Abstract
Background
Viruses are commonly detected in children with acute respiratory illnesses (ARI) and in asymptomatic children. Longitudinal studies of viral detections during asymptomatic periods surrounding ARI could facilitate interpretation of viral detections but are currently scant.
Methods
We used reverse transcription-polymerase chain reaction (RT-PCR) to analyze respiratory samples from young Andean children for viruses during asymptomatic periods within 8-120 days of index ARI (cough or fever). We compared viral detections over time within children and explored RT-PCR cycle thresholds (CT) as surrogates for viral loads.
Results
At least one respiratory virus was detected in 367 (43%) of 859 samples collected during asymptomatic periods, with more frequent detections in periods with rhinorrhea (49%) than those without (34%, p<0.001). Relative to index ARI with human rhinovirus (HRV), adenovirus (AdV), respiratory syncytial virus (RSV), and parainfluenza virus (PIV) detected, the same virus was also detected during 32%, 22%, 10%, and 3% of asymptomatic periods, respectively. RSV was only detected 8-30 days after index RSV ARI, whereas HRV and AdV were detected throughout asymptomatic periods. Human metapneumovirus (MPV) and influenza were rarely detected during asymptomatic periods (<3%). No significant differences were observed in the CT for HRV or AdV during asymptomatic periods relative to ARI. For RSV, CT were significantly lower during ARI relative to the asymptomatic period (p=0.03).
Conclusions
These findings indicate that influenza, MPV, PIV, and RSV detections in children with ARI usually indicate a causal relationship. When HRV or AdV is detected during ARI, the causal relationship is less certain.
Keywords: acute respiratory illness, children, Peru, respiratory virus
BACKGROUND
Increasing availability of molecular diagnostic methods has enhanced detection of viruses in the upper respiratory tracts of patients with acute respiratory illnesses (ARI). However, viral detection in a patient with an ARI does not confirm an etiologic role for the virus. Viral detection may, alternatively, represent coincidental asymptomatic infection or persistent shedding after a prior infection.(1, 2)
Understanding the role of viral detection in ARI is essential to inform clinical management decisions and research priorities. Many published studies, including some of our own, have evaluated patients presenting or admitted to healthcare centers with respiratory infections in developed countries and compared them with different groups of healthy patients without respiratory symptoms. However, potential underlying differences between these groups could impact the validity of these comparisons.
Longitudinal assessments of the same individual could address some of these concerns by collecting repeated respiratory samples over time within a given individual during both symptomatic and asymptomatic periods. Nevertheless, most available longitudinal studies have evaluated only a limited number of viruses, were conducted in urban, high population-density areas in developed countries, and surveillance was performed in health care facilities, not in the patient homes, which potentially impacts the generalizability of those data. (3-13)
Our prospective household-based cohort study of young children from communities in the Peruvian Andes(14) is uniquely suited to provide information about the detection of common respiratory viruses during symptomatic ARI and from sequential respiratory samples obtained from the same child during prospective follow-up in a rural, high-altitude setting. The objective of the current study was to evaluate the presence and persistence of respiratory viral detections in individual children during asymptomatic periods relative to periods of symptomatic ARI.
MATERIALS AND METHODS
Study Design
This study utilizes data from the study of Respiratory Infections in Andean Peruvian children (RESPIRA-PERU), a prospective cohort study designed to evaluate the epidemiology of common respiratory viruses, the distribution of colonizing bacteria, the role of respiratory viruses in pneumococcal colonization, the impact of environmental factors on bacterial colonization, and the incidence and severity of ARI among young Andean children.(15-20) This study was approved by the Vanderbilt Institutional Review Board and by the Ethics Committee of the Instituto de Investigacion Nutricional.
Study Setting
RESPIRA PERU was conducted in the Province of San Marcos, Department of Cajamarca, located in the northern highlands of Peru. San Marcos includes areas ranging from approximately 1500-4000 meters above sea level. The population is primarily rural, with low income, low educational level, and limited access to health-care services, as previously described.(20)
Enrollment and Follow-up
After a local census, broad selection criteria were used for enrollment, consisting of: 1) families with children aged <3 years; and 2) intention to remain in the study area for the next year. Children born during the study period in the study communities were enrolled to replace children who attained 3 years of age, with the aim of maintaining a dynamic cohort with approximately 500 children <3 years under observation at any time. Weekly active household-based ARI surveillance was conducted from May 1, 2009 through September 30, 2011 by trained field workers. ARI was defined as the presence of either cough or fever.(21) An ARI episode encompassed the period from the date of symptom onset to the last day of ARI symptoms that was followed by 7 or more days free of ARI symptoms. Children were followed until their third birthday, loss to follow-up, withdrawal of consent, death, or the end of the study, whichever came first.
Household Visits
Trained field workers visited the home of each enrolled child weekly to collect information on respiratory signs and symptoms through the use of a standardized questionnaire. At each visit, field workers assessed from the caretaker whether the children were experiencing ARI symptoms on the day of or up to 7 days prior to the household visit. In children with ARI symptoms on the day of or the day before the visit, the presence of World Health Organization (WHO) danger signs or lower respiratory tract infection (LRTI) was assessed by physical examination. If these signs were present, the field workers notified a study physician or nurse by cell phone to arrange a household visit or to coordinate referral to a healthcare center.
Respiratory Sample Collection
Nasal swab collection
During weekly household visits, field workers collected a nasal swab (NS) from any child with an ARI, following procedures previously reported.(22) In brief, one non-flocked polyester-tipped swab was placed into each nostril sequentially and rotated beneath the turbinates to collect epithelial cells and absorb secretions. After each nostril was swabbed, the swab was inserted into a tube with Remel M4RT® viral transport medium and transported in ice packages to the local research laboratory within 8 hours of sample collection. Samples were preserved at −70°C prior to viral diagnostic testing. Nasal swabs were collected for each new episode of ARI, but were not collected in consecutive weeks unless a new ARI developed.
Nasopharyngeal swab collection
Nasopharyngeal (NP) swabs were collected monthly from each child under observation, whether or not respiratory symptoms were present, and during complicated ARI, defined by the presence of WHO danger or LRTI signs (subcostal retractions, nasal flaring, etc.).(23) NP samples were processed according to WHO recommendations for identifying pneumococcal colonization.(24, 25) Briefly, samples were collected with a non-flocked deep nasopharyngeal Rayon swab and then immediately placed in 1 mL of Skim Milk-Tryptone-Glucose-Glycerol (STGG) transport medium. Specimens were transported in cold packs to the laboratory within 8 hours of collection and then preserved at −70°C.
Selection of Nasopharyngeal Samples
Because NP samples were collected monthly from all subjects under observation, multiple asymptomatic NP specimens were available from most subjects. We restricted sample selection for this study to observation periods after a first ARI so that the temporal relationship with an ARI was always known. For analysis of viral detections during asymptomatic periods, we selected a random sample of NP specimens collected during asymptomatic periods 8-30 or 31-120 days before or after an ARI (hereafter referred to as index ARI) (Figure 1).
Figure 1.
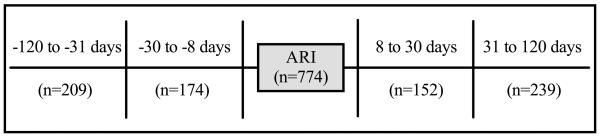
Selection of nasopharyngeal samples from four asymptomatic time periods within 120 days of an index acute respiratory illness (ARI).
Viral Detection Techniques
Both NS and NP samples were analyzed for the identification of influenza viruses (A and B), respiratory syncytial virus (RSV), human metapneumovirus (MPV), rhinovirus (HRV), adenovirus (AdV), and parainfluenza viruses 1-3 (PIV) using nucleic acid extraction and real-time reverse transcription-polymerase chain reaction (RT-PCR) as previously described.(22, 26-29) Despite different collection methods, prior studies by our group and others have demonstrated a very high agreement (89%-99%) in the detection of common respiratory viruses between NS and NP samples collected in this manner.(16, 30) For samples with virus detections, RT-PCR cycle thresholds (CT) from asymptomatic and ARI periods were considered as surrogates for viral loads, with higher CT indicating lower viral load. CT values of ≤40 were used to define sample positivity. Each RT-PCR was capable of detecting less than 50 RNA copies based on RNA runoff transcripts.
Statistical Analysis
The prevalence of detection of each respiratory virus during four asymptomatic periods relative to an index ARI within a given child was calculated (Figure 1). We also compared viral detections during asymptomatic periods between children with specific virus-positive and virus-negative ARI using the Fisher’s exact test. Viral detection during asymptomatic periods was also stratified by the presence or absence of rhinorrhea and compared using the Fisher’s exact test. CT for viral detections from asymptomatic and ARI periods were compared using the Wilcoxon sign-rank test.
Comparison of viral detections between symptomatic and asymptomatic periods
To assess the association between viral detections and symptomatic periods, we compared the proportion of specific viral detections between symptomatic and asymptomatic periods. For this, we used univariate logistic regression models and calculated odds ratios (ORs) for the detections of each virus. The dependent variable in each model was symptomatic (i.e. ARI) or asymptomatic period (all combined) at the time of sample collection, and the independent variable was detection of each individual virus (positive or negative RT-PCR). Given the within-subject nature of our assessments, all regression models accounted for the clustering of observations at the individual level using the Huber-White sandwich variance estimator.(31) In addition, we estimated attributable fractions for each virus and expressed those as percentages using the equation: AF% = (OR – 1)*100 / OR.(32-34) Thus, for each group of symptomatic periods with positive detections for a specific virus, the AF% represented the proportion that could be attributed to the virus detected (i.e. considering that viruses could also be detected during asymptomatic periods). For example, if the odds of a viral detection were the same during symptomatic and asymptomatic periods, the odds ratio would be 1.0; accordingly, the corresponding AF would be 0% suggesting that the symptomatic periods might not be attributed to viral detections. In contrast, an OR of 5.0 yields an AF% of 80 indicating that 80% of symptomatic periods with virus detected might be attributed to the detected virus.
All analyses were done in Stata 13 (StataCorp, College Station, TX).
RESULTS
Characteristics of the Study Population
In total, 892 children representing 810 households in 58 communities were enrolled in the RESPIRA-PERU study. A detailed description of enrolled households and children has been published previously.(20) A total of 4475 ARI episodes were observed, with at least one virus detected in 67% of ARI. HRV was the most common virus identified, detected during 32% of ARI, followed by AdV (5%), PIV (5%), influenza (5%), RSV (3%), and MPV (2%).(15) Detection of two or more viruses was observed in approximately 13% of ARI.(15)
Viral Detection During Asymptomatic Periods
Nine hundred nine (909) NP samples collected during asymptomatic periods were randomly selected from a total of 3470 samples to undergo viral testing. Of those, 859 had enough residual material for comprehensive viral testing. At least one virus was detected in 42.7% of these 859 NP samples, while co-detection of more than one virus was observed in 48 samples (5.7%). HRV and AdV were detected most commonly, in 270 (31.4%) and 95 (11.0%) asymptomatic periods, respectively, followed by RSV in 29 (3.4%), MPV in 13 (1.5%), PIV in 11 (1.3 %), and influenza in 6 (0.7%) asymptomatic periods (Table 1).
TABLE 1.
Viral detections in asymptomatic time periods stratified by age.
| Number of detections (%)* |
||||
|---|---|---|---|---|
| Virus | All ages (n=859) |
0-5 months (n=64) |
6-11 months (n=178) |
12-35 months (n=605) |
| HRV | 270 (31.4) | 19 (29.7) | 57 (32.0) | 194 (32.1) |
| AdV | 95 (11.0) | 1 (1.5) | 25 (14.0) | 69 (11.4) |
| RSV | 29 (3.4) | 2 (3.1) | 7 (3.9) | 20 (3.3) |
| MPV | 13 (1.5) | 1 (1.5) | 2 (1.1) | 10 (1.7) |
| PIV | 11 (1.3) | 1 (1.5) | 4 (2.2) | 6 (1.0) |
| Influenza | 6 (0.7) | 1 (1.5) | 1 (0.6) | 4 (0.7) |
| Any Virus | 367 (42.7) | 23 (35.9) | 82 (46.1) | 263 (43.5) |
| ≥ 2 Viruses | 48 (5.6) | 2 (3.1) | 14 (7.9) | 32 (5.3) |
Viruses were detected more frequently in children with rhinorrhea than those without (p<0.001; Table 2). The association of viral detection with rhinorrhea was primarily driven by HRV and AdV. This association was not observed for MPV, RSV, PIV, and influenza; however, these viruses were rarely detected during asymptomatic periods and thus, those comparisons had limited power.
TABLE 2.
Viral detections in asymptomatic time periods stratified by rhinorrhea.
| Any Virus | HRV | AdV | RSV | MPV | PIV | Influenza | |
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Rhinorrhea (n=446) | 219 (49.1) | 163 (37.1) | 60 (13.5) | 12 (2.7) | 7 (1.6) | 9 (2.0) | 3 (0.7) |
| No rhinorrhea (n=403) | 138 (34.2) | 102 (25.4) | 35 (8.7) | 14 (3.5) | 3 (0.7) | 2 (0.5) | 3 (0.7) |
| p<0.001 | p<0.001 | p=0.03 | p=0.55 | p=0.35 | p=0.07 | p=1.0 | |
Viral Detections During Asymptomatic Periods Relative to Index ARI
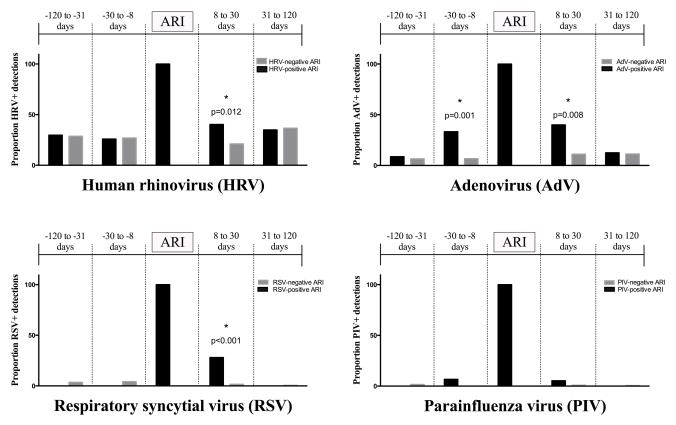
Seven hundred seventy-four (774) observations in which NP samples were available from an asymptomatic period and within 120 days of an index ARI were identified, representing 774 unique ARI-asymptomatic sample pairs (Figure 1). Figure 2 displays the proportions of viral detection during each asymptomatic period relative to an index ARI. Black bars indicate observations in which a given virus was detected, both during the index ARI and asymptomatic periods. Gray bars represent detection of a given virus during an asymptomatic time period relative to an index ARI in which the same virus was not detected. For ARI in which HRV, AdV, RSV, and PIV were detected, the overall proportions of detection of the same virus during asymptomatic periods within 120 days of the ARI in a given subject were 32%, 22%, 10%, and 3%, respectively (Table 3). No subject who had a virus-positive ARI for influenza (n=25) or MPV (n=32) had the same virus detected during an asymptomatic time period.
Figure 2.
Detection of virus during asymptomatic periods relative to virus-positive and virus-negative index ARI.
TABLE 3.
Proportion of viral detections relative to virus-positive index ARI and CT differences according to virus and time period.
| Days −120 to −31 |
Days −30 to −8 |
Days 8 to 30 |
Days 31-120 |
All time points | |
|---|---|---|---|---|---|
|
|
|||||
| HRV+ ARI (n=330) | 30/101 (29.7) | 21/81 (25.9) | 25/62 (40.3) | 30/86 (34.9) | 106/330 (32.1) |
| Median CT Asymptomatic (IQR) | 29.4 (25.5-36.8) | 29.6 (25.4-34.9) | 28.4 (23.8-31.5) | 30.3 (24.1-34.7) | 29.7 (24.4-34.7) |
| Median CT ARI (IQR) | 25.6 (21.9-30.0) | 28.2 (24.4-32.6) | 28.0 (25.2-34.2) | 27.3 (23.4-31.1) | 27.2 (23.2-32.1) |
| p=0.29 | p=0.52 | p=0.17 | p=0.38 | p=0.49 | |
| RSV+ ARI (n=69) | No detection | No detection | 7/25 (28.0) | No detection | 7/69 (10.1) |
| Median CT Asymptomatic (IQR) | 28.8 (25.0-38.5) | 28.8 (25.0-38.5) | |||
| Median CT ARI (IQR) | 23.4 (18.9-27.0) | 24.4 (18.9-29.3) | |||
| p=0.028 | p=0.028 | ||||
| PIV+ ARI (n=75) | No detection | 1/15 (6.7) | 1/19 (5.3) | No detection | 2/75 (2.6) |
| Median CT Asymptomatic (IQR) | 20.7 | 38.9 | 29.8 (20.7-38.9) | ||
| Median CT ARI (IQR) | 25.6 (23.8-35.1) | 21.5 (19.5-28.5) | 24.2 (21.0-30.8) | ||
| p=0.32 | p=0.32 | p=0.65 | |||
| AdV+ ARI (n=83) | 2/23 (8.7) | 7/21 (33.3) | 6/15 (40.0) | 3/24 (12.5) | 18/83 (21.7) |
| Median CT Asymptomatic (IQR) | 33.0 (28.5-37.5) | 36.0 (35.2-37.1) | 33.3 (31.6-35.3) | 36.8 (23.2-38.7) | 35.6 (31.6-37.1) |
| Median CT ARI (IQR) | 35.9 (33.1-37.6) | 36.1 (31.4-37.0) | 32.8 (30.0-36.5) | 36.7 (34.8-37.8) | 36.1 (32.7-37.3) |
| p=0.65 | p=0.40 | p=0.75 | p=0.59 | p=0.78 | |
| MPV+ ARI (n=32) | No detection | No detection | No detection | No detection | No detection |
| Flu+ ARI (n=25) | No detection | No detection | No detection | No detection | No detection |
Viral detections varied during asymptomatic periods. Asymptomatic detections of RSV were restricted to the 8-30 day period after RSV-positive ARI. Although detections of HRV and AdV were observed throughout the asymptomatic periods, HRV was significantly more likely to occur in the 8-30 day asymptomatic period after HRV-positive ARI than after ARI in which HRV was not detected. Detection of AdV was significantly more likely to occur in the 8-30 day periods both before and after an index ARI in which AdV was detected. Detection of PIV was rare during asymptomatic periods relative to ARI in which PIV was detected.
Comparison of viral detections between symptomatic and asymptomatic periods
In regression models evaluating symptomatic ARI and asymptomatic sample pairs, detections of PIV, MPV, and influenza were most highly associated with ARI, resulting in AFs of 91% (95% confidence interval (CI): 80, 86), 79% (CI: 46, 92), and 73% (CI: 21, 90), respectively (Table 4). RSV and HRV detections were also significantly associated with ARI, but with lower AF% of 67% (CI: 43, 81) and 41% (CI: 22, 54), respectively. Only AdV was not significantly associated with ARI in this cohort, with an AF% of 5% (CI: −37, 34), meaning its detection was not significantly increased during symptomatic periods relative to asymptomatic periods.
TABLE 4.
Prevalence of respiratory virus detection by RT-samples and samples from ARI, unadjusted odds ratios (OR (AF). PCR in paired asymptomatic ), and attributable fractions
| Asymptomatic period, n (%) |
ARI, n (%) |
p | OR (95% CI) |
AF (95% CI) |
|
|---|---|---|---|---|---|
|
|
|||||
| HRV | 237 (30.6) | 330 (42.6) | <0.001 | 1.68 (1.30, 2.18) |
0.41 (0.22, 0.54) |
| AdV | 80 (10.3) | 83 (10.8) | 0.773 | 1.06 (0.73, 1.53) |
0.05 (−0.37, 0.34) |
| RSV | 24 (3.1) | 69 (8.9) | <0.001 | 3.06 (1.75, 5.35) |
0.67 (0.43, 0.81) |
| MPV | 7 (0.9) | 32 (4.1) | 0.001 | 4.73 (1.85, 12.0) |
0.79 (0.46, 0.92) |
| PIV | 7 (0.9) | 75 (9.7) | <0.001 | 11.76 (4.96, 27.89) |
0.91 (0.80, 0.96) |
| Influenza | 7 (0.9) | 25 (3.2) | 0.017 | 3.66 (1.26, 10.62) |
0.73 (0.21, 0.90) |
RT-PCR CT for Viral Detections during ARI and Asymptomatic Periods
No significant differences were observed in CT values during asymptomatic periods [median 29.7, (IQR 24.4-34.7)] relative to index ARI [27.2 (23.2-32.1), p=0.49] in which HRV was detected (Table 3). Similarly, no differences were observed in CT values for AdV during asymptomatic periods [median 35.6, (IQR 31.6-37.1)] relative to index ARI’s [36.1 (32.7-37.3), p=0.78]. However, in the 7/25 (28%) samples in which RSV was detected during the asymptomatic period 8-30 days after RSV-positive index ARI, the median CT values were significantly higher during asymptomatic periods than ARI, indicating lower viral loads during these asymptomatic periods following ARI [CT 28.8 (25.0-38.5)] than during ARI [CT 24.4 (18.9-29.3); p=0.028]. The median duration of RSV detection after a symptomatic ARI was 15 days (IQR 14-21).
DISCUSSION
We compared the prevalence of detection of specific respiratory viruses during asymptomatic periods compared to ARI within individual children <3 years of age. We found that detection of respiratory viruses during asymptomatic periods was common, occurring in over 40% of samples, and each one of the viruses evaluated was detected in asymptomatic periods. The majority of detections that occurred in asymptomatic periods were HRV and AdV, consistent with findings from other studies.(1, 35, 36) All asymptomatic RSV detections occurred shortly following index ARI in which RSV was detected. Detections of PIV, MPV, and influenza were rare during asymptomatic periods.
The prevalence of asymptomatic detection for both HRV and AdV varied significantly relative to the timing of index ARI. HRV was detected more frequently in the 8-30 days after ARI in which HRV was detected than following an ARI in which HRV was not detected, consistent with findings from other studies that have demonstrated that rhinovirus RNA may persist for up to 30 days or even longer after HRV symptomatic infection in young infants.(12, 37-40) There was also variation in the prevalence of asymptomatic AdV detection relative to the timing of index AdV-positive ARI, with AdV detected in 33% and 40%, respectively, of asymptomatic periods 8-30 days before and 8-30 days after AdV-positive ARI but only in 13% or less of asymptomatic samples obtained greater than 30 days apart from ARI. Additionally, detection of AdV during the 8-30 days asymptomatic periods before and after ARI was significantly more frequent surrounding AdV-positive ARI compared to AdV-negative ARI, suggesting a relationship between asymptomatic detection and ARI. While the prolonged presence of AdV following ARI has not been thoroughly evaluated, prolonged shedding has been described,(3) and AdV has been found in tonsil and adenoid tissue of asymptomatic children undergoing routine tonsillectomy,(41) suggesting that this mucosal lymphoid tissue may harbor AdV and allow intermittent shedding before or after symptomatic disease.
However, the relative viral loads of HRV and AdV, as estimated by CT values, were not higher during index ARI compared to asymptomatic periods before or after ARI, arguing against the possibility that these detections represented persistence of infection due to the same HRV or AdV strain.(42-45) Recently, Loeffelholz et al. reported that prolonged presence of the same HRV strain for >30 days was uncommon in young children, occurring in less than 5% of HRV detections.(12) Another study by the same group evaluating repeated AdV detections within individual children demonstrated that repeat detection of the same strain of AdV following AdV-associated symptomatic disease occurred, either continuously or intermittently, for more than four months in 10 of 16 (62.5%) instances, while other repeated detections represented acquisition of new AdV strains.(46) Additional genotyping of HRV and AdV detections in our cohort would be necessary to conclusively clarify these observations.
RSV was only detected in the 8-30 day asymptomatic period after an RSV-associated ARI. These RSV detections in asymptomatic periods shortly after an RSV-associated ARI were associated with significantly higher CT, suggesting declining viral shedding. Our observed median duration of detection 15 days after symptomatic ARI is consistent with previous studies that have demonstrated that the duration of RSV shedding after infection in healthy young children is usually 4-10 days but may persist up to 3-4 weeks.(47-50)
We also found that viruses were detected more frequently during non-ARI periods in which rhinorrhea was present. This observation was primarily driven by HRV and AdV. Although a similar trend was observed for MPV and PIV, the small sample sizes for asymptomatic detection of these viruses may have limited our power to detect significant differences. While infection with some of these viruses may be associated with rhinorrhea alone,(51, 52) or rhinorrhea may enhance viral detection from the nasopharynx, the interpretation of this finding should take into account that our definition of ARI only included cough and fever, but not rhinorrhea.
We also examined the association of specific viral detections and symptomatic respiratory illness. Most viruses including MPV, PIV, and influenza were detected commonly during symptomatic periods but uncommonly during asymptomatic periods, suggesting a direct etiological role when detected in children with ARI. In contrast, HRV and especially AdV were frequently detected during aggregated asymptomatic periods in addition to ARI periods, yielding much lower odds ratios and lower AFs. An important caveat for the interpretation of these estimates is the variable detection of these viruses during asymptomatic periods over time. In particular, the lack of a significant association between AdV detections and symptomatic illness as determined by OR and AF calculations must be interpreted with caution, as the prevalence of asymptomatic AdV detection varied significantly in different asymptomatic periods. The AF measurement is based on a simple model of causation. For example, it did not take into account data on CT values for RSV, thus likely underestimating the true fraction of disease due to RSV. In addition, previous experimental and observational studies have demonstrated a clear pathogenic role of specific strains of HRV and AdV.(46, 53-58) Whether some specific viral strains may be more pathogenic than others remains unclear and requires further scrutiny.
Our study took place in a rural high altitude setting, with approximately 75% of our cohort residing at altitudes higher than 2314 meters above sea level. The physiologic effects of altitude are multiple, including lower baseline oxygen saturations,(59, 60) impaired respiratory ciliary function,(61) and hypoxia-driven pulmonary vasoconstriction, and may impact susceptibility to respiratory illness or disease severity. In our cohort, residence at higher altitude (≥2315-2865 meters compared to <2314 meters) was an independent risk factor for RSV infection, but not for MPV infection.(62) Choudhuri et al.(63) similarly demonstrated increased RSV-associated hospitalization rates of 25% and 53% for every 1000-meter increase in altitude among children less than one and children aged 1 to 4 years, respectively. The highest risk for RSV-associated hospitalization occurred at elevations >2500 meters. However, there were no data for other viruess. Whether altitude and other factors that could influence susceptibility to specific viral infections also impact respiratory viral disease severity requires further exploration.
The current study has several important strengths. First, the RESPIRA-PERU study is one of few large prospective intensive evaluations of mild-to-moderate respiratory illness in a rural, high-altitude setting. Additionally, rather than utilizing an external control cohort, the study collected prospective data from individual subjects over a period of longitudinal follow-up wherein each subject served as his own control, directly accounting for inter-subject variability. Rigorous active household surveillance combined with sensitive viral detection techniques enabled a highly sensitive assessment of viruses associated with both asymptomatic and ARI conditions.
Our study also has several limitations. First, limited information regarding host immunological factors and viral-bacterial interactions in individuals over time is available, which may influence patterns of respiratory viral detection.(64-68) Additionally, no detailed molecular characterizations of each viral strain were conducted to inform interpretation of whether repeated detections in an individual are due to prolonged shedding with a single viral strain or frequent acquisition of new strains. Our highly sensitive ARI definition (fever or cough) may have resulted in noninfectious causes of fever or cough to be counted as ARI. Finally, although we tested for a large panel of respiratory viruses, we did not attempt to identify other viruses (such as coronaviruses and bocaviruses) that may have been detectable in this cohort.
Taken together, our findings established from within-person comparisons indicate that detections of HRV and AdV were common during asymptomatic periods and varied over time, occurring closely before and after index viral ARI, with similar CTs across these periods, and with lower attributable fractions for symptomatic diseases than the other viruses. RSV was infrequently detected during asymptomatic periods except in the 8-30 days following an index RSV-positive ARI. In contrast, detection of influenza, MPV and PIV during asymptomatic periods was very uncommon, indicating that detection of influenza, MPV, PIV, and RSV in a child with an ARI usually indicates a causal relationship. The causal relationship between HRV or AdV detections and ARI is less certain, and the patterns of the detection of different strains of these viruses over time requires further investigation.
ACKNOWLEDGMENTS
On behalf of the study of Respiratory Infections in Andean Peruvian children (RESPIRA-PERU): Vanderbilt University: Marie R. Griffin, John V. Williams, Kathryn M. Edwards, Philip J. Budge, Yuwei Zhu, Monika Johnson, Carlos G. Grijalva; Emory University: Jorge E. Vidal, Keith P. Klugman; Instituto de Investigacion Nutricional: Hector Verastegui, Stella M. Hartinger, Ana I. Gil, Claudio F. Lanata. We are indebted to the communities of San Marcos, Cajamarca Peru for their participation in this study. We also acknowledge the approval and continuous support of the Cajamarca Health Region authorities. We are also indebted to the field workers and field supervisors whose efforts in difficult geographical areas and harsh weather conditions allowed the conduct of this study.
This work was supported by the Vanderbilt University CTSA grant UL1 RR024975-01 from NIH, an investigator initiated grant from Pfizer (IIR WS1898786(0887X1-4492)) and a research grant from the Thrasher Research Fund (02832-9). L.M.H was supported by Fogarty International Center Grant Number R25TW009337 from National Institutes of Health through Vanderbilt University.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jartti T, Jartti L, Peltola V, Waris M, Ruuskanen O. Identification of respiratory viruses in asymptomatic subjects: asymptomatic respiratory viral infections. The Pediatric infectious disease journal. 2008;27(12):1103–7. doi: 10.1097/INF.0b013e31817e695d. doi: 10.1097/INF.0b013e31817e695d. PubMed PMID: 18978518. [DOI] [PubMed] [Google Scholar]
- 2.Jansen RR, Wieringa J, Koekkoek SM, Visser CE, Pajkrt D, Molenkamp R, et al. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. Journal of clinical microbiology. 2011;49(7):2631–6. doi: 10.1128/JCM.02094-10. doi: 10.1128/JCM.02094-10. PubMed PMID: 21543571; PubMed Central PMCID: PMC3147826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jartti T, Lee WM, Pappas T, Evans M, Lemanske RF, Jr., Gern JE. Serial viral infections in infants with recurrent respiratory illnesses. The European respiratory journal. 2008;32(2):314–20. doi: 10.1183/09031936.00161907. doi: 10.1183/09031936.00161907. PubMed PMID: 18448489; PubMed Central PMCID: PMC2843696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peltola V, Waris M, Osterback R, Susi P, Ruuskanen O, Hyypia T. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. The Journal of infectious diseases. 2008;197(3):382–9. doi: 10.1086/525542. doi: 10.1086/525542. PubMed PMID: 18248302. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida LM, Suzuki M, Thiem VD, Smith WP, Tsuzuki A, Huong VT, et al. Population based cohort study for pediatric infectious diseases research in Vietnam. Tropical medicine and health. 2014;42(2 Suppl):47–58. doi: 10.2149/tmh.2014-S07. doi: 10.2149/tmh.2014-S07. PubMed PMID: 25425951; PubMed Central PMCID: PMC4204059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay IM, Lambert SB, Faux CE, Arden KE, Nissen MD, Sloots TP, et al. Community-wide, contemporaneous circulation of a broad spectrum of human rhinoviruses in healthy Australian preschool-aged children during a 12-month period. The Journal of infectious diseases. 2013;207(9):1433–41. doi: 10.1093/infdis/jis476. doi: 10.1093/infdis/jis476. PubMed PMID: 22829638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Zalm MM, Wilbrink B, van Ewijk BE, Overduin P, Wolfs TF, van der Ent CK. Highly frequent infections with human rhinovirus in healthy young children: a longitudinal cohort study. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2011;52(4):317–20. doi: 10.1016/j.jcv.2011.09.003. doi: 10.1016/j.jcv.2011.09.003. PubMed PMID: 21982210. [DOI] [PubMed] [Google Scholar]
- 8.Blomqvist S, Roivainen M, Puhakka T, Kleemola M, Hovi T. Virological and serological analysis of rhinovirus infections during the first two years of life in a cohort of children. Journal of medical virology. 2002;66(2):263–8. doi: 10.1002/jmv.2140. PubMed PMID: 11782938. [DOI] [PubMed] [Google Scholar]
- 9.Chonmaitree T, Alvarez-Fernandez P, Jennings K, Trujillo R, Marom T, Loeffelholz MJ, et al. Symptomatic and asymptomatic respiratory viral infections in the first year of life: association with acute otitis media development. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60(1):1–9. doi: 10.1093/cid/ciu714. doi: 10.1093/cid/ciu714. PubMed PMID: 25205769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grijalva CG, Weinberg GA, Bennett NM, Staat MA, Craig AS, Dupont WD, et al. Estimating the undetected burden of influenza hospitalizations in children. Epidemiology and infection. 2007;135(6):951–8. doi: 10.1017/S095026880600762X. doi: 10.1017/S095026880600762X. PubMed PMID: 17156502; PubMed Central PMCID: PMC2870647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinberg GA, Hall CB, Iwane MK, Poehling KA, Edwards KM, Griffin MR, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. The Journal of pediatrics. 2009;154(5):694–9. doi: 10.1016/j.jpeds.2008.11.034. doi: 10.1016/j.jpeds.2008.11.034. PubMed PMID: 19159905. [DOI] [PubMed] [Google Scholar]
- 12.Loeffelholz MJ, Trujillo R, Pyles RB, Miller AL, Alvarez-Fernandez P, Pong DL, et al. Duration of rhinovirus shedding in the upper respiratory tract in the first year of life. Pediatrics. 2014;134(6):1144–50. doi: 10.1542/peds.2014-2132. doi: 10.1542/peds.2014-2132. PubMed PMID: 25404719; PubMed Central PMCID: PMC4243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chonmaitree T, Revai K, Grady JJ, Clos A, Patel JA, Nair S, et al. Viral upper respiratory tract infection and otitis media complication in young children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46(6):815–23. doi: 10.1086/528685. doi: 10.1086/528685. PubMed PMID: 18279042; PubMed Central PMCID: PMC2744371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huicho L, Trelles M, Gonzales F. National and sub-national under-five mortality profiles in Peru: a basis for informed policy decisions. BMC public health. 2006;6:173. doi: 10.1186/1471-2458-6-173. doi: 10.1186/1471-2458-6-173. PubMed PMID: 16820049; PubMed Central PMCID: PMC1524945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budge PJ, Griffin MR, Edwards KM, Williams JV, Verastegui H, Hartinger SM, et al. A household-based study of acute viral respiratory illnesses in Andean children. The Pediatric infectious disease journal. 2014;33(5):443–7. doi: 10.1097/INF.0000000000000135. doi: 10.1097/INF.0000000000000135. PubMed PMID: 24378948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grijalva CG, Griffin MR, Edwards KM, Johnson M, Gil AI, Verastegui H, et al. Concordance between RT-PCR-based detection of respiratory viruses from nasal swabs collected for viral testing and nasopharyngeal swabs collected for bacterial testing. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2014;60(3):309–12. doi: 10.1016/j.jcv.2014.04.011. doi: 10.1016/j.jcv.2014.04.011. PubMed PMID: 24875136; PubMed Central PMCID: PMC4055503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grijalva CG, Griffin MR, Edwards KM, Williams JV, Gil AI, Verastegui H, et al. The Study of Respiratory Pathogens in Andean Children. International journal of epidemiology. 2013 doi: 10.1093/ije/dyt065. doi: 10.1093/ije/dyt065. PubMed PMID: 23771719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budge PJ, Griffin MR, Edwards KM, Williams JV, Verastegui H, Hartinger SM, et al. Impact of home environment interventions on the risk of influenza-associated ARI in Andean children: observations from a prospective household-based cohort study. PloS one. 2014;9(3):e91247. doi: 10.1371/journal.pone.0091247. doi: 10.1371/journal.pone.0091247. PubMed PMID: 24622044; PubMed Central PMCID: PMC3951509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grijalva CG, Griffin MR, Edwards KM, Williams JV, Gil AI, Verastegui H, et al. The role of influenza and parainfluenza infections in nasopharyngeal pneumococcal acquisition among young children. Clin Infect Dis. 2014;58(10):1369–76. doi: 10.1093/cid/ciu148. doi: 10.1093/cid/ciu148. PubMed PMID: 24621951; PubMed Central PMCID: PMC4001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grijalva CG, Griffin MR, Edwards KM, Williams JV, Gil AI, Verastegui H, et al. Cohort profile: The study of respiratory pathogens in Andean children. International journal of epidemiology. 2014;43(4):1021–30. doi: 10.1093/ije/dyt065. doi: 10.1093/ije/dyt065. PubMed PMID: 23771719; PubMed Central PMCID: PMC4121548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanata CF, Rudan I, Boschi-Pinto C, Tomaskovic L, Cherian T, Weber M, et al. Methodological and quality issues in epidemiological studies of acute lower respiratory infections in children in developing countries. International journal of epidemiology. 2004;33(6):1362–72. doi: 10.1093/ije/dyh229. doi: 10.1093/ije/dyh229. PubMed PMID: 15166188. [DOI] [PubMed] [Google Scholar]
- 22.Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, et al. The underrecognized burden of influenza in young children. The New England journal of medicine. 2006;355(1):31–40. doi: 10.1056/NEJMoa054869. doi: 10.1056/NEJMoa054869. PubMed PMID: 16822994. [DOI] [PubMed] [Google Scholar]
- 23.Integrated management of childhood illness: conclusions WHO Division of Child Health and Development. Bulletin of the World Health Organization. 1997;75(Suppl 1):119–28. PubMed PMID: 9529725; PubMed Central PMCID: PMC2486994. [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien KL, Nohynek H, World Health Organization Pneumococcal Vaccine Trials Carraige Working G Report from a WHO working group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. The Pediatric infectious disease journal. 2003;22(2):133–40. doi: 10.1097/01.inf.0000048676.93549.d1. doi: 10.1097/01.inf.0000048676.93549.d1. PubMed PMID: 12586977. [DOI] [PubMed] [Google Scholar]
- 25.Satzke C, Turner P, Virolainen-Julkunen A, Adrian PV, Antonio M, Hare KM, et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine. 2013;32(1):165–79. doi: 10.1016/j.vaccine.2013.08.062. doi: 10.1016/j.vaccine.2013.08.062. PubMed PMID: 24331112. [DOI] [PubMed] [Google Scholar]
- 26.Ali SA, Gern JE, Hartert TV, Edwards KM, Griffin MR, Miller EK, et al. Real-world comparison of two molecular methods for detection of respiratory viruses. Virology journal. 2011;8:332. doi: 10.1186/1743-422X-8-332. doi: 10.1186/1743-422X-8-332. PubMed PMID: 21714915; PubMed Central PMCID: PMC3154182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kodani M, Yang G, Conklin LM, Travis TC, Whitney CG, Anderson LJ, et al. Application of TaqMan low-density arrays for simultaneous detection of multiple respiratory pathogens. Journal of clinical microbiology. 2011;49(6):2175–82. doi: 10.1128/JCM.02270-10. doi: 10.1128/JCM.02270-10. PubMed PMID: 21471348; PubMed Central PMCID: PMC3122721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klemenc J, Asad Ali S, Johnson M, Tollefson SJ, Talbot HK, Hartert TV, et al. Real-time reverse transcriptase PCR assay for improved detection of human metapneumovirus. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2012;54(4):371–5. doi: 10.1016/j.jcv.2012.05.005. doi: 10.1016/j.jcv.2012.05.005. PubMed PMID: 22677006; PubMed Central PMCID: PMC3412361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu X, Holloway B, Dare RK, Kuypers J, Yagi S, Williams JV, et al. Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. Journal of clinical microbiology. 2008;46(2):533–9. doi: 10.1128/JCM.01739-07. doi: 10.1128/JCM.01739-07. PubMed PMID: 18057136; PubMed Central PMCID: PMC2238069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner P, Po L, Turner C, Goldblatt D, Nosten F. Detection of respiratory viruses by PCR assay of nasopharyngeal swabs stored in skim milk-tryptone-glucose-glycerol transport medium. Journal of clinical microbiology. 2011;49(6):2311–3. doi: 10.1128/JCM.00224-11. doi: 10.1128/JCM.00224-11. PubMed PMID: 21450959; PubMed Central PMCID: PMC3122715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkwood BRSJ. Essential Medical Statistics. 2nd Blackwell Science; Massachusetts: 2003. [Google Scholar]
- 32.Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. American journal of epidemiology. 1974;99(5):325–32. doi: 10.1093/oxfordjournals.aje.a121617. PubMed PMID: 4825599. [DOI] [PubMed] [Google Scholar]
- 33.Singleton RJ, Bulkow LR, Miernyk K, DeByle C, Pruitt L, Hummel KB, et al. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol. 2010;82(7):1282–90. doi: 10.1002/jmv.21790. doi: 10.1002/jmv.21790. PubMed PMID: 20513097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feikin DR, Njenga MK, Bigogo G, Aura B, Aol G, Audi A, et al. Etiology and Incidence of viral and bacterial acute respiratory illness among older children and adults in rural western Kenya, 2007-2010. PloS one. 2012;7(8):e43656. doi: 10.1371/journal.pone.0043656. doi: 10.1371/journal.pone.0043656. PubMed PMID: 22937071; PubMed Central PMCID: PMC3427162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feikin DR, Njenga MK, Bigogo G, Aura B, Aol G, Audi A, et al. Viral and bacterial causes of severe acute respiratory illness among children aged less than 5 years in a high malaria prevalence area of western Kenya, 2007-2010. Pediatr Infect Dis J. 2013;32(1):e14–9. doi: 10.1097/INF.0b013e31826fd39b. doi: 10.1097/INF.0b013e31826fd39b. PubMed PMID: 22914561. [DOI] [PubMed] [Google Scholar]
- 36.Winther B, Alper CM, Mandel EM, Doyle WJ, Hendley JO. Temporal relationships between colds, upper respiratory viruses detected by polymerase chain reaction, and otitis media in young children followed through a typical cold season. Pediatrics. 2007;119(6):1069–75. doi: 10.1542/peds.2006-3294. doi: 10.1542/peds.2006-3294. PubMed PMID: 17545372. [DOI] [PubMed] [Google Scholar]
- 37.Peltola V, Waris M, Kainulainen L, Kero J, Ruuskanen O. Virus shedding after human rhinovirus infection in children, adults and patients with hypogammaglobulinaemia. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2013;19(7):E322–7. doi: 10.1111/1469-0691.12193. doi: 10.1111/1469-0691.12193. PubMed PMID: 23490188. [DOI] [PubMed] [Google Scholar]
- 38.Pathak AK, Adams RH, Shah NC, Gustin KE. Persistent human rhinovirus type C infection of the lower respiratory tract in a pediatric cord blood transplant recipient. Bone marrow transplantation. 2013;48(5):747–8. doi: 10.1038/bmt.2012.226. doi: 10.1038/bmt.2012.226. PubMed PMID: 23165503. [DOI] [PubMed] [Google Scholar]
- 39.Kaiser L, Aubert JD, Pache JC, Deffernez C, Rochat T, Garbino J, et al. Chronic rhinoviral infection in lung transplant recipients. American journal of respiratory and critical care medicine. 2006;174(12):1392–9. doi: 10.1164/rccm.200604-489OC. doi: 10.1164/rccm.200604-489OC. PubMed PMID: 17008640. [DOI] [PubMed] [Google Scholar]
- 40.Kainulainen L, Vuorinen T, Rantakokko-Jalava K, Osterback R, Ruuskanen O. Recurrent and persistent respiratory tract viral infections in patients with primary hypogammaglobulinemia. The Journal of allergy and clinical immunology. 2010;126(1):120–6. doi: 10.1016/j.jaci.2010.04.016. doi: 10.1016/j.jaci.2010.04.016. PubMed PMID: 20541246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garnett CT, Erdman D, Xu W, Gooding LR. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. Journal of virology. 2002;76(21):10608–16. doi: 10.1128/JVI.76.21.10608-10616.2002. PubMed PMID: 12368303; PubMed Central PMCID: PMC136639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin ET, Kuypers J, Heugel J, Englund JA. Clinical disease and viral load in children infected with respiratory syncytial virus or human metapneumovirus. Diagnostic microbiology and infectious disease. 2008;62(4):382–8. doi: 10.1016/j.diagmicrobio.2008.08.002. doi: 10.1016/j.diagmicrobio.2008.08.002. PubMed PMID: 18842376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houben ML, Coenjaerts FE, Rossen JW, Belderbos ME, Hofland RW, Kimpen JL, et al. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. Journal of medical virology. 2010;82(7):1266–71. doi: 10.1002/jmv.21771. doi: 10.1002/jmv.21771. PubMed PMID: 20513094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franz A, Adams O, Willems R, Bonzel L, Neuhausen N, Schweizer-Krantz S, et al. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2010;48(4):239–45. doi: 10.1016/j.jcv.2010.05.007. doi: 10.1016/j.jcv.2010.05.007. PubMed PMID: 20646956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuller JA, Njenga MK, Bigogo G, Aura B, Ope MO, Nderitu L, et al. Association of the CT values of real-time PCR of viral upper respiratory tract infection with clinical severity, Kenya. Journal of medical virology. 2013;85(5):924–32. doi: 10.1002/jmv.23455. doi: 10.1002/jmv.23455. PubMed PMID: 23508918. [DOI] [PubMed] [Google Scholar]
- 46.Kalu SU, Loeffelholz M, Beck E, Patel JA, Revai K, Fan J, et al. Persistence of adenovirus nucleic acids in nasopharyngeal secretions: a diagnostic conundrum. The Pediatric infectious disease journal. 2010;29(8):746–50. doi: 10.1097/INF.0b013e3181d743c8. doi: 10.1097/INF.0b013e3181d743c8. PubMed PMID: 20308936; PubMed Central PMCID: PMC3206289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall CB, Douglas RG, Jr., Geiman JM. Respiratory syncytial virus infections in infants: quantitation and duration of shedding. The Journal of pediatrics. 1976;89(1):11–5. doi: 10.1016/s0022-3476(76)80918-3. PubMed PMID: 180274. [DOI] [PubMed] [Google Scholar]
- 48.Okiro EA, White LJ, Ngama M, Cane PA, Medley GF, Nokes DJ. Duration of shedding of respiratory syncytial virus in a community study of Kenyan children. BMC infectious diseases. 2010;10:15. doi: 10.1186/1471-2334-10-15. doi: 10.1186/1471-2334-10-15. PubMed PMID: 20096106; PubMed Central PMCID: PMC2822777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King JC, Jr., Burke AR, Clemens JD, Nair P, Farley JJ, Vink PE, et al. Respiratory syncytial virus illnesses in human immunodeficiency virus- and noninfected children. The Pediatric infectious disease journal. 1993;12(9):733–9. doi: 10.1097/00006454-199309000-00006. PubMed PMID: 8414800. [DOI] [PubMed] [Google Scholar]
- 50.von Linstow ML, Eugen-Olsen J, Koch A, Winther TN, Westh H, Hogh B. Excretion patterns of human metapneumovirus and respiratory syncytial virus among young children. European journal of medical research. 2006;11(8):329–35. PubMed PMID: 17052968. [PubMed] [Google Scholar]
- 51.Manoha C, Espinosa S, Aho SL, Huet F, Pothier P. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2007;38(3):221–6. doi: 10.1016/j.jcv.2006.12.005. doi: 10.1016/j.jcv.2006.12.005. PubMed PMID: 17241812. [DOI] [PubMed] [Google Scholar]
- 52.Hara M, Takao S, Shimazu Y, Nishimura T. Three-year study of viral etiology and features of febrile respiratory tract infections in Japanese pediatric outpatients. The Pediatric infectious disease journal. 2014;33(7):687–92. doi: 10.1097/INF.0000000000000227. doi: 10.1097/INF.0000000000000227. PubMed PMID: 24378946. [DOI] [PubMed] [Google Scholar]
- 53.Edwards KM, Thompson J, Paolini J, Wright PF. Adenovirus infections in young children. Pediatrics. 1985;76(3):420–4. PubMed PMID: 6900. [PubMed] [Google Scholar]
- 54.Moore ML, Stokes KL, Hartert TV. The impact of viral genotype on pathogenesis and disease severity: respiratory syncytial virus and human rhinoviruses. Current opinion in immunology. 2013;25(6):761–8. doi: 10.1016/j.coi.2013.09.016. PubMed PMID: 24455766; PubMed Central PMCID: PMC4097120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin EK, Kuypers J, Chu HY, Lacombe K, Qin X, Strelitz B, et al. Molecular epidemiology of human rhinovirus infections in the pediatric emergency department. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2015;62:25–31. doi: 10.1016/j.jcv.2014.11.006. doi: 10.1016/j.jcv.2014.11.006. PubMed PMID: 25542466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller EK, Edwards KM, Weinberg GA, Iwane MK, Griffin MR, Hall CB, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123(1):98–104 e1. doi: 10.1016/j.jaci.2008.10.007. Epub 2008/11/26. doi: S0091-6749(08)01847-2 [pii] 10.1016/j.jaci.2008.10.007. PubMed PMID: 19027147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller EK, Mackay IM. From sneeze to wheeze: what we know about rhinovirus Cs. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2013;57(4):291–9. doi: 10.1016/j.jcv.2013.04.015. doi: 10.1016/j.jcv.2013.04.015. PubMed PMID: 23714395. [DOI] [PubMed] [Google Scholar]
- 58.Wong S, Pabbaraju K, Pang XL, Lee BE, Fox JD. Detection of a broad range of human adenoviruses in respiratory tract samples using a sensitive multiplex real-time PCR assay. Journal of medical virology. 2008;80(5):856–65. doi: 10.1002/jmv.21136. doi: 10.1002/jmv.21136. PubMed PMID: 18360899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nicholas R, Yaron M, Reeves J. Oxygen saturation in children living at moderate altitude. The Journal of the American Board of Family Practice / American Board of Family Practice. 1993;6(5):452–6. PubMed PMID: 8213235. [PubMed] [Google Scholar]
- 60.Gamponia MJ, Babaali H, Yugar F, Gilman RH. Reference values for pulse oximetry at high altitude. Archives of disease in childhood. 1998;78(5):461–5. doi: 10.1136/adc.78.5.461. PubMed PMID: 9659095; PubMed Central PMCID: PMC1717583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barry PW, Mason NP, O'Callaghan C. Nasal mucociliary transport is impaired at altitude. The European respiratory journal. 1997;10(1):35–7. doi: 10.1183/09031936.97.10010035. PubMed PMID: 9032488. [DOI] [PubMed] [Google Scholar]
- 62.Wu AB,PJ, Williams JV, Griffin MR, Edwards KM, Johnson M, Zhu Y, Hartinger S, Verastegui H, Gil AI, Lanata CF, Grijalva CG. Incidence and risk factors for respiratory syncytial virus and human metapneumovirus infections among children in the remote highlands of Peru. doi: 10.1371/journal.pone.0130233. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choudhuri JA, Ogden LG, Ruttenber AJ, Thomas DS, Todd JK, Simoes EA. Effect of altitude on hospitalizations for respiratory syncytial virus infection. Pediatrics. 2006;117(2):349–56. doi: 10.1542/peds.2004-2795. doi: 10.1542/peds.2004-2795. PubMed PMID: 16452353. [DOI] [PubMed] [Google Scholar]
- 64.Allen EK, Koeppel AF, Hendley JO, Turner SD, Winther B, Sale MM. Characterization of the nasopharyngeal microbiota in health and during rhinovirus challenge. Microbiome. 2014;2:22. doi: 10.1186/2049-2618-2-22. doi: 10.1186/2049-2618-2-22. PubMed PMID: 25028608; PubMed Central PMCID: PMC4098959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Babiuk LA, Lawman MJ, Ohmann HB. Viral-bacterial synergistic interaction in respiratory disease. Advances in virus research. 1988;35:219–49. doi: 10.1016/S0065-3527(08)60713-7. PubMed PMID: 3148270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bosch AA, Biesbroek G, Trzcinski K, Sanders EA, Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS pathogens. 2013;9(1):e1003057. doi: 10.1371/journal.ppat.1003057. doi: 10.1371/journal.ppat.1003057. PubMed PMID: 23326226; PubMed Central PMCID: PMC3542149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakwinska O, Bastic Schmid V, Berger B, Bruttin A, Keitel K, Lepage M, et al. Nasopharyngeal microbiota in healthy children and pneumonia patients. Journal of clinical microbiology. 2014;52(5):1590–4. doi: 10.1128/JCM.03280-13. doi: 10.1128/JCM.03280-13. PubMed PMID: 24599973; PubMed Central PMCID: PMC3993659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Degre M. Interaction between viral and bacterial infections in the respiratory tract. Scandinavian journal of infectious diseases Supplementum. 1986;49:140–5. PubMed PMID: 2434989. [PubMed] [Google Scholar]