Abstract
Environmental exposures to many phenols are documented worldwide and exposures can be quite high (>1 micromolar of urine metabolites). Phenols have a range of hormonal activity, but knowledge of effects on child reproductive development is limited, coming mostly from cross-sectional studies. We undertook a prospective study of pubertal development among 1239 girls recruited at three U.S. sites when they were 6–8 years old and were followed annually for 7 years to determine age at first breast or pubic hair development. Ten phenols were measured in urine collected at enrollment (benzophenone-3, enterolactone, bisphenol A, three parabens (methyl-, ethyl-, propyl-), 2,5-dichlorophenol, triclosan, genistein, daidzein). We used multivariable adjusted Cox proportional hazards ratios (HR (95% confidence intervals)) and Kaplan-Meier survival analyses to estimate relative risk of earlier or later age at puberty associated with phenol exposures. For enterolactone and benzophenone-3, girls experienced breast development 5–6 months later, adjusted HR 0.79 (0.64–0.98) and HR 0.80 (0.65–0.98) respectively for the 5th vs 1st quintiles of urinary biomarkers (μg/g-creatinine). Earlier breast development was seen for triclosan and 2,5- dichlorophenol: 4–9 months sooner for 5th vs 1st quintiles of urinary concentrations (HR 1.17 (0.96–1.43) and HR 1.37 (1.09–1.72), respectively). Association of breast development with enterolactone, but not the other three phenols, was mediated by body size. These phenols may be antiadipogens (benzophenone-3 and enterolactone) or thyroid agonists (triclosan and 2,5- dichlorophenol), and their ubiquity and relatively high levels in children would benefit from further investigation to confirm these findings and to establish whether there are certain windows of susceptibility during which exposure can affect pubertal development.
Keywords: phenols, breast development, puberty, environment
1. Introduction
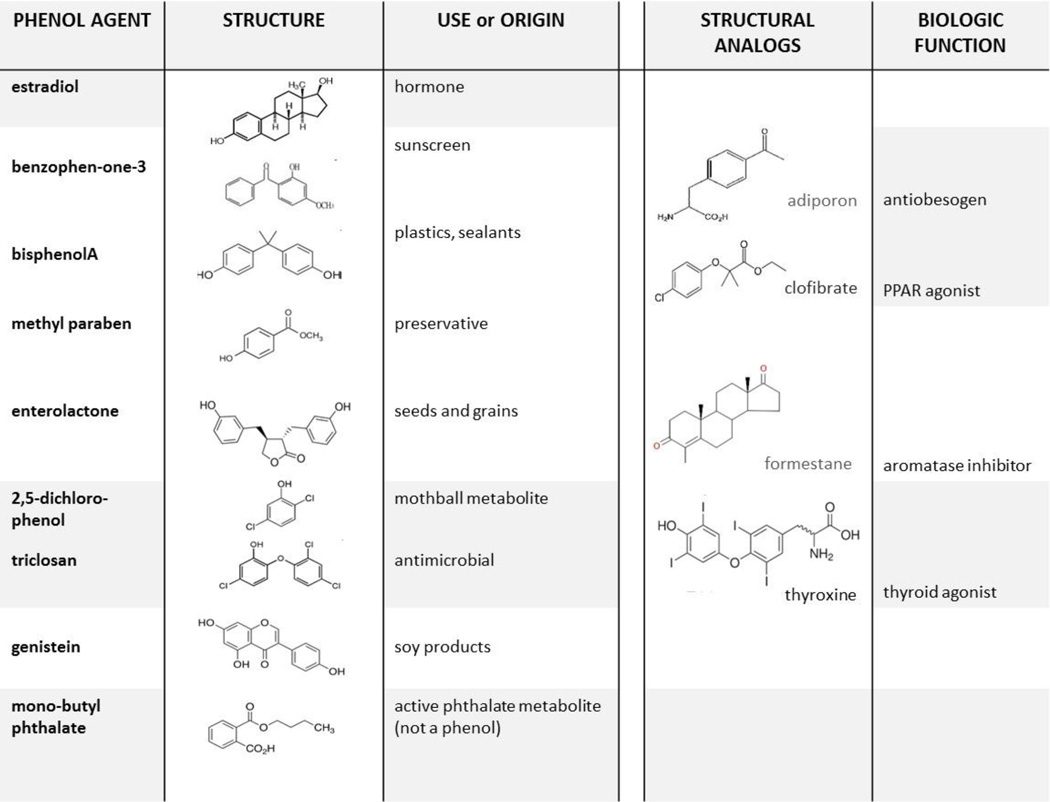
During the past 20 years, a new generation of environmental contaminants has emerged, including metabolites of chemicals used widely in commerce and derived from a variety of sources (Wolff, 2006). Human exposure exists universally, as documented by detection of urinary metabolites around the world (CDC, 2009; Moos et al., 2014; Philippat et al., 2012; Engel et al., 2014; Nahar et al., 2012; Xue et al., 2015). Reported urinary biomarkers include more than a dozen phenols, which may be a parent compound or metabolite, including phytoestrogen polyphenols that are dietary in origin. Biological effects have been seen in a variety of experimental models, potentially related to several hormonal mechanisms in humans including thyroid agonists and obesogens (Witorsch and Thomas, 2010). Structural homology with agents of known function suggests that varying responses exist for different phenols including possible mechanisms for common urinary phenols (Fig. 1). Several polyphenols resemble antiobesogens and aromatase inhibitors, and agents with a chorophenol moiety are similar to thyroid hormone (Buzdar and Howell, 2001; Gross and Staels, 2007; Okada-Iwabu et al., 2013).
Fig. 1.
Chemical structures of environmental phenols, examples of representative sources, and possible mechanistic parallels for converse associations with hormonal outcomes, including adiponectin agonists or aromatase inhibitors. (Buzdar and Howell, 2001; Okada-Iwabu et al., 2013). In addition, enterolactone is derived from lignans and both genistein and daidzein are isoflavone phytoestrogens mainly found in soy products and legumes.
Puberty is a reproductive milestone that signals the onset of maturity, and early puberty is likely a risk for metabolic disease and breast cancer (Biro and Wolff, 2011; Bodicoat et al., 2014). Action of environmental agents during puberty may be an indirect pathway to later disease. In particular, hormonal effects of environmental agents are relevant to breast development as well as changes in body size or obesity. Both earlier and later pubertal milestones have been seen with a number of phenols, mainly in cross-sectional studies (Biro and Wolff, 2011; Buttke et al., 2012). We reported previously on exposures to ten phenols among girls in the Breast Cancer and Environment Research Program (BCERP) Puberty Study when girls in the cohort were <10 years of age (Wolff et al., 2010). In this new analysis, we have investigated timing of pubertal onset across 7 years of follow-up, during which stage 2 of breast or pubic hair was reached by >85% of girls. Thus, we can now examine associations of exposures measured at enrollment in relation to actual ages for these benchmarks across the window of development, and with respect to changes in body size during this period.
2. Materials and Methods
2.1. Study design and data collection
The BCERP Puberty Study is a cohort that has followed girls enrolled starting in 2004. This report includes data collected through 2012 (up to seven years’ follow-up). Study sites included Icahn School of Medicine at Mount Sinai (MSSM) that recruited black or Hispanic girls mainly from East Harlem in New York City; Cincinnati Children’s Hospital (Cincinnati) recruited from the greater Cincinnati metropolitan area; and Kaiser Permanente Northern California (KPNC) that recruited members of the KPNC Health Plan in the San Francisco Bay Area. Eligible girls were 6–8 years of age without serious endocrine medical conditions. Informed consent was obtained from parent or guardian administered by the institutional IRBs. The Centers for Disease Control and Prevention (CDC) IRB approved the urine specimen analysis, which had no personal identifiers. For this report, we used demographic, anthropometric, and pubertal assessments and urinary phenol metabolites for which complete protocols and analytic methods have been described previously along with quality control measures (Biro et al., 2010; Wolff et al., 2010). As detailed there, we obtained age at stage 2, the first appearance of breast (B2) or pubic hair (PH2) development (vs stage 1, B1 or PH1, no development), as well as age- and sex-specific body mass index percentiles (BMI% at the last B1 or PH1 visit) calculated using the CDC growth charts (CDC, 2000). Urine specimens collected at enrollment were analyzed using well-established analytic methods at the CDC National Center for Environmental Health laboratory that were available for 10 metabolites that represent several families of phenols (benzophenone-3, enterolactone, bisphenol A, methyl-, ethyl-, propyl-parabens, 2,5-dichlorophenol, triclosan, genistein, and daidzein). We summed the paraben metabolites based on molecular weight, expressed as propyl paraben (molecular weight 180.2 g/mol). Phenols were detected in >80% of samples except for butyl paraben (48%). Concentrations below the limit of detection (LOD) were assigned the value LOD/√2. Concentrations (ln-μg/L) were normalized for urine dilution in linear models using ln-creatinine as a covariate or, in models with quintiles, using cutpoints based on creatinine-corrected biomarkers (μg/g-creatinine). There were 1170 girls with at least one exposure biomarker and pubertal stage information. The urinary phenol assays were the same as those reported earlier (Wolff et al., 2010), with a few analyses that were added at a later date; see Suppl Table 1 for a summary.
2.2. Statistical analyses
Analyses were performed with SAS (version 9.4; SAS Institute, Inc). We modeled relative risk for age when girls advanced from B1 to B2 or PH1 to PH2 using Cox Proportional Hazards models to compute Hazard ratios (HR) and 95% confidence intervals (CI) in relation to phenol exposures. More details on the Cox methods and handling of censored pubertal age values are given in (Wolff et al., 2014). We obtained adjusted median pubertal ages for quintiles of urinary concentrations using survival function estimates from the baseline survivor function of multivariable adjusted Cox models also previously described (Wolff et al., 2014). We obtained p-trend for quintiles by including the natural log of the median quintile urinary phenol concentrations as a continuous variable. The first and fifth quintile medians approximated the 10th and 90th percentile of concentrations in this cohort. Potential confounders were selected based on biological plausibility in the exposure-puberty pathway and knowledge of relationships among the variables (Table 1). We retained in the final models covariates that altered the estimates by more than 10% or improved precision of models, which were girl’s race/ethnicity and caregiver education. We did not include study site as a confounder as it has no characteristic causally related to puberty and phenols aside from the main covariates with which site was collinear (BMI%, caregiver education, and race) and because the study design variable, site, was intended to provide a range of exposures. BMI% may be in the biological pathway, and thus inclusion in the model may result in overadjustment. Also, the BMI trajectory, its consequent hormones, and pubertal timing differ for girls with high and low BMI (Biro et al., 2014). Therefore, we investigated the influence of BMI as a potential biological intermediate in four ways. We compared models with and without adjusting for BMI%; we evaluated BMI% as an effect modifier by adding an interaction term to the model (phenol concentration × BMI% dichotomized at the median, or 71st percentile, which we also used to designate low- (leaner) and high- (heavier) BMI girls); we tested whether BMI effects were stronger at younger ages (B2-age<120 months); and finally we undertook diagnostic mediation analyses to support the first three approaches. The mediation analyses followed that described elsewhere (Burns et al., 2014) using the R Package for Causal Mediation Analysis (Imai et al., 2010; Tingley D et al., 2013) which allowed for a survival outcome of B2 age, and a linear mediator, BMI%. Results were complementary to the other analyses, and are included in the supplemental material with further annotation (Suppl Table 2). We conducted additional sensitivity analyses to eliminate alternative explanations, including race, site, extremes of creatinine, and length of time within the study; none of these explained the findings. In addition, to explore whether exposures affected each other, we ran the model including all ten exposure biomarkers (as continuous variables) with age-at-B2; HR estimates were very similar to those for the individual biomarker models presented in Table 2.
Table 1.
Geometric means (95% CI) of creatinine-corrected phenol biomarker concentrations in urine (µg/g creatinine) collected at baseline in relation to covariates.
| Covariate | N | Benzophenone-3 | Enterolactone | 2,5-Dichlorophenol | Triclosan | |
|---|---|---|---|---|---|---|
| Age at baseline urine, years | 6.0–6.9 | 307 | 44.9 (35.4–56.8)* | 408 ( 357, 467)* | 22.2 (17.8, 27.6)* | 17.4 (14.6, 20.8) |
| 7.0–7.9 | 609 | 43.1 (36.5–51.0) | 516 ( 468, 568) | 11.3 (9.70, 13.2) | 17.6 (15.5, 20.1) | |
| >=8.0 | 254 | 21.5 (16.6–27.9) | 374 ( 322, 434) | 29.4 (23.1, 37.3) | 20.1 (16.5, 24.5) | |
| Race/ethnicity | Asian | 56 | 104 (62.1, 176)* | 484 ( 355, 660)* | 5.96 (3.81, 9.31)* | 14.1 (9.29, 21.5) |
| Black | 366 | 17.0 (13.8, 20.8) | 446 ( 394, 505) | 34.0 (28.6, 40.5) | 18.8 (16.0, 22.2) | |
| Hispanic | 360 | 25.7 (20.9, 31.5) | 349 ( 309, 395) | 40.0 (33.5, 47.7) | 16.5 (14.0, 19.5) | |
| White | 388 | 97.1 (79.7, 118) | 575 ( 511, 647) | 4.32 (3.64, 5.12) | 19.7 (16.8, 23.2) | |
| BMI% at last B1 visit | <50th Percentile | 390 | 47.4 (38.4, 58.6)* | 564 ( 501, 635)* | 13.1 (10.8, 15.9)* | 19.8 (16.9, 23.2) |
| 50–85th Percentile | 376 | 35.2 (28.4, 43.6) | 477 ( 423, 539) | 15.4 (12.6, 18.8) | 17.4 (14.7, 20.4) | |
| >=85th Percentile | 403 | 31.7 (25.8, 39.0) | 345 ( 307, 388) | 22.5 (18.6, 27.3) | 17.4 (14.8, 20.3) | |
| BMI% at last PH1 visit | <50th Percentile | 387 | 49.8 (40.3, 61.5)* | 566 ( 503, 637)* | 12.3 (10.1, 15.0)* | 19.5 (16.6, 22.9) |
| 50–85th Percentile | 374 | 33.3 (26.9, 41.3) | 483 ( 428, 546) | 16.2 (13.3, 19.8) | 17.3 (14.7, 20.3) | |
| >=85th Percentile | 403 | 31.7 (25.8, 39.0) | 339 ( 301, 381) | 22.6 (18.7, 27.5) | 17.7 (15.1, 20.7) | |
| Caregiver education | <= HS | 348 | 20.1 (16.1, 25.1)* | 344 ( 303, 390)* | 43.1 (35.3, 52.6)* | 17.8 (15.0, 21.0) |
| > HS | 794 | 50.2 (43.4, 58.1) | 512 ( 471, 556) | 10.8 (9.51, 12.4) | 18.0 (16.1, 20.2) | |
| Site | California | 434 | 106 (88.5, 128)* | 627 ( 561, 701)* | 5.09 (4.39, 5.89)* | 13.2 (11.4, 15.3)* |
| New York | 405 | 14.8 (12.2, 17.9) | 336 ( 300, 377) | 86.6 (74.4, 101) | 17.1 (14.7, 19.9) | |
| Ohio | 331 | 29.8 (24.1, 36.8) | 426 ( 375, 484) | 10.4 (8.77, 12.3) | 29.0 (24.5, 34.4) | |
| Season at urine | Fall | 229 | 24.8 (19.4, 31.7)* | 503 ( 428, 592) | 26.0 (20.1, 33.5) | 16.1 (13.0, 20.0) |
| Spring | 388 | 24.4 (20.2, 29.4) | 440 ( 390, 496) | 13.5 (11.1, 16.4) | 19.4 (16.5, 22.8) | |
| Summer | 322 | 175 ( 142, 215) | 429 ( 376, 489) | 11.8 (9.53, 14.6) | 17.3 (14.5, 20.6) | |
| Winter | 228 | 13.4 (10.5, 17.2) | 459 ( 392, 538) | 24.8 (19.2, 32.0) | 19.0 (15.4, 23.4) | |
| ALL | 1170 | 37.5 (33.2, 42.3) | 452 ( 422, 484) | 16.6 (14.8, 18.6) | 18.1 (16.5, 19.8) | |
Asterisks after the first category indicates that the biomarker varies by that characteristic, p<.05 for unadjusted geometric means by ANOVA. There were 1170 girls with at least one biomarker and creatinine. There were 1170 observations with at least one biomarker and creatinine. Not all girls had all biomarkers, as detailed in Suppl Table 1.
Table 2.
Association of phenol urinary biomarker concentrations (ln-µg/L) with age at first breast or first pubic hair development (stage 2), BCERP cohort 2004–2011
| AGE AT B2 | adjusted for ln-creatinine | further adjusted for race/ethnicity & caregiver education |
|||||
|---|---|---|---|---|---|---|---|
| biomarker | HR (95% CI) | P_value | N | HR (95% CI) | P_value | N | P- interaction BMI |
| benzophenone-3 | 0.93 ( 0.90 – 0.96 ) | <.0001 | 1169 | 0.95 ( 0.92 – 0.98 ) | 0.001 | 1141 | 0.034 |
| enterolactone | 0.95 ( 0.91 – 1.00 ) | 0.055 | 1147 | 0.94 ( 0.90 – 0.99 ) | 0.032 | 1119 | 0.338 |
| Bisphenol A | 1.00 ( 0.93 – 1.08 ) | 0.960 | 1169 | 0.99 ( 0.91 – 1.06 ) | 0.709 | 1141 | 0.869 |
| Paraben Sum | 1.06 ( 1.01 – 1.11 ) | 0.012 | 1079 | 1.01 ( 0.96 – 1.06 ) | 0.668 | 1051 | 0.209 |
| 2,5-dichlorophenol | 1.07 ( 1.04 – 1.11 ) | <.0001 | 1169 | 1.05 ( 1.01 – 1.09 ) | 0.008 | 1141 | 0.017 |
| Triclosan | 1.06 ( 1.02 – 1.10 ) | 0.005 | 1157 | 1.05 ( 1.01 – 1.09 ) | 0.013 | 1129 | 0.240 |
| Genistein | 0.98 ( 0.94 – 1.02 ) | 0.279 | 1147 | 1.00 ( 0.96 – 1.04 ) | 0.989 | 1119 | 0.582 |
| Daidzein | 0.99 ( 0.95 – 1.03 ) | 0.599 | 1147 | 1.01 ( 0.97 – 1.04 ) | 0.740 | 1119 | 0.790 |
| AGE AT PH2 | adjusted for ln-creatinine |
further adjusted for race/ethnicity & caregiver education |
|||||
| biomarker | HR (95% CI) | P_value | N | P_value | N | ||
| benzophenone-3 | 0.96 ( 0.93 – 0.99 ) | 0.005 | 1164 | 1.00 ( 0.97 – 1.03 ) | 0.972 | 1136 | 0.562 |
| enterolactone | 0.95 ( 0.91 – 1.00 ) | 0.064 | 1143 | 0.93 ( 0.88 – 0.98 ) | 0.011 | 1115 | 0.303 |
| Bisphenol A | 1.03 ( 0.96 – 1.11 ) | 0.416 | 1164 | 1.02 ( 0.95 – 1.10 ) | 0.564 | 1136 | 0.624 |
| Paraben Sum | 1.10 ( 1.05 – 1.15 ) | <.0001 | 1074 | 1.02 ( 0.97 – 1.07 ) | 0.388 | 1046 | 0.997 |
| 2,5-DCP | 1.09 ( 1.05 – 1.12 ) | <.0001 | 1164 | 1.05 ( 1.01 – 1.09 ) | 0.013 | 1136 | 0.704 |
| Triclosan | 1.01 ( 0.98 – 1.06 ) | 0.464 | 1153 | 1.01 ( 0.97 – 1.05 ) | 0.771 | 1125 | 0.666 |
| Genistein | 0.97 ( 0.94 – 1.01 ) | 0.156 | 1143 | 1.00 ( 0.97 – 1.04 ) | 0.845 | 1115 | 0.821 |
| Daidzein | 0.98 ( 0.94 – 1.02 ) | 0.228 | 1143 | 0.99 ( 0.96 – 1.03 ) | 0.785 | 1115 | 0.979 |
Estimates are derived from Cox proportional hazards models, with age at stage 2 as outcome and urinary phenol as the exposure (ln-ug/L). Adjusted models excluded 28 observations with missing caregiver education. PH staging was missing on 5 girls, 4 with phytoestrogens. Interaction models include a term for biomarker concentration × median BMI-% (<, ≥ 71%)) and are adjusted for ln-creatinine, race/ethnicity and caregiver education. Paraben sum is the molar sum of methyl-, ethyl-, and propyl-parabens, expressed as propylparaben.
3. Results
The BCERP cohort includes approximately equal numbers of black, Hispanic and white girls, with few Asians. Concentrations of the four phenols that are the focus of this paper are shown in Table 1 in relation to key characteristics (unadjusted). Adjusted geometric means of all ten phenols differed by race/ ethnicity (except triclosan), education (except 2,5-dichlorophenol and triclosan), season of urine collection (benzophenone-3, 2,5-dichlorophenol), BMI% (enterolactone only), as reported (Wolff et al., 2010).
3.1
Urinary concentrations of four phenols were associated with age-at-B2 and two phenols with age-at-PH2 in adjusted proportional hazards models (ln-μg/L, continuous variable; Table 2). Paraben concentrations were associated with earlier age-at-B2 and -PH2 in creatinine-only adjusted models, but there was no association after further adjustment for race/ethnicity and caregiver education (Table 2). No associations with pubertal ages were found for bisphenol A or the two isoflavones (genistein and daidzein).
3.2
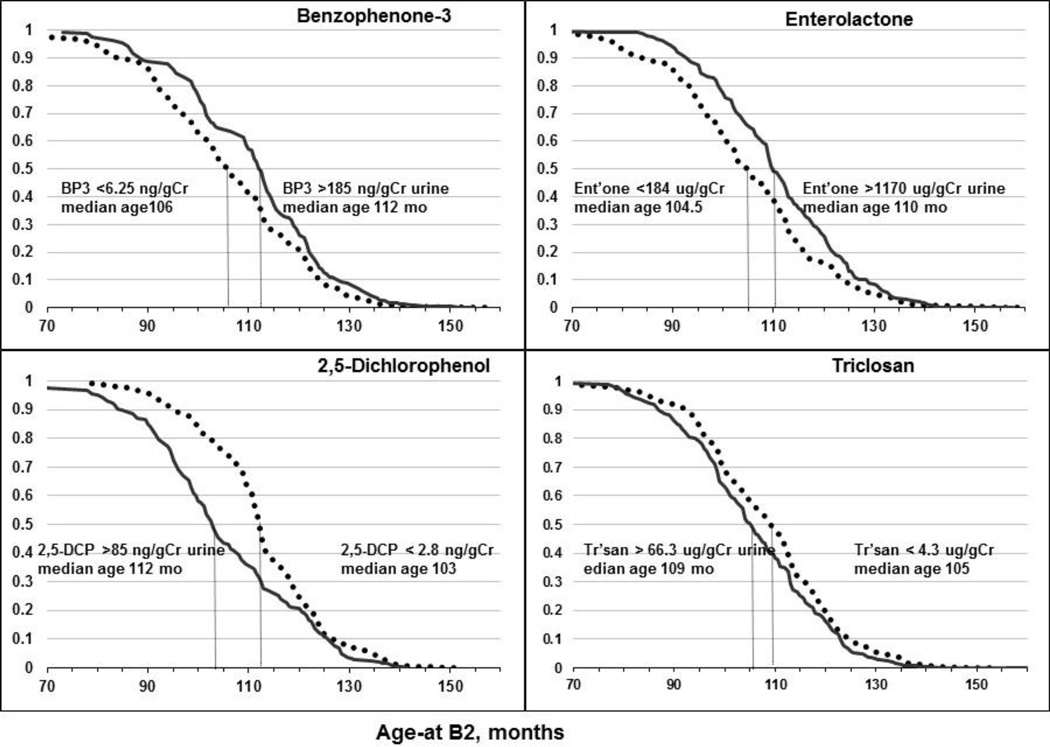
Benzophenone-3 and enterolactone, which may share certain mechanisms related to obesity antagonists (Fig. 1), were associated with older age-at-B2 (Tables 2, 3). For each phenol, the reduced risk for age at transition from B1 to B2 amounted to 5–6 months’ later age-at-B2 when comparing girls in the 5th vs 1st quintile of urinary concentrations (bottom of Table 3 and Fig. 2). Age-at-PH2 was also later for enterolactone with estimates similar to those for age-at-B2 (approximately 20% reduced risk for the 5th vs 1st quintile; Fig. 3). Of note, the concentration range across quintiles was wide for benzophenone-3 and enterolactone (200-fold and 20-fold, respectively). Both urinary phenols had concentrations >5-micromolar in the fifth quintile.
Table 3.
Quintiles of urinary phenol concentrations and risk for age to achieve first breast development (stage 2, B2).
| Hazards ratios for phenol biomarkers and age-at-b2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benzophenone-3 (molecular weight = 228.24) |
Enterolactone (molecular weight 298.33) |
2,5-Dichlorophenol (molecular weight 163) |
Triclosan (molecular weight 289.54) |
|||||||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||||||
| ln-urinary biomarker, ug/L | 0.95 (0.92–0.98) | 0.94 (0.90–0.99) | 1.05 (1.01–1.09) | 1.05 (1.01–1.09) | ||||||||
| Biomarker medians | Biomarker medians: | Biomarker medians: | Biomarker medians: | |||||||||
| Quintile | ug/gCr | um/gCr | ug/gC | um/gCr | ug/gCr | um/gCr | ug/gCr | um/gCr | ||||
| 1 | 3.4 | 0.01 | 1 (ref) | 92 | 0.31 | 1 (ref) | 1.6 | 0.01 | 1 (ref) | 2.6 | 0.01 | 1 (ref) |
| 2 | 10 | 0.04 | 1.08 (0.89–1.33) | 280 | 0.94 | 1.02 (0.83–1.25) | 4.6 | 0.03 | 1.07 (0.88–1.30) | 7 | 0.02 | 0.96 (0.79–1.17) |
| 3 | 27 | 0.12 | 1.15 (0.94–1.41) | 500 | 1.7 | 0.92 (0.75–1.12) | 13 | 0.08 | 1.34 (1.08–1.65) | 15 | 0.05 | 1.05 (0.86–1.28) |
| 4 | 86 | 0.38 | 0.80 (0.65–0.98) | 870 | 2.9 | 1.01 (0.82–1.24) | 45 | 0.28 | 1.31 (1.04–1.64) | 38 | 0.13 | 1.17 (0.96–1.42) |
| 5 | 780 | 3.4 | 0.79 (0.64–0.98) | 1,800 | 6.0 | 0.80 (0.65–0.98) | 220 | 1.40 | 1.37 (1.09–1.72) | 170 | 0.60 | 1.17 (0.96–1.43) |
| p-trend for log medians |
0.0007 | 0.041 | 0.0037 | 0.027 | ||||||||
| Median age-at-b2 by phenol quintiles (adjusted) | ||||||||||||
| Quintile | ug/gCr | um/gCr | Ages at Median Survival, months |
ug/gCr | um/gCr | Ages at Median Survival, months |
ug/gC | um/gCr | Ages at Median Survival, months |
ug/gCr | um/gCr | Ages at Median Survival, months |
| 1 | 3.4 | 0.01 | 106 | 92 | 0.31 | 104.5 | 1.6 | 0.01 | 112 | 2.6 | 0.01 | 109 |
| 2 | 10 | 0.04 | 103 | 280 | 0.94 | 105 | 4.6 | 0.03 | 107.5 | 7 | 0.02 | 109.5 |
| 3 | 27 | 0.12 | 104.5 | 500 | 1.7 | 105.5 | 13 | 0.08 | 105.5 | 15 | 0.05 | 107 |
| 4 | 86 | 0.38 | 111 | 870 | 2.9 | 108 | 45 | 0.28 | 105 | 38 | 0.13 | 103.5 |
| 5 | 780 | 3.4 | 112 | 1,800 | 6.0 | 110 | 220 | 1.40 | 103 | 170 | 0.60 | 105 |
| p for Q5 vs Q1 | 0.032 | p for Q5 vs Q1 | 0.032 | p for Q5 vs Q1 | 0.006 | p for Q5 vs Q1 | 0.113 | |||||
| N | 1141 | N | 1119 | N | 1141 | N | 1128 | |||||
| p-interaction BMI × quintiles 0.302 | p-interaction 0.557 | p-interaction 0.061 | p-interaction 0.342 | |||||||||
Models are adjusted for race/ethnicity and caregiver education. Interaction models included a term for biomarker quintile × median BMI-% (< and ≥ 71%)). P-trends derive from models that used ln-quintile medians as a continuous variable instead of quintiles as class variables.
Abbreviation for concentrations is ug/gCr (microgram per gram creatinine), um/gCr (micromole per gram creatinine).
Fig. 2.
Adjusted Kaplan-Meier curves for age-at-B2 in relation to 1st and 5th quintiles of four urinary phenol biomarkers. See Table 3 for details. Median concentrations for the quintiles are shown, and the vertical lines approximate the age-at-B2 at median survival.
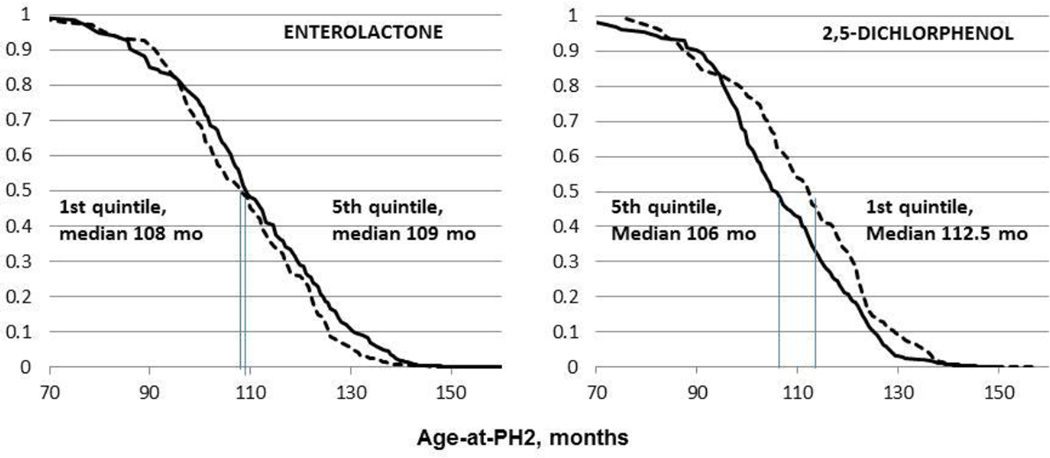
Fig. 3.
Adjusted Kaplan-Meier plots of age-at-PH2 by quintiles of urinary phenol biomarkers (adjusted for race/ethnicity and caregiver education). For enterolactone age was later (HR for 5th vs 1st quintile = 0.81 (0.66–0.99) p trend .039); for 2,5-DCP age was earlier HR= 1.24 (0.99–1.56), p-trend .013 (Table 3).
3.3
For the two possible thyroid agonists, 2,5-dichlorophenol and triclosan (Fig. 1), higher concentrations of 2,5-dichlorophenol and triclosan were associated with younger age-at-B2. Risks were greater for 2,5-dichlorophenol than triclosan (37% vs 17% increase in 5th quintiles respectively), with significant trends for both. Similarly, age-at-B2 was 9 months earlier for 2,5-dichlorophenol but only 4 months earlier for triclosan in the 5th quintile vs 1st quintile (Table 3). Exposure effects are further illustrated in the survival curves for age-at- B2 which are farther apart for 2,5-dichlorophenol quintiles than for triclosan (Fig. 2). 2,5-Dichlorophenol (but not triclosan) was also associated with earlier age-at-PH2 (approximately 25% increased risk for the 5th vs 1st quintile; Fig. 3). The weaker association of triclosan may be due to the smaller concentration range (70-fold for the median of 5th vs 1st quintiles) compared with 2,5-dichlorophenol (130-fold, Table 3).
3.4.1
BMI adjustment attenuated enterolactone relationships with age-at-B2 and -PH2 but did not alter associations of benzophenone-3, 2,5-dichlorophenol, or triclosan with pubertal onset. The increasing dose-response of median ages with enterolactone quintiles, as presented in Table 3, did not change upon BMI% adjustment, but trends were no longer significant. The accompanying HRs for enterolactone-B2 models were attenuated and the CIs were wider, although the change in risk was small. Thus, for continuous enterolactone with age-at-B2 the BMI-adjusted estimate moved toward the null by ~4%, with HR 0.98 (CI 0.93–1.04, not shown) vs. not-BMI-adjusted (shown in Table 2, HR 0.94, CI 0.90–0.99); CIs closely overlapped. Similarly, the BMI%-adjusted HR for PH2-age was 0.97 (0.92–1.03) (continuous variables, ln-μg/L, not shown, compared to the significant HR in Table 2).
3.4.2
Interactions (phenol ×BMI%) did not explain associations of phenol quintiles with pubertal ages (Table 3). The two significant interactions for models using continuous urinary concentration (Table 2) were null in models with biomarker quintiles (Table 3). The precision of estimates across phenol quintiles was better in the high- than low- BMI strata. For example, the B2-ages declined with 2,5-dichlorophenol quintiles among all girls (p trend=0.006, Table 3); the decline was similar among high-BMI (p=0.001) but not low-BMI girls (p= 0.143; not shown). Similarly, among all girls the median age-at-B2 was 9 months younger for 5th than 1st quintile of 2,5-dichlorophenol (Table 3), while in high-BMI girls, median ages-at-B2 were 12 months younger but only 2 months younger in low-BMI (not shown).
3.4.3
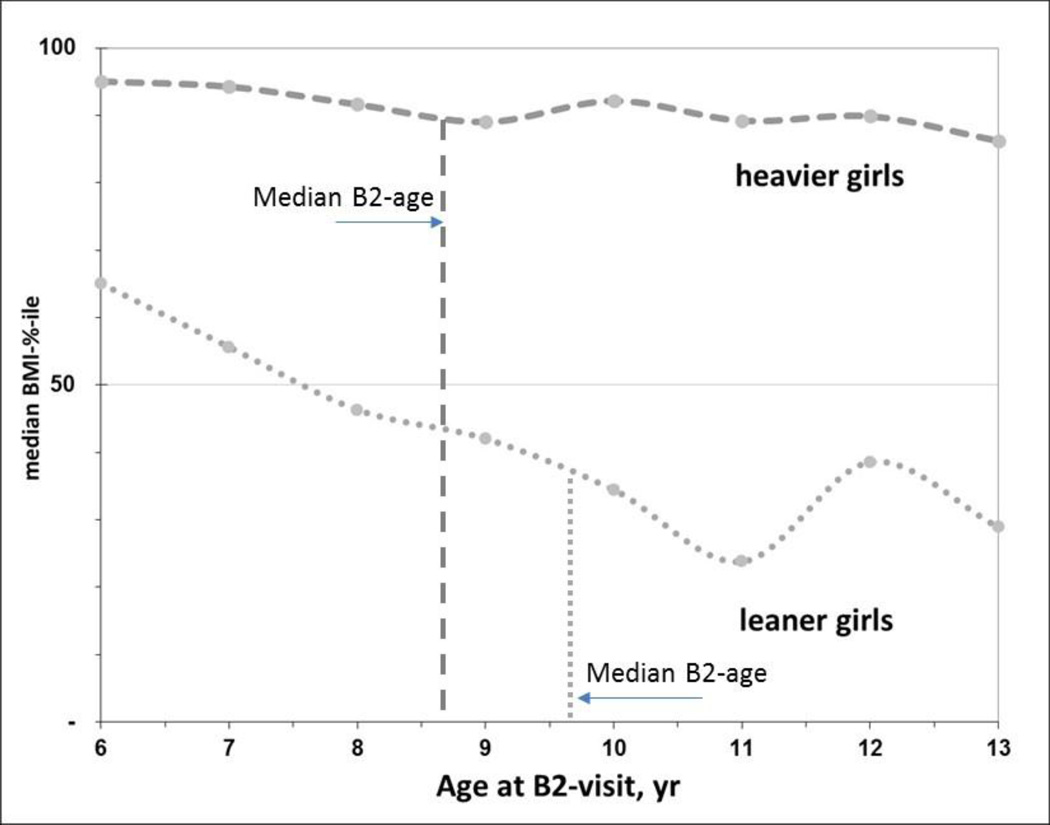
In secondary analyses we explored associations of the four phenols with B2-age over a shorter interval after baseline, when the urinary phenol measurements were made. At 120 months (10 yr) the survival curves for age-at-B2 between exposure quintiles became closer, as seen in the Kaplan-Meier plots for 5th vs 1st quintiles of four phenols (Fig. 2). When we restricted models to this interval (age-at-B2 <120 mo), BMI adjustment no longer altered any of the phenol associations with B2-age; estimates were not attenuated, and precision was similar or better than those in Table 2 (not shown). In addition, the BMI trajectory over this window differed markedly for leaner and heavier girls (<71% vs ≥71% BMI) (Fig. 4). Heavier girls matured earlier and half had reached B2 by age 8.7 years, whereas leaner girls developed one year later (12 months; Fig. 4). Notably, among heavier girls pre-pubertal BMI% remained fairly constant over this interval (medians hover around the 90th percentile, Fig. 4). But among leaner girls (<71%-BMI), median BMI% decreased from 65th to 34th percentile for age-at-B2 occurring between 72 and 132 months of age (6–11 years, Fig. 4). Thus, in leaner girls, those who matured earlier had relatively higher BMI%, e.g. lean girls maturing at 6–7 years had median BMI% > 50 while at 10–11 years girls median BMI% was <40. Among lighter-weight girls maturing after 132 months of age (11 years), there was a slight increase in BMI%, viz. from median 24% at age 11 to 34% at age 12 years (Fig. 4). Finally, we undertook formal mediation analyses to determine if BMI were in the pathway between exposure and B2. Enterolactone was the only phenol with an indirect, or BMI-mediating, effect on age-at-B2 (i.e., indicating a pathway of enterolactone → BMI →B2-age), but this mediation was much weaker among girls with B2 before 120 months (Suppl Table 2). For age-at-PH2, survival curves did not converge at later ages as they did for B2-age (5th vs 1st quintiles of 2,5-dichlorophenol and enterolactone biomarkers; Fig. 3). Age-at-PH2 associations with phenols were less influenced by BMI, possibly as pubarche is governed more by androgen than by estrogen (Biro et al., 2014).
Fig. 4.
BMI varies considerably during puberty among lean but not heavy girls. Shown is the median BMI-percentile for heavier (≥71%ile BMI, dashed line) and leaner girls (<71%-ile BMI, dotted line) at the B2-visit-age (integer years). The median ages-at-B2 differed by 12 months between heavier and leaner girls in this sample, estimated from Kaplan-Meier analyses. Thus the vertical lines at 8.7 and 9.7 years are when half of the girls had reached B2 by that age. The slight upswing in median BMI% for leaner girls from 11–13 years of age is similar to the rising pattern of estrogen levels found among leaner girls by Biro et al., 2014, suggesting a reciprocal relationship between BMI and estradiol among lean girls with later age-at-B2.
4. Discussion and Conclusions
In this prospective study, four phenols were found to be associated with onset of puberty. For breast development, altered risk estimates of ±20–40% were seen between the 90th vs 10th percentiles of urinary phenol concentrations. Age-at-B2 was 5–6 months later over this exposure range for benzophenone-3 and enterolactone where a possible mechanism may be their antiobesogenic properties. B2 occurred 4–9 months earlier for 2,5-dichlorophenol and triclosan possibly related to thyroid regulation. These risks are smaller but similar in magnitude to the two strongest risk factors for puberty, race/ethnicity and BMI. In our cohort age-at-B2 was 9 months younger among black than white girls (Biro et al., 2014), and historically menarche has been earlier by approximately six months in black girls (Biro and Wolff, 2011). Heavier girls experience B2 about 12 months earlier than leaner girls (Fig. 4) (Biro et al., 2013).
A benefit of longitudinal analyses was that we could examine exposures, B2-age and BMI over 7 years of observation. We found stronger associations of phenols with B2 among younger, heavier girls. Possibly the exposure window favored associations among girls who matured nearer to the exposure measures, who were also higher BMI. Similarly, BMI-exposure relationships may also differ at various moments in the pubertal window. Also, statistical imbalance may exist for adjustment by BMI that we could not identify and may be greater at later ages when there are very lean and very heavy girls. The markedly steep trajectory of BMI% among leaner girls during the pubertal window suggests that developmental pathways may differ over time (Fig. 4). In addition a bump in the BMI trajectory late in puberty is similar to a reported upswing in estradiol around onset of B2 among low- BMI girls (Biro et al., 2014), offering a unique window of development for girls with low BMI. If so, such a window offers an additional reasons why later B2-age associations with exposure may be weaker, because a rise in endogenous estrogen among lean girls at older ages may overwhelm any impact by less potent environmental hormone modulators (Biro and Wolff, 2011; Witorsch and Thomas, 2010).
Our results suggest that enterolactone may have an indirect or mediating effect on the association between enterolactone and pubertal onset that is more pronounced at earlier ages. Younger girls who reached B2 had higher BMI, i.e., maybe favoring a pathway of enterolactone → BMI →B2-age. Enterolactone is thought to inhibit aromatase formation of estrogen in peripheral adipose (Buzdar and Howell, 2001), which might be more likely in the earlier window. This result is consistent with our earlier analysis of this cohort where the main finding was a strong inverse association of enterolactone with age-at-B2 among high-BMI girls that we interpreted as attenuation of the BMI-effect on breast development (enterolactone × BMI p-interaction <.001). At the time of that analysis, the median age of the cohort was 8.5 yrs, when the influence of BMI on development was strong. In that cross-sectional analysis of phenols few girls had reached B2 (26%) or PH2 (18%), and we used prevalence ratios (PR) to estimate associations (Wolff et al., 2010). In the current analysis of girls with urinary phenols, 84% of girls had reached stage PH2 and 88% were B2. Overall, the 2010 cross-sectional findings are similar to the longitudinal results of this paper for B2 but not PH2, possibly due to the low frequency of PH2 in the 2010 data. In models with the 2010 data for phenols as continuous variables controlling for the same covariates, the PRs for B2 (dichotomous outcome) were the same in terms of direction and significance as those for the HR models in Table 2 (age-at-B2), while the PRs for PH2 were null (data not shown). Other reports have found similar associations with phthalate exposure biomarkers and pubertal onset using cross-sectional or longitudinal data (Wolff et al., 2010; Wolff et al., 2014; Frederiksen et al., 2012).
In a recent review, we listed evidence for fourteen chemical and physical agents that have been associated with either earlier or later puberty as well as with altered risk for breast cancer (Biro and Wolff, 2011). Included were polyphenols such as enterolactone and isoflavones. We observed no associations with puberty for the two isoflavone urinary biomarkers genistein and daidzein in either analysis of our cohort. Others have reported delay of breast development by phytoestrogens in smaller cross-sectional studies (Wolff et al., 2008; Bandera et al., 2011), and dietary phytoestrogen intake has also been associated with delayed development (Mervish et al., 2013; Cheng et al., 2010). Associations have been reported in cross-sectional data between urinary 2,5-dichlorophenol and earlier menarche (Buttke et al., 2012). A possible reason for not observing associations with parabens in our adjusted models is that we measured the parent phenols but not secondary metabolites. Although the parent parabens are exposure-specific biomarkers, they may represent a small proportion of the metabolized agents (Wang and Kannan, 2013). Bisphenol A urinary concentrations were quite low compared to other phenols in this cohort, comparable to the low levels seen in most reports, which may limit power to observe associations. Among possible limitations, there is concern about using a single biomarker for estimation of exposures to a chemical that is short-lived in the body and possibly episodic in nature, but we and others have shown acceptable intra-individual variability over more than a year and excellent ranking over time (Teitelbaum et al., 2008; Engel et al., 2014; Calafat et al., 2015).
Finally, several phenol exposures reported in this study are very common worldwide, are elevated in children, and urinary concentrations approach levels relevant to biologic activity in experimental models (i.e. the top quintile of four biomarkers had a median concentration in the range of 1–5 micromolar, Table 3). If associations with phenol xenobiotics are found that alter risk by 20% for the rather inexact outcome of pubertal onset, then perhaps larger effects on more subtle endpoints can be expected. Intermediate links between exposure and puberty may involve adipogenesis or thyroid action, mechanisms that can be investigated using physiologic biomarker measurements, hormones or genetic variants, or other methods such as molecular homology and toxicology. Cofactors such as nutrition that are relevant for the biological action of obesogens and thyroid agonists may also be of interest for further research on these common phenols. Consideration may also be required to more precisely ascertain when effective exposure may occur during the trajectory of pubertal development, as the BMI-effect may be greater at older ages for low- than high-BMI girls.
Supplementary Material
Highlights.
Phenols are associated with timing of breast and pubic hair development in girls
Associations suggest earlier or later pubertal development for different phenols
Associations of phenols with breast development may be mediated by body size
Associations may differ for different timeframes of the developmental window
Acknowledgments
We gratefully acknowledge our collaborators at the centers involved in this research including Jessica Montana, Nancy Mervish, Cheryl Stein, Rochelle Osborne, Lisa Boguski, Joel Forman, and Barbara Brenner (MSSM); Gayle Greenberg, Peggy Monroe, Bob Bornschein (Cincinnati); Robert Hiatt, Louise Greenspan, Julie Deardorff, Janice Barlow (Kaiser Permanente). We also thank Daniel L. Parker, Xiaoyun Ye, Amber Bishop, and Tao Jia for measurement of the phenol metabolites. This research was supported by the Breast Cancer and the Environment Research Program (BCERP) award numbers U01ES012770, U01ES012771, U01ES012800, U01ES012801, U01ES019435, U01ES019453, U01ES019454, U01ES019457, R827039 and P01ES009584, P30ES006096, P30ES023515 from the National Institute of Environmental Health Sciences (NIEHS), the National Cancer Institute (NCI), EPA, NIH, DHHS, CSTA-UL1RR029887, NYS Empire Clinical Research Investigator Program, Pediatric Environmental Health Fellowship HD049311, and the Avon Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NCI, the National Institutes of Health, the Centers for Disease Control and Prevention, or the California Department of Public Health.
ABBREVIATIONS
- BMI
body mass index
- B1, B2
breast stage 1, 2
- PH1, 2
pubic hair stage 1,2
- LOD
limit of detection
- CDC
Centers for Disease Control and Prevention
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mary S. Wolff, Email: mary.wolff@mssm.edu.
Susan L. Teitelbaum, Email: susan.teitelbaum@mssm.edu.
Kathleen McGovern, Email: kathleen.mcgovern@mssm.edu.
Susan M. Pinney, Email: pinneysm@ucmail.uc.edu.
Gayle C. Windham, Email: gayle.windham@cdph.ca.gov.
Maida Galvez, Email: maida.galvez@mssm.edu.
Ashley Pajak, Email: ashley.pajak7@mssm.edu.
Michael Rybak, Email: szr8@cdc.gov.
Antonia M. Calafat, Email: aic7@cdc.gov.
Lawrence H. Kushi, Email: larry.kushi@kp.org.
Frank M. Biro, Email: frank.biro@cchmc.org.
Reference List
- Bandera EV, Chandran U, Buckley B, Lin Y, Isukapalli S, Marshall I, King M, Zarbl H. Urinary Mycoestrogens, Body Size and Breast Development in New Jersey Girls. Sci Total Environ. 2011;409:5221–5227. doi: 10.1016/j.scitotenv.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM, Galvez MP, Greenspan LC, Succop PA, Vangeepuram N, Pinney SM, Teitelbaum S, Windham GC, Kushi LH, Wolff MS. Pubertal Assessment Methods and Baseline Characteristics in a Mixed Longitudinal Study of Girls. Pediatrics. 2010;126:e583–e590. doi: 10.1542/peds.2009-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM, Greenspan LC, Galvez MP, Teitelbaum SL, Windham GC, Deardorff J, Hertzberg VS, Succop PA, Hiatt RA, Kushi LH, et al. Onset of Breast Development in a Longitudinal Cohort. Pediatrics. 2013;132:1019–1027. doi: 10.1542/peds.2012-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM, Pinney SM, Huang B, Baker ER, Walt CD, Dorn LD. Hormone Changes in Peripubertal Girls. J Clin Endocrinol Metab. 2014;99:3829–3835. doi: 10.1210/jc.2013-4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM, Wolff MS. Puberty as a Window of Susceptibility. In: Russo J, editor. Environment and Breast Cancer. Springer; 2011. pp. 29–42. [Google Scholar]
- Bodicoat DH, Schoemaker MJ, Jones ME, McFadden E, Griffin J, Ashworth A, Swerdlow AJ. Timing of Pubertal Stages and Breast Cancer Risk: the Breakthrough Generations Study. Breast Cancer Res. 2014;16:R18. doi: 10.1186/bcr3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JS, Williams PL, Korrick SA, Hauser R, Sergeyev O, Revich B, Lam T, Lee MM. Association Between Chlorinated Pesticides in the Serum of Prepubertal Russian Boys and Longitudinal Biomarkers of Metabolic Function. Am J Epidemiol. 2014;180:909–919. doi: 10.1093/aje/kwu212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttke DE, Sircar K, Martin C. Exposures to Endocrine-Disrupting Chemicals and Age of Menarche in Adolescent Girls in NHANES (2003–2008) Environ Health Perspect. 2012;120:1613–1618. doi: 10.1289/ehp.1104748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzdar A, Howell A. Advances in Aromatase Inhibition: Clinical Efficacy and Tolerability in the Treatment of Breast Cancer. Clin Cancer Res. 2001;7:2620–2635. [PubMed] [Google Scholar]
- Calafat AM, Longnecker M, Koch HM, Swan SH, Hauser R, Goldman LR, Lanphear BP, Rudel RA, Engel SM, Teitelbaum SL, et al. Optimal Exposure Biomarkers for Nonpersistent Chemicals in Environmental Epidemiology. Environ Health Perspect. 2015;123:A166–A188. doi: 10.1289/ehp.1510041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. 2000 CDC Growth Charts: United States. 2000 http://www.cdc.gov/growthcharts/.
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. 2009 http://www.cdc.gov/exposurereport/pdf/FourthReport.pdf. [PubMed]
- Cheng G, Remer T, Prinz-Langenohl R, Blaszkewicz M, Degen GH, Buyken AE. Relation of Isoflavones and Fiber Intake in Childhood to the Timing of Puberty. Am J Clin Nutr. 2010;92:556–564. doi: 10.3945/ajcn.2010.29394. [DOI] [PubMed] [Google Scholar]
- Engel LS, Buckley JP, Yang G, Liao LM, Satagopan J, Calafat AM, Matthews CE, Cai Q, Ji BT, Cai H, et al. Predictors and Variability of Repeat Measurements of Urinary Phenols and Parabens in a Cohort of Shanghai Women and Men. Environ Health Perspect. 2014;122:733–740. doi: 10.1289/ehp.1306830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, Sorensen K, Mouritsen A, Aksglaede L, Hagen CP, Petersen JH, Skakkebaek NE, Andersson AM, Juul A. High Urinary Phthalate Concentration Associated With Delayed Pubarche in Girls. Int J Androl. 2012;35:216–226. doi: 10.1111/j.1365-2605.2012.01260.x. [DOI] [PubMed] [Google Scholar]
- Gross B, Staels B. PPAR Agonists: Multimodal Drugs for the Treatment of Type-2 Diabetes. Best Pract Res Clin Endocrinol Metab. 2007;21:687–710. doi: 10.1016/j.beem.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Imai K, Keele L, Tingley D. A General Approach to Causal Mediation Analysis. Psychol Methods. 2010;15:309–334. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- Mervish NA, Gardiner EW, Galvez MP, Kushi LH, Windham GC, Biro FM, Pinney SM, Rybak ME, Teitelbaum SL, Wolff MS. Dietary Flavonol Intake Is Associated With Age of Puberty in a Longitudinal Cohort of Girls. Nutr Res. 2013;33:534–542. doi: 10.1016/j.nutres.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RK, Angerer J, Wittsiepe J, Wilhelm M, Bruning T, Koch HM. Rapid Determination of Nine Parabens and Seven Other Environmental Phenols in Urine Samples of German Children and Adults. Int J Hyg Environ Health. 2014;217:845–853. doi: 10.1016/j.ijheh.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Nahar MS, Soliman AS, Colacino JA, Calafat AM, Battige K, Hablas A, Seifeldin IA, Dolinoy DC, Rozek LS. Urinary Bisphenol A Concentrations in Girls From Rural and Urban Egypt: a Pilot Study. Environ Health. 2012;11:20. doi: 10.1186/1476-069X-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, et al. A Small-Molecule AdipoR Agonist for Type 2 Diabetes and Short Life in Obesity. Nature. 2013;503:493–499. doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
- Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, Silva MJ, Brambilla C, Pin I, Charles MA, et al. Exposure to Phthalates and Phenols During Pregnancy and Offspring Size at Birth. Environ Health Perspect. 2012;120:464–470. doi: 10.1289/ehp.1103634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS. Temporal Variability in Urinary Concentrations of Phthalate Metabolites, Phytoestrogens and Phenols Among Minority Children in the United States. Environ Res. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Tingley D, Yamamoto TiK, Hirose K, Keele L, Ima K. Mediation: R Package for Causal Mediation Analysis. R package version 4.4.2. 2013 In . [Google Scholar]
- Wang L, Kannan K. Alkyl Protocatechuates As Novel Urinary Biomarkers of Exposure to P-Hydroxybenzoic Acid Esters (Parabens) Environ Int. 2013;59:27–32. doi: 10.1016/j.envint.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Witorsch RJ, Thomas JA. Personal Care Products and Endocrine Disruption: A Critical Review of the Literature. Crit Rev Toxicol. 2010;40(Suppl 3):1–30. doi: 10.3109/10408444.2010.515563. [DOI] [PubMed] [Google Scholar]
- Wolff MS. Endocrine Disruptors: Challenges for Environmental Research in the 21st Century. Ann N Y Acad Sci. 2006;1076:228–238. doi: 10.1196/annals.1371.009. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Britton JA, Boguski L, Hochman S, Maloney N, Serra N, Liu Z, Berkowitz G, Larson S, Forman J. Environmental Exposures and Puberty in Inner-City Girls. Environ Res. 2008;107:393–400. doi: 10.1016/j.envres.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, McGovern K, Windham GC, Pinney SM, Galvez M, Calafat AM, Kushi LH, Biro FM. Phthalate Exposure and Pubertal Development in a Longitudinal Study of US Girls. Hum Reprod. 2014;29:1558–1566. doi: 10.1093/humrep/deu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, Pinney SM, Windham G, Liao L, Biro F, Kushi LH, Erdmann C, Hiatt RA, Rybak ME, et al. Investigation of Relationships Between Urinary Biomarkers of Phytoestrogens, Phthalates, and Phenols and Pubertal Stages in Girls. Environ Health Perspect. 2010;118:1039–1046. doi: 10.1289/ehp.0901690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Wu Q, Sakthivel S, Pavithran PV, Vasukutty JR, Kannan K. Urinary Levels of Endocrine-Disrupting Chemicals, Including Bisphenols, Bisphenol A Diglycidyl Ethers, Benzophenones, Parabens, and Triclosan in Obese and Non-Obese Indian Children. Environ Res. 2015;137:120–128. doi: 10.1016/j.envres.2014.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.