due to chronic interactions with ICOS+ cells at sites (Witsch et al., 2002), we examined the expression of ICOSL transcripts by RNA-FISH in frozen human tonsil tissues. We found that ICOSL transcript was expressed within GCs at higher density than outside GCs (fig. S1). The expression of ICOSL transcript was largely consistent with the expression of CD19 transcript, suggesting that the major source of ICOSL transcripts was B cells (fig. S1).
Fig. 1. Increased OX40L expression by myeloid APCs in inflammatory tonsils.
(A) Expression of OX40L, ICOSL, 4-1BBL and GITRL on myeloid CD11c+HLA-DR+ APCs from pediatric tonsils. A representative result out of 9 independent experiments.
(B) Frequency of OX40L+, ICOSL+, GITRL+, and 4-1BBL+ cells within tonsillar myeloid APCs. Mean ± s.d., n=9. One way ANOVA. *** p<0.001.
(C) OX40L+CD11c+ APCs in inflammatory tonsils. GC: germinal center; MZ: mantle zone; Epi: Epithelial layers. The scale bars on the top and the bottom panels shows 100 μm and 10 μm, respectively.
We found instead that CD11c+HLA-DR+ myeloid APCs, in particular CD14+ cells, expressed the co-stimulatory molecule OX40L (9.3 ± 7.1% of CD11c+HLA-DR+ cells, Mean ± s.d., n=9. Fig. 1A). Other TNF ligand family molecules such as GITRL and 4-1BBL were undetectable or expressed only minimally (Fig. 1A, B). OX40L expression by myeloid APCs was nearly absent in spleen (0.3 ± 0.5% of CD11c+HLA-DR+ cells, Mean ± s.d., n=4), where Tfh and GC responses are much less evident than in pediatric tonsils (Bentebibel et al., 2013). This suggests that among secondary lymphoid organs, the presence of OX40L+ myeloid APCs is limited to those with strong inflammatory response.
To determine the localization, tonsil tissues were stained with anti-OX40L and anti-CD11c and analyzed by immunofluorescence microscopy. We found that OX40L was abundantly expressed in tonsils, in particular subepithelial area, T cell zones, and mantle zones; but less in GCs (Fig. 1C). OX40L+ CD11c+ myeloid APCs were mainly found in the T cell zone (Fig. 1C). The frequency of OX40L+ cells among myeloid APCs (CD11c+) was 20.5 ± 10.4 % (Mean ± s.d., n=6. Range 10.7-35.5%). RNA-FISH analysis also demonstrated the presence of cells expressing both CD14 and OX40L transcript mainly in T cell zone (fig. S1). Consistent with the fact that OX40L can be expressed by a broad range of immune cells including B cells, vascular endothelial cells, mast cells, activated NK cells, and activated Th cells (Croft, 2010), OX40L was also expressed by CD11c- cells, including B cells. The frequency of OX40L+ B cells largely varied among tonsil tissues and among GCs in a given tonsil tissue (20.2 ± 15.4 % (Mean ± s.d.), n=11. Range 2.3-51.6%). Our observation suggests that inflammatory environment induces upregulation of OX40L expression on multiple types of cells.
Myeloid APCs from active SLE patients express OX40L
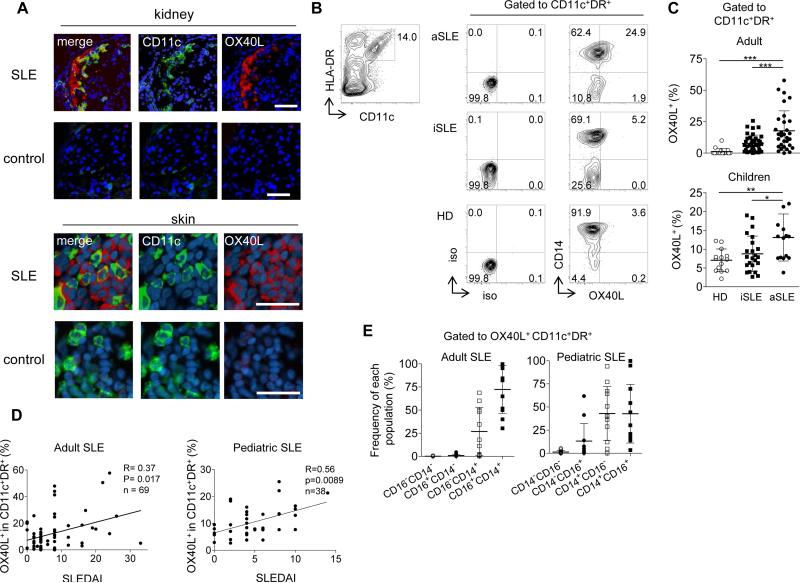
Given prominent expression of OX40L in inflamed tonsils, we wondered whether OX40L was also expressed in inflammatory tissues from SLE patients. We found that OX40L was abundantly expressed by CD11c+ myeloid APCs in inflammatory kidney tissues from active adult SLE patients with nephritis, but absent in tissues from subjects without autoimmune diseases (Fig. 2A). OX40L+ myeloid APCs were also found in skin biopsy samples from SLE patients, but not from controls (Fig. 2A). ICOSL expression was not detected by any cells (not shown). Similar to tonsils, OX40L+ CD11c- cells were also present in both tissues from SLE patients, but no OX40L+ B cells were found in any tissues (fig. S2A)
Fig. 2. OX40L expression by myeloid APCs from SLE patients.
(A) OX40L+ myeloid APCs in skin and kidney biopsies from adult SLE patients and subjects without autoimmune diseases. A representative result of 5 skin and 3 kidney biopsy samples from SLE patients and 5 skin and 2 kidney biopsy samples from controls. Scale bar=100 μm.
(B) Representative flow data on OX40L expression by blood myeloid CD11c+HLA-DR+ APCs from the three groups: healthy donors (HD), inactive (iSLE) and active (aSLE) SLE patients.
(C) Frequency of OX40L+ cells within blood myeloid APCs in the three groups in adult and pediatric cohorts. Top: the adult cohort; 16 HD, 38 iSLE, and 31 aSLE samples. Bottom: the children cohort; 14 HD, 20 iSLE, and 14 aSLE samples. One-way ANOVA. * p <0.05, ** p <0.01, *** p <0.001.
(D) Correlation between the percentage of OX40L+ cells within CD11c+HLA-DR+ myeloid APCs (adults: n=69 and children: n=38) and disease activity assessed by the SLEDAI. Statistical analysis was performed with the Spearman test.
(E) Composition of blood OX40L+ myeloid APCs by different subsets (CD14+CD16-, CD14+CD16+, CD14-CD16-, CD14-CD16+) in adult (n=28) and pediatric (n=34) SLE patients. Mean ± s.d.
We next analyzed whether peripheral myeloid APCs in patients with SLE also express OX40L. OX40L expression was significantly increased on the surface of blood myeloid APCs from adult and pediatric patients with active SLE compared to healthy subjects, inactive SLE patients, and other autoimmune disease patients (Fig. 2B, and fig. S2B). Similar to tonsillar myeloid APCs (Fig. 1A, B), we did not observe the expression of ICOSL, GITRL, or 4-1BBL on blood myeloid APCs (fig. S2C, D). To determine whether OX40L expression was also increased on B cells in SLE patients, we analyzed the expression of OX40L on blood CD11c+ APCs and B cells side-by-side by including markers in a same staining panel. Both in adult and pediatric SLE blood samples, OX40L expression by B cells was minimal, and significantly lower than CD11c+ APCs (Percentage of OX40L+ cells in CD11c+ APCs and B cells: 11.0 ± 2.5% vs. 0.6 ± 0.3% in adult SLE (Mean ± s.e.m., n=19, p<0.0001 by paired t-test), and 10.5 ± 1.3% vs. 2.7 ± 0.6% in pediatric SLE (n=28, p<0.0001). fig. S2E).
The percentage of OX40L+ myeloid APCs in blood was significantly higher in active patients (assessed by the SLE Disease Activity Index (SLEDAI)) than in inactive patients, both in adult and pediatric SLE (Fig. 2C). Furthermore, the frequency of OX40L+ cells within myeloid APCs correlated with disease activity as assessed by the SLEDAI in both adult and pediatric SLE (Fig. 2D). OX40L was mainly expressed by CD14+CD16+ and CD14+CD16- monocytes in blood (Fig. 2E, and fig. S2F). In a longitudinal follow-up of 10 flaring and previously untreated adult SLE patients, the percentage of OX40L+ myeloid APC substantially decreased after treatment along with the decrease in disease activity (fig. S2G, p<0.01). Taken together, these results show that OX40L is expressed on blood and tissue-infiltrating myeloid APCs, but not on B cells, in active SLE patients.
OX40 signals promote the expression of Tfh genes in naïve and memory T cells
The presence of OX40L+ myeloid APCs in blood and inflamed tissues suggests that OX40L expression is globally increased on myeloid APCs in active SLE patients. In particular, inflamed tissues in SLE patients appear to create an OX40L-rich environment where Th cells receive OX40 signals from multiple cell sources (Fig. 2A). While being important for proliferation and survival, OX40 signals also regulate the differentiation of Th cells in collaboration with other factors derived from APCs, microenvironment, and Th cells themselves (Croft, 2010). We hypothesized that OX40 signals might display an intrinsic property to promote the differentiation of human Th cells towards the Tfh lineage. To address this hypothesis, we applied an APC-free system to avoid the contribution of factors from APCs and microenvironment, and cultured naïve and memory Th cells with anti-CD3 and anti-CD28 in the presence of agonistic soluble OX40L (sOX40L). To minimize the influence of T cell-intrinsic factors, we analyzed the gene expression profiles at 48 h of culture by NanoString. We found that OX40 signaling upregulated multiple Tfh genes, including CXCR5, BCL6, IL21, CXCL13, and PDCD1 (encoding PD-1) in both naïve and memory Th cells (Fig. 3A). Furthermore, OX40L stimulation downregulated the expression of PRDM1 (encoding Blimp-1), the transcription repressor that inhibits Tfh generation (Crotty, 2014).
Fig. 3. OX40 signals induce upregulation of Tfh genes.
(A) Tfh gene expression by naïve and memory Th cells (from three donors) activated with anti-CD3 and anti-CD28 in the presence or absence of sOX40L for 48 h. Transcript counts in the cultured Th cells are shown after normalization to housekeeping genes. Mean ± s.d., n=3. Paired t-test. * p <0.05, ** p <0.01.
(B) Tfh gene expression profiles by naïve and memory Th cells activated with anti-CD3 and anti-CD28 in the presence of indicated reagents for 48 h. Transcript counts in Th cells cultured in the presence of the indicated reagents were normalized to those in control Th cells in each donor.
(C) Transcript counts in memory Th cells activated with anti-CD3 and anti-CD28 in the presence of indicated reagents. Mean ± s.d., n=3. One-way ANOVA. * p <0.05, ** p <0.01, *** p <0.001.
Previously we and others show that IL-12 induces activated human naïve Th cells to express multiple Tfh molecules including IL-21, ICOS, CXCR5, and Bcl-6 at higher levels than other cytokines (Schmitt et al., 2013; Schmitt et al., 2014; Schmitt et al., 2009). Subjects deficient of IL-12 receptor β1 (IL-12Rβ1) chain display reduced Tfh and GC responses (in particular children), providing in vivo evidence that signals via IL-12 receptor is essential for the generation of Tfh cell differentiation in humans (Schmitt et al., 2013). Thus, we compared the expression of Tfh genes between OX40- and IL-12-stimulated Th cells. To our surprise, OX40 signals induced naïve Th cells to express multiple Tfh genes at equivalent levels with IL-12 signals (fig. S3). Furthermore, overall expression patterns of Tfh genes were similar between OX40- and IL-12-stimulated naive Th cells (Fig. 3B, left). While mouse studies suggest the positive role of IFN–γ for the generation of Tfh cells (Lee et al., 2012), the upregulation of Tfh molecules in these cells was not due to IFN–γ secreted in the cultures, as IFN-γ-stimulated naïve Th cells did not show the similar gene patterns (Fig. 3B, left). The combination of the two signals further increased the expression of IL21, but not other Tfh molecules (fig. S3).
Importantly, in contrast to the observation with naïve Th cells, OX40 signals were more potent than IL-12 signals at inducing memory Th cells to upregulate Tfh genes (BCL6, CXCR5, IL-21, CXCL13, and PDCD1) and to downregulate PRDM1 (Fig. 3B, 3C). It was notable that OX40 signals differentially modulated the expression of MAF and BATF, genes associated with Tfh development and functions (Crotty, 2014), between naïve and memory Th cells. OX40 signals induced upregulation of the two genes in naïve Th cells, but downregulation in memory Th cells (Fig. 3B). Nonetheless, IL-12 signals cooperated with OX40 signals to increase the expression of CXCR5 and IL21 by memory Th cells (Fig. 3C).
OX40 signals promote the generation of functional helpers
To analyze the expression of Tfh molecules at protein levels, naïve and memory Th cells were activated by anti-CD3 and anti-CD28 in the presence or absence of sOX40L for 3 days, and the phenotype was analyzed by flow cytometry. Consistent with transcriptional data (Fig. 3A, B), OX40 signals promoted both naïve and memory Th cells to express Tfh molecules including CXCR5, CD40L, and IL-21, and increased the generation of CXCR5+ cells co-expressing IL-21, CD40L,ICOS, and Bcl-6 (Fig. 4A and 4B; fig. S4A). Of note, in addition to IL-21, OX40 signals induced the expression of IL-2 and TNF-α, but not IFN-γ or IL-4 (fig. S4B) despite increased T-bet expression (Fig. 4A and 4B). OX40 signals also weakly increased the expression of RORγt, but did not induce IL-17A expression (fig. S4C). OX40L stimulation also induced naïve Th cells to downregulate the expression of CCR7 on CXCR5+ cells (Fig. S4A), and increased the generation of CXCR5+CCR7- cells, a chemokine receptor expression profile required for homing to B cell follicles (Haynes et al., 2007). This was not due to an enhanced expression of achaete-scute homologue 2 (Ascl2), the transcription factor important for initiation of the murine Tfh cell development (Liu et al., 2014), because Ascl2 transcript expression was completely absent in any culture conditions (not shown).
Fig. 4. OX40L stimulation promotes the differentiation of naïve and memory T cells into Tfh-like cells.
(A) Expression of CXCR5, IL-21, CD40L, Bcl-6, and T-bet by naïve and memory Th cells activated with anti-CD3 and anti-CD28 in the presence or absence of sOX40L and/or IL-12. Gated to FSChiSSChi activated cells. A representative result out of 3 independent experiments is shown.
(B) Frequency of CXCR5+IL-21+,CXCR5+CD40L+, CXCR5+Bcl-6+,and CXCR5+T-bet+ cells developed in naïve or memory Th cells after activation with anti-CD3 and anti-CD28 in the presence or absence of sOX40L and/or IL-12. One-way ANOVA. * p<0.05, ** p<0.01, *** p<0.001, n=3.
(C) Naïve or memory Th cells were activated for 4 days with anti-CD3 and anti-CD28 in the presence of sOX40L and/or IL-12, and then cultured with autologous memory B cells. IgG concentrations in the supernatant of each well are shown. A representative result out of 2 independent experiments is shown.
Strikingly, OX40 signals induced memory Th cells to express Tfh molecules including CXCR5, CD40L, and IL-21 more efficiently than IL-12 signals (Fig. 4B). We noticed that OX40 signals decreased the expression of ICOS on memory Th cells compared to the control culture (fig. S4A), which was consistent with the transcriptional data (Fig. 3B). However, ICOS expression levels remained high, and more than 80% of CXCR5+ cells stimulated with OX40 signals expressed ICOS.
We wondered whether OX40 signals are sufficient to induce Th cells to become functional helpers. To this end, stimulated Th cells were co-cultured with autologous B cells and the produced IgG were measured at day 14. OX40 signals were sufficient to induce both naïve and memory Th cells to become B cell helpers (Fig. 4C). Notably, OX40 signals were more efficient than IL-12 signals to induce memory Th cells to become helpers (Fig. 4C). These results show that OX40L stimulation promotes naïve and memory Th cells to differentiate into Tfh-like cells.
Collectively, these results show that OX40 signals display an intrinsic property to induce human naïve and memory Th cells to express multiple Tfh molecules and to become functional B cell helpers.
OX40 signals promote the expression of Tfh molecules by enhancing TCR signals
OX40 signals activate canonical and non-canonical NF-κB pathways (Croft, 2010). To determine the mechanism by which OX40 signals promote the expression of Tfh molecules, we first analyzed whether inhibition of either canonical or non-canonical NF-κB pathway affects the expression of Tfh molecules by OX40-stimulated human naïve Th cells. We inhibited the expression of NF-κB signaling molecules by transfecting specific siRNA, including NF-κB1, RelA (included in canonical pathway), NF-κB2, RelB (involved in non-canonical pathway). As a previous mouse study demonstrated that TRAF6 was essential for OX40 signals to promote the generation of Th9 cells (Xiao et al., 2012), we also tested the role of TRAF6. We confirmed that siRNA transfection substantially inhibited the expression of the target protein (fig. S5A). Naive Th cells transfected siRNA were stimulated with CD3-CD28 mAbs in the presence or absence of sOX40L, and the expression of Tfh molecules were analyzed by flow cytometry.
Blocking molecules of canonical (NF-κB1, RelA) and non-canonical (NF-κB2) pathways weakly but significantly inhibited the Bcl-6 expression by OX40-stimulated CD4+ T cells (Fig. 5A). However, this did not appear to depend on OX40 signals, as a similar trend was observed with the Th cells cultured in the control condition (no sOX40L). Similarly, while inhibition of NF-κB2 upregulated the expression of CXCR5 and IL-21 and downregulated the expression of CD40L and ICOS, this was independent of OX40 signals (fig. S5B). These results suggest that while NF-κB pathway can regulate the expression of Tfh molecules, this may not be the dominant one by which OX40 signals induce naïve Th cells to upregulate Tfh molecules.
Fig. 5. Strong TCR stimulation induce naïve Th cells to express Tfh molecules.
(A) Bcl-6 expression by naïve Th cells transfected with the indicated siRNA and cultured for 3 days with anti-CD3 and CD28 ± sOX40L. Gated to FSChiSSChi activated cells. Mean ± s.e.m., n=3.
(B) Expression of the indicated markers by naïve Th cells activated for 4 days with the indicated number of anti-CD3 and anti-CD28-coated beads. Gated to FSChiSSChi activated cells. A representative result out of 3 independent experiments is shown.
Recent studies demonstrate that the strength and the duration of signals through T cell receptor (TCR) play a major role in determining the fate of primed Th cells (Tubo et al., 2013; van Panhuys et al., 2014). In this regard, strong and durable TCR signals promote Th differentiation towards the Tfh lineage and their proliferation (Deenick et al., 2010; Fazilleau et al., 2009; Tubo et al., 2013). It is known that OX40 signals augment TCR signals via the PI3K-Akt pathway (So et al., 2011). Furthermore, a recent study shows that blood memory Th cells in pediatric SLE patients constitutively express higher levels of phosphorylated Akt, and OX40 signals further enhance Akt activation of these cells (Kshirsagar et al., 2013). Therefore, it is possible that OX40 signals promote the expression of Tfh molecules by enhancing TCR signals. However, whether strong TCR signals promote human Th cells to express Tfh molecules or not remains unknown. Therefore, we stimulated naïve Th cells with titrated numbers of anti-CD3 and anti-CD28-coated beads and analyzed the expression of Tfh molecules. We found that the stimulation with anti-CD3-CD28 beads promoted the expression of multiple Tfh molecules including CXCR5, IL-21, CD40L, and Bcl-6 in a dose-dependent manner (Fig. 5B). Stronger TCR signals also increased OX40 expression. In contrast, the expression of IFN-γ, IL-4, and IL-17A was not modified by the number of anti-CD3-CD28 beads (fig. S5C). Thus, strong TCR signals promote human naïve Th cells to express multiple Tfh molecules, but not other Th molecules.
Collectively, these results suggest that OX40 signals promote the expression of Tfh molecules by enhancing TCR signals.
The frequency of OX40L+ APCs correlates with that of ICOS+CXCR5+Tfh cells in blood
Previous studies showed that active SLE patients display an increased frequency of blood Tfh cells with active phenotype (ICOS+CXCR5+) (He et al., 2013; Simpson et al., 2010). We were able to confirm this observation in our cohort (Fig. 6A). Importantly, we found that the frequency of ICOS+ cells within blood Tfh cells positively correlated with the frequency of OX40L+ cells within blood myeloid APCs (Fig. 6B). The frequency of OX40L+ APCs also positively correlated with the frequency of blood Tfh cells (CXCR5+ in total Th cells) (fig. S6A), but showed no correlation with the frequency of blood CXCR5- Th1 (CXCR3+CCR6-), Th2 (CXCR3-CCR6-) Th17 (CXCR3-CCR6+) cells (Morita et al., 2011) (fig. S6B). These results suggest that OX40L-expressing myeloid APCs from SLE patients promote the development and/or the activation of Tfh cells.
Fig. 6. The frequency of OX40L+ myeloid APCs correlates with the frequency of ICOS+ blood Tfh cells in human SLE.
(A) Expression of ICOS on blood Tfh cells in the three groups; aSLE, iSLE, and HD. A representative flow result is shown.
(B) Correlation between the frequency of OX40L+ cells within blood myeloid APCs and the frequency of ICOS+ cells within blood Tfh cells in SLE patients. Spearman correlation test, n=19.
RNP-anti-RNP ICs promote OX40L expression through TLR7 activation
We wondered which mechanism is involved in OX40L expression by myeloid APCs in active SLE patients. We previously demonstrated that SLE sera induce monocytes to acquire the properties of DCs (Blanco et al., 2001). Therefore, we hypothesized that SLE sera might contain components that induce OX40L expression by myeloid APCs. Accordingly, we found that SLE sera, but not control sera, induced OX40L expression on healthy donor monocytes at variable levels (Fig. 7A). Upon co-culture with allogeneic naïve Th cells, monocytes exposed to SLE sera promoted the expression of IL-21 in a manner partly dependent on OX40L (fig. S7A, B). We suspected the involvement of immune complexes (ICs) containing self nucleic acid, because activation of APCs through endosomal nucleic acid sensors play a key role in SLE pathogenesis (Barrat and Coffman, 2008). Indeed, stimulation with agonist of TLR7, but not TLR9 and 3, induced healthy donor monocytes to express OX40L (Fig. 7B). Of note, B cells did not express OX40L in response to stimulation with any of these TLR ligands (Fig. S7C and not shown). While previous mouse and human studies show that CD40 signal induces DCs to upregulate OX40L expression (Fillatreau and Gray, 2003; Murata et al., 2000; Ohshima et al., 1997), CD40 signal by itself was insufficient to induce monocytes to express OX40L (fig. S7D). To test whether TLR7 was directly implicated in OX40L upregulation by SLE sera, we exposed monocytes to SLE sera in the presence of a specific TLR7 inhibitor IRS-661 (Barrat et al., 2005) or RNase. Both TLR7 inhibitor and RNase significantly reduced the ability of SLE sera to induce OX40L expression (Fig. 7C; fig S7E), suggesting the major role by ICs containing RNA. In agreement with this hypothesis, we observed that the presence of anti-ribonucleoprotein (RNP), but not anti-DNA, antibodies in SLE sera was associated with the increased ability to promote OX40L expression on monocytes (Fig. 7D).
Fig. 7. RNP-anti-RNP ICs promote OX40L expression by myeloid APCs in a TLR7-dependent manner.
(A) Expression of OX40L (MFI) by purified healthy donor monocytes exposed to control sera (n=7) or SLE sera (n=21). Mann-Whitney U-test. ** p <0.01. A representative staining is shown on the left panel.
(B) OX40L expression upon stimulation of purified healthy donor monocytes by TLR3, TLR7 or TLR9 agonists. A representative staining out of 4 different experiments is shown.
(C) Fold change in OX40L expression (MFI) in healthy donor monocytes exposed to SLE sera (n=7) in the presence or not of a TLR7 inhibitor. Paired t-test. **** p <0.0001. A representative staining is shown on the left panel.
(D) OX40L expression (MFI) in healthy donor monocytes exposed to anti-RNPneg SLE sera (n=5) or anti-RNPpos SLE sera (n=16). Mann-Whitney U-test. ** p <0.01.
(E) OX40L expression of purified healthy donor monocytes exposed to anti-RNPneg SLE serum, the serum supplemented with anti-RNP-containing IgG, the serum spiked with anti-RNP-containing IgG in the presence of a TLR7 inhibitor. A representative staining out of three independent experiments is shown.
To validate whether RNP-anti-RNP ICs were directly involved in OX40L expression, healthy donor monocytes were cultured with anti-RNP negative SLE sera, and purified IgG containing RNP-anti-RNP ICs were spiked into the cultures. We found that the supplementation with RNP-anti-RNP ICs rendered anti-RNP negative SLE sera able to promote OX40L expression (Fig. 7E). This effect was dependent on TLR7, as addition of TLR7-specific inhibitor abrogated the upregulation of OX40L (Fig. 7E).
These data show that RNP-anti-RNP ICs promote OX40L expression through TLR7 activation in myeloid APCs in active SLE.
DISCUSSION
Autoreactive antibody production is a hallmark of a variety of autoimmune diseases including SLE. Our study provides evidence that the OX40L-OX40 axis contributes to lupus pathogenesis by promoting the generation of Tfh cells.
The expression of OX40L by myeloid APCs, but not B cells, was increased in blood in active SLE patients. OX40L+ myeloid APCs in blood of active SLE patients were largely confined to CD14+CD16- and CD14+CD16+ monocyte populations. OX40L+ myeloid APCs in pediatric tonsils were also largely limited to the CD14+ population. Increased OX40L expression on blood monocyte populations was also reported in patients with sepsis (Karulf et al., 2010) and patients with chronic hepatitis C (Zhang et al., 2013). Interestingly, both disease conditions are known to be often associated with hyper gammaglobulinemia. These observations suggest that monocytes and macrophages upregulate OX40L in inflammatory environment, and contribute to antibody responses in humans. Furthermore, various types of cells upregulate OX40L expression in tonsils and inflammatory tissues of SLE patients, and therefore might also provide OX40 signals to T cells.
The pathogenic roles of ICs containing self nucleic acid are well established in SLE. The ICs activate plasmacytoid DCs via TLR9 and TLR7, and induce them to produce large amounts of type I interferons (Lovgren et al., 2006). Type I IFN induces neutrophils to upregulate TLR7, and renders them able to respond to RNP-anti-RNP ICs. Then neutrophils produce DNA-containing components that activate pDCs (Garcia-Romo et al., 2011; Lande et al., 2011). RNP-anti-RNP ICs also target the CD16+CD14dim monocyte population, and induce these cells to produce cytokines that damage the endothelium, including TNF–α, IL-1, and CCL3 (Cros et al., 2010). While these mechanisms involve the activation of the innate immune system and consequent inflammation, our study shows that RNP-anti-RNP ICs also activate the adaptive immune system. We found that RNP-anti-RNP ICs contribute to OX40L expression by monocytes and macrophages via TLR7. Tfh responses increased by the RNP-anti-RNP IC - OX40L axis further accelerate the generation of autoantibodies including those against self nucleic acid. Therefore, the RNP-anti-RNP IC - OX40L axis appear to provide an amplification loop of the generation of autoantibodies in SLE.
We showed that OX40 signals together with TCR and CD28 signals promote naïve and memory Th cells to express multiple Tfh molecules, including CXCR5, IL-21, and Bcl-6. Remarkably, OX40 signals were more potent than IL-12 signals to induce memory Th cells to express Tfh molecules, and were sufficient to render them to become efficient B cell helpers. These results show that OX40 signals display intrinsic property to promote Th differentiation towards the Tfh lineage in humans. Our study further suggests that this property is mainly mediated by an enhancement of TCR signals rather than that of the NF-κB pathway. In this line, strong TCR signals induced human naïve Th cells to express multiple Tfh molecules including CXCR5, IL-21, CD40L, and Bcl-6, but not other Th molecules. Thus, the OX40L-OX40 axis contributes to Tfh development in a manner independent of cytokine signals that activate STAT3 and STAT4 (Schmitt et al., 2014). These two mechanisms likely cooperate, because human Th cells stimulated with both OX40 signals and IL-12 signals further upregulated IL-21 expression.
While previous mouse studies demonstrated a fundamental role of ICOSL-expressing DCs for the development of Tfh cells (Choi et al., 2011), to our surprise, we were not able to detect myeloid APCs or B cells highly expressing ICOSL in inflamed tonsils or SLE samples. Nonetheless, considering that ICOS deficiency in humans results in absence of mature Tfh cells and GCs (Bossaller et al., 2006), we do not argue the contribution of ICOSL+ APCs for Tfh cell development in humans or for lupus pathogenesis. Our study rather highlights the additional contribution of OX40L+ DCs to the development of Tfh response in humans. Whether, when, and how OX40 signals and ICOS signals contribute to the development and/or maintenance of aberrant Tfh response in human autoimmune diseases remain to be addressed. Nonetheless, given that both OX40 signals and ICOS signals enhance the PI3K-Akt pathway which plays an important role for the expression of IL-21 (Gigoux et al., 2009), these two pathways might cooperate and/or be complementary to each other for the development of Tfh cells. Importantly, in contrast to ICOS deficiency, a recent report shows that human OX40 deficiency seems to have intact Tfh and antibody responses (Byun et al., 2013). This suggests that OX40 signals are not essential for Tfh cell development or sufficient to compensate ICOS deficiency in vivo in humans. Thus, we surmise that OX40 signals cause aberrant Tfh response and autoimmunity in humans only when excessive. The positive correlation between the frequency of ICOS+ blood Tfh cells and the frequency of OX40L+ myeloid APCs in active SLE patients supports this hypothesis.
Mouse models so far provided mixed results regarding the role of the OX40-OX40L axis on the regulation of Tfh cell responses. Early studies showed that OX40L stimulation promotes mouse naïve Th cells to express CXCR5 (Flynn et al., 1998), and their migration into B cell follicles (Brocker et al., 1999; Fillatreau and Gray, 2003). Furthermore, an OX40L-transgenic mice model (T cell-specific overexpression) showed development an autoimmune-like disease characterized by interstitial pneumonia, colitis, and high levels of anti-nuclear antibodies (Murata et al., 2002). Recent studies show that the mutation of Roquin gene in sanroque mice causes upregulation of OX40 on Th cells, suggesting the positive role of OX40 signals for the generation of Tfh cells (Pratama et al., 2013; Vogel et al., 2013). On the other hand, at least two studies concluded that the absence of OX40 signals did not affect CXCR5 expression by Th cells, Tfh differentiation, GC development, or antibody generation (Akiba et al., 2005; Kopf et al., 1999). Furthermore, in vivo treatment with agonistic OX40 mAb inhibited Tfh cell generation in mice in an acute viral infection model (Boettler et al., 2013) and in a listeria infection model (Marriott et al., 2014). Boettler et al. showed that agonistic anti-OX40 mAb enhanced the expression of Blimp-1 by specific Th cells while suppressing the expression of Bcl-6 in vivo (Boettler et al., 2013), contrary to our observations with human Th cells in vitro. Given that OX40 signals regulate Th differentiation in collaboration with other factors derived from APCs, microenvironment, and Th cells themselves (Croft, 2010), it is possible that OX40 signals promote or suppress Tfh cell differentiation according to the microenvironment where Th cells interact with APCs. For example, in an acute viral infection model, it is possible that OX40 signals enhanced Blimp-1 expression due to the co-presence of Type I interferons, which strongly promote Blimp-1 expression (Schmitt et al., 2014). It is yet possible that OX40 signals differentially modulate the expression of Tfh molecules between human and mouse Th cells.
Our conclusion is also supported by the findings in GWAS in autoimmune diseases. TNFSF4 (encoding OX40L) polymorphism has been found to confer susceptibility to SLE (Cunninghame Graham et al., 2008; Delgado-Vega et al., 2009) and other autoimmune diseases, such as Sjögren syndrome, and rheumatoid arthritis (Kim et al., 2015; Nordmark et al., 2011). Furthermore, copy number variations and/or polymorphism at the TLR7 locus has been shown to associate with SLE susceptibility (Shen et al., 2010). Our study provides a rationale that therapeutic modalities targeting the RNP-containing IC-OX40L-OX40 axis and TLR7 could impact the development of autoantibodies and therefore be beneficial for human SLE.
Experimental Procedures
Patient Samples
Adult SLE patients (total 61: 53 female and 8 male) and pediatric SLE patients (total 38: 34 female and 4 male) who met the American College of Rheumatology revised criteria for SLE were enrolled. All clinical and biologically relevant information of the patients is shown in Table S1 and S2. Clinical disease activity was assessed using the SLE Disease Activity Index (SLEDAI). Active patients were defined as SLEDAI score ≥ 6.
Phenotyping of blood immune cells by flow cytometry
For the analysis of OX40L expression, whole blood samples were stained with anti-CD14-PC5, CD16-FITC, CD11c-APC, HLA-DR-PC7, and OX40L-PE mAbs, and red blood cells were lysed with Versalyse (Beckman Coulter). For the analysis of blood Tfh cells, whole blood samples were stained with anti-CXCR5-AF488, CCR6-PE, CXCR3-PC5, CCR4-PC7, CD3-AF700, CD8-APCH7, CD4-Pacific Blue (all from Becton Dickinson), CD45RA-ECD (Beckman Coulter), ICOS-APC (Biolegend) and CD45-Pacific Orange (Invitrogen). Data were collected using a BD LSR II instrument (BD Biosciences) and analyzed with Flowjo software (Tree Star Inc.).
Culture of Th cells
Naïve (CD45RA+CCR7+) and memory (CD45RA-) Th cells were sorted by flow cytometry as described before (Schmitt et al., 2009). Th cells were stimulated overnight with CD3/CD28 Dynabeads (Invitrogen) in RPMI complete medium supplemented with 10% FCS. In some experiments, overnight stimulated naïve Th cells were transfected with either control or TRAF6 (s14389), NFκB1 (s9504), NFκB2 (s9507), RelA (s11916) or RelB (s11919) specific siRNA (Life Technologies) as previously described (Schmitt et al., 2009). Cells were then transferred to flat-bottomed 96 well plates coated with CD3 mAb (5 μg /ml, OKT3) supplemented with soluble CD28 mAb (1 μg/ml, CD28.2), in the presence or absence of recombinant IL-12 (100 pg/ml), and/or soluble OX40L (100 ng/ml, R&D systems). T cells were harvested at day 4 (for CD3/CD28 stimulated T cells) or at day 7 (for monocyte-T co-culture) for phenotyping with anti-CXCR5 AF647, anti-CD40L APC-eFluor 780 and anti-ICOS biotin / Streptavidin-PerCP; and for co-culture with B cells. For the assessment of IL-21 expression (with anti-IL21-PE), cultured cells were re-stimulated with 25 ng/ml PMA, 1 μg/ml ionomycin for 6 hours in the presence of brefeldine and monensin for the last 4 hours.
Co-culture of Th and B cells
Activated Th cells were co-cultured with autologous memory B cells (5 × 103 T cells for 40 × 103 memory B cells per well) in 96-well round-bottom plates in Yssel medium/10% FBS in the presence of endotoxin-reduced SEB (0.25 ng/ml; Toxin technology, Inc.). IgG produced in the cultures were analyzed by ELISA at day 14.
Culture of monocytes
CD14+ monocytes were purified from blood samples from healthy donors by negative selection (Stemcell) and then exposed to SLE serum (10%) or control serum for 3 days in a 6 well-plate. The phenotype was analyzed by FACS with anti-CD14-PC5, anti-HLADR-PC7, and anti-OX40L-PE. TLR3 (poly-IC, 10 μg/ml), TLR7 (R837, 5 μg/ml), TLR9 (ODN2216, 10 μg/ml) agonists were purchased from InvivoGen. The TLR7 inhibitor IRS-661 (1 μM) (Barrat et al., 2005) was incubated for 10 min with the monocytes before the addition of SLE serum or anti-RNP IgG (50 μg/ml).
Statistical analysis
When the normality of the distribution was rejected, non-parametric paired Wilcoxon test or unpaired Mann-Whitney U tests were used. One-way ANOVA with multiple comparison tests was used to compare more than three parameters. Correlation between variables was determined by using the Spearman test.
Supplementary Material
Acknowledgements
We thank J-C. Caron, K.Eschel, M. Gassie, N. Pic and C. Cognet for the technical help for flow cytometry. We thank E. Kowalski, S. Coquery, N. Loof and K. Kayembe for cell sorting. We thank K. Palucka, and Y-J Liu for discussions. We thank S. Clayton for confocal imaging. This study was supported by research funding from Centre national pour la Recherche Scientifique, the Société Française de Rhumatologie and Arthritis Fondation Courtin; Ministère de la Recherche et de l’Enseignement supérieur; NIH grants U19-AI057234, U19-AI082715, U19-AI089987, Alliance for Lupus Research, and Baylor Health Care System.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: C.J., C. C-B., J.S., T.M., D.D., E.S.D., and L.R. analyzed the phenotype and performed the statistical analysis of blood samples. N.S. performed the in vitro experiments with naive and memory CD4+ T cells. Y.L. performed tissue staining and RNA-FISH. P.N., C.J., and N.S. performed tonsillar cell analysis. T.M., E.S.D. and I.D performed experiments with monocytes. H.D and S.M were involved in experimental design. L.R., C.R., E.L., P.D, ME.T, L.K, P.M, L.C, P.M, T.S, JF.V, JL.P and JF.M provided adult SLE samples and clinical information. V.P. provided pediatric SLE blood samples and clinical information. S.Z. generated anti-OX40L mAb. R.L.C. provided a TLR7 inhibitor and contributed to the design of the experiments. C.J., N.S., H.U., and P.B. wrote the manuscript. H.U. and P.B. conceived the project and oversaw the entire work.
Additional Supplemental Experimental Procedures are available online.
References
- Aicher A, Hayden-Ledbetter M, Brady WA, Pezzutto A, Richter G, Magaletti D, Buckwalter S, Ledbetter JA, Clark EA. Characterization of human inducible costimulator ligand expression and function. J Immunol. 2000;164:4689–4696. doi: 10.4049/jimmunol.164.9.4689. [DOI] [PubMed] [Google Scholar]
- Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, Ma J, Tezuka K, Yagita H, Okumura K. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunological reviews. 2008;223:271–283. doi: 10.1111/j.1600-065X.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, Chang B, Duramad O, Coffman RL. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. The Journal of experimental medicine. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Science Translational Medicine. 2013;5:176ra132. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentebibel SE, Schmitt N, Banchereau J, Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proceedings of the National Academy of Sciences. 2011;108:E488–497. doi: 10.1073/pnas.1100898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science (New York, N.Y. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008;19:41–52. doi: 10.1016/j.cytogfr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettler T, Choi YS, Salek-Ardakani S, Cheng Y, Moeckel F, Croft M, Crotty S, von Herrath M. Exogenous OX40 stimulation during lymphocytic choriomeningitis virus infection impairs follicular Th cell differentiation and diverts CD4 T cells into the effector lineage by upregulating Blimp-1. J Immunol. 2013;191:5026–5035. doi: 10.4049/jimmunol.1300013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, Durandy A, Baumann U, Schlesier M, Welcher AA, et al. ICOS Deficiency Is Associated with a Severe Reduction of CXCR5+CD4 Germinal Center Th Cells. J Immunol. 2006;177:4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- Boumpas DT, Furie R, Manzi S, Illei GG, Wallace DJ, Balow JE, Vaishnaw A. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis and rheumatism48. 2003:719–727. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- Brocker T, Gulbranson-Judge A, Flynn S, Riedinger M, Raykundalia C, Lane P. CD4 T cell traffic control: in vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. European journal of immunology. 1999;29:1610–1616. doi: 10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC, 3rd, Leonard WJ, Roopenian DC. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proceedings of the National Academy of Sciences. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun M, Ma CS, Akcay A, Pedergnana V, Palendira U, Myoung J, Avery DT, Liu Y, Abhyankar A, Lorenzo L, et al. Inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood. The Journal of experimental medicine. 2013;210:1743–1759. doi: 10.1084/jem.20130592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, Kaverina N, Utset TO, Meehan SM, Quigg RJ, et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol. 2011;186:1849–1860. doi: 10.4049/jimmunol.1001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134). Annual review of immunology. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunninghame Graham DS, Graham RR, Manku H, Wong AK, Whittaker JC, Gaffney PM, Moser KL, Rioux JD, Altshuler D, Behrens TW, Vyse TJ. Polymorphism at the TNF superfamily gene TNFSF4 confers susceptibility to systemic lupus erythematosus. Nat Genet. 2008;40:83–89. doi: 10.1038/ng.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Vega AM, Abelson AK, Sanchez E, Witte T, D'Alfonso S, Galeazzi M, Jimenez-Alonso J, Pons-Estel BA, Martin J, Alarcon-Riquelme ME. Replication of the TNFSF4 (OX40L) promoter region association with systemic lupus erythematosus. Genes Immun. 2009;10:248–253. doi: 10.1038/gene.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nature immunology. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillatreau S, Gray D. T cell accumulation in B cell follicles is regulated by dendritic cells and is independent of B cell activation. The Journal of experimental medicine. 2003;197:195–206. doi: 10.1084/jem.20021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. The Journal of experimental medicine. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Science Translational Medicine. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW, Suh WK. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proceedings of the National Academy of Sciences. 2009;106:20371–20376. doi: 10.1073/pnas.0911573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, et al. Circulating Precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T Cells Indicate Tfh Cell Activity and Promote Antibody Responses upon Antigen Reexposure. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol. 2007;178:3822–3830. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
- Kalled SL, Cutler AH, Datta SK, Thomas DW. Anti-CD40 ligand antibody treatment of SNF1 mice with established nephritis: preservation of kidney function. J Immunol. 1998;160:2158–2165. [PubMed] [Google Scholar]
- Karulf M, Kelly A, Weinberg AD, Gold JA. OX40 ligand regulates inflammation and mortality in the innate immune response to sepsis. J Immunol. 2010;185:4856–4862. doi: 10.4049/jimmunol.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Bang SY, Lee HS, Cho SK, Choi CB, Sung YK, Kim TH, Jun JB, Yoo DH, Kang YM, et al. High-density genotyping of immune loci in Koreans and Europeans identifies eight new rheumatoid arthritis risk loci. Ann Rheum Dis. 2015;74:e13. doi: 10.1136/annrheumdis-2013-204749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, Odermatt B, Bachmann MF. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL Responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- Kshirsagar S, Binder E, Riedl M, Wechselberger G, Steichen E, Edelbauer M. Enhanced activity of Akt in Teff cells from children with lupus nephritis is associated with reduced induction of tumor necrosis factor receptor-associated factor 6 and increased OX40 expression. Arthritis and rheumatism. 2013;65:2996–3006. doi: 10.1002/art.38089. [DOI] [PubMed] [Google Scholar]
- Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Science Translational Medicine. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Silva DG, Martin JL, Pratama A, Hu X, Chang PP, Walters G, Vinuesa CG. Interferon-gamma excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity. 2012;37:880–892. doi: 10.1016/j.immuni.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Liarski VM, Kaverina N, Chang A, Brandt D, Yanez D, Talasnik L, Carlesso G, Herbst R, Utset TO, Labno C, et al. Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Science Translational Medicine. 2014;6:230ra246. doi: 10.1126/scitranslmed.3008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. The Journal of experimental medicine. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen X, Zhong B, Wang A, Wang X, Chu F, Nurieva RI, Yan X, Chen P, van der Flier LG, et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature. 2014;507:513–518. doi: 10.1038/nature12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovgren T, Eloranta ML, Kastner B, Wahren-Herlenius M, Alm GV, Ronnblom L. Induction of interferon-alpha by immune complexes or liposomes containing systemic lupus erythematosus autoantigen- and Sjogren's syndrome autoantigen-associated RNA. Arthritis and rheumatism. 2006;54:1917–1927. doi: 10.1002/art.21893. [DOI] [PubMed] [Google Scholar]
- Marriott CL, Mackley EC, Ferreira C, Veldhoen M, Yagita H, Withers DR. OX40 controls effector CD4 T-cell expansion, not follicular T helper cell generation in acute Listeria infection. European journal of immunology. 2014 doi: 10.1002/eji.201344211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K, Ishii N, Takano H, Miura S, Ndhlovu LC, Nose M, Noda T, Sugamura K. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. The Journal of experimental medicine. 2000;191:365–374. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K, Nose M, Ndhlovu LC, Sato T, Sugamura K, Ishii N. Constitutive OX40/OX40 ligand interaction induces autoimmune-like diseases. J Immunol. 2002;169:4628–4636. doi: 10.4049/jimmunol.169.8.4628. [DOI] [PubMed] [Google Scholar]
- Murphy G, Lisnevskaia L, Isenberg D. Systemic lupus erythematosus and other autoimmune rheumatic diseases: challenges to treatment. Lancet. 2013;382:809–818. doi: 10.1016/S0140-6736(13)60889-2. [DOI] [PubMed] [Google Scholar]
- Nordmark G, Kristjansdottir G, Theander E, Appel S, Eriksson P, Vasaitis L, Kvarnstrom M, Delaleu N, Lundmark P, Lundmark A, et al. Association of EBF1, FAM167A(C8orf13)-BLK and TNFSF4 gene variants with primary Sjogren's syndrome. Genes Immun. 2011;12:100–109. doi: 10.1038/gene.2010.44. [DOI] [PubMed] [Google Scholar]
- Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. The Journal of experimental medicine. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- Pratama A, Ramiscal RR, Silva DG, Das SK, Athanasopoulos V, Fitch J, Botelho NK, Chang PP, Hu X, Hogan JJ, et al. Roquin-2 shares functions with its paralog Roquin-1 in the repression of mRNAs controlling T follicular helper cells and systemic inflammation. Immunity. 2013;38:669–680. doi: 10.1016/j.immuni.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Schmitt N, Bustamante J, Bourdery L, Bentebibel SE, Boisson-Dupuis S, Hamlin F, Tran MV, Blankenship D, Pascual V, Savino DA, et al. IL-12 receptor beta1 deficiency alters in vivo T follicular helper cell response in humans. Blood. 2013;121:3375–3385. doi: 10.1182/blood-2012-08-448902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, Banchereau J, Ueno H. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nature immunology. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura E, Valladeau-Guilemond J, Donnadieu MH, Sastre-Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. The Journal of experimental medicine. 2012;209:653–660. doi: 10.1084/jem.20111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen N, Fu Q, Deng Y, Qian X, Zhao J, Kaufman KM, Wu YL, Yu CY, Tang Y, Chen JY, et al. Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proceedings of the National Academy of Sciences. 2010;107:15838–15843. doi: 10.1073/pnas.1001337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, Manku H, Vyse TJ, Roncador G, Huttley GA, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis and rheumatism. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- So T, Choi H, Croft M. OX40 complexes with phosphoinositide 3-kinase and protein kinase B (PKB) to augment TCR-dependent PKB signaling. J Immunol. 2011;186:3547–3555. doi: 10.4049/jimmunol.1003156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl W, Hiepe F, Latinis KM, Thomas M, Scheinberg MA, Clarke A, Aranow C, Wellborne FR, Abud-Mendoza C, Hough DR, et al. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis and. 2012;64:2328–2337. doi: 10.1002/art.34400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly - TFH cells in human health and disease. Nat Rev Immunol. 2013;13:412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokos GC. Systemic lupus erythematosus. The New England journal of medicine. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nature immunology. 2015;16:142–152. doi: 10.1038/ni.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Panhuys N, Klauschen F, Germain RN. T-cell-receptor-dependent signal intensity dominantly controls CD4(+) T cell polarization In Vivo. Immunity. 2014;41:63–74. doi: 10.1016/j.immuni.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG, Cyster JG. How T cells earn the follicular rite of passage. Immunity. 2011;35:671–680. doi: 10.1016/j.immuni.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Vogel KU, Edelmann SL, Jeltsch KM, Bertossi A, Heger K, Heinz GA, Zoller J, Warth SC, Hoefig KP, Lohs C, et al. Roquin paralogs 1 and 2 redundantly repress the Icos and Ox40 costimulator mRNAs and control follicular helper T cell differentiation. Immunity. 2013;38:655–668. doi: 10.1016/j.immuni.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Witsch EJ, Peiser M, Hutloff A, Buchner K, Dorner BG, Jonuleit H, Mages HW, Kroczek RA. ICOS and CD28 reversely regulate IL-10 on re-activation of human effector T cells with mature dendritic cells. European journal of immunology. 2002;32:2680–2686. doi: 10.1002/1521-4141(200209)32:9<2680::AID-IMMU2680>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Xiao X, Balasubramanian S, Liu W, Chu X, Wang H, Taparowsky EJ, Fu YX, Choi Y, Walsh MC, Li XC. OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nature immunology. 2012;13:981–990. doi: 10.1038/ni.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Wu XL, Yang B, Wang Y, Feng GH, Jiang TJ, Zeng QL, Xu XS, Li YY, Jin L, et al. Upregulation of OX40 ligand on monocytes contributes to early virological control in patients with chronic hepatitis C. European journal of immunology. 2013;43:1953–1962. doi: 10.1002/eji.201243097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.