Abstract
Intensity-modulated radiation therapy (IMRT), an innovative treatment option for prostate cancer, has rapidly diffused over the past decade. To inform our understanding of racial disparities in prostate cancer treatment and outcomes, this study compared diffusion of IMRT in African American (AA) and Caucasian American (CA) prostate cancer patients during the early years of IMRT diffusion using the Surveillance, Epidemiology and End Results (SEER)–Medicare linked database. A retrospective cohort of 947 AA and 10,028 CA patients diagnosed with localized prostate cancer from 2002 through 2006, who were treated with either IMRT or non-IMRT as primary treatment within 1 year of diagnoses was constructed. Logistic regression was used to examine potential differences in diffusion of IMRT in AA and CA patients, while adjusting for socioeconomic and clinical covariates. A significantly smaller proportion of AA compared with CA patients received IMRT for localized prostate cancer (45% vs. 53%, p < .0001). Racial differences were apparent in multivariable analysis though did not achieve statistical significance, as time and factors associated with race (socioeconomic, geographic, and tumor related factors) explained the preponderance of variance in use of IMRT. Further research examining improved access to innovative cancer treatment and technologies is essential to reducing racial disparities in cancer care.
Keywords: Surveillance, Epidemiology and End Results (SEER)–Medicare, radiation therapy, diffusion, intensity-modulated radiation therapy (IMRT)
Introduction
African American (AA) men in the United States experience significantly greater prostate cancer incidence and mortality compared with Caucasian American (CA) males (American Cancer Society, 2013a, 2013b). The reasons for this disparity are complex and not fully understood, but are believed to include differential access to care, including differences in cancer treatment (American Cancer Society, 2013b). Prior studies have demonstrated that compared with CA men, AA men are diagnosed at a later stage (Cohen et al., 2006; Du et al., 2006; Hoffman et al., 2001; Mullins, Onukwugha, Bikov, Seal, & Hassain, 2010) receive less aggressive treatment (Ellis et al., 2013; Godley et al., 2003; Hayn et al., 2011; Holmes et al., 2009; Pisu et al., 2010) and also experience a longer delay from diagnosis to start of treatment (Gross, Smith, Wolf, & Andersen, 2008; Stokes et al., 2013).
Treatment options for prostate cancer differ depending on the age and general health of the patient, and the characteristics of the cancer itself. The primary treatment options for early-stage disease—which is the focus of this study—include prostatectomy (surgical removal of the prostate gland), brachytherapy (surgical implantation of radioactive seeds), and external beam radiation therapy. Because these prostate cancer treatment options show comparable survival benefits and there is no definitive “best” treatment, treatment selection is commonly a matter of the patient's preference and his physician's referral.
In addition, within the treatment options of prostatectomy, brachytherapy, and radiation—there has been dramatic technological advancements over the past 15 years. For radiation therapy the advent of computed tomography–based treatment planning, three-dimensional conformal radiotherapy allowed for a higher dose of radiation to be delivered to the prostate safely compared with the previous technology of two-dimensional radiation planning (Cahlon, Hunt, & Zelefsky, 2008). More recent, the advent of more sophisticated treatment planning and delivery, namely intensity-modulated radiation therapy (IMRT; Cahlon et al., 2008; De Meerleer et al., 2000; Ling et al., 1996; Sheets, Hendrix, Allen, & Chen, 2013) further reduces unnecessary radiation exposure to organs and tissue adjacent to the prostate (Cahlon et al., 2008; De Meerleer et al., 2000; Ling et al., 1996). Compared with the older conformal radiotherapy, IMRT has been demonstrated to be more effective in treating prostate cancer and causes fewer side effects (Forsythe, Blacksburg, Stone, & Stock, 2012; Sheets et al., 2012).
The use of IMRT technology for the treatment of prostate cancer has diffused rapidly over the past decade, and is now the standard type of radiation used to treat this disease (Jacobs et al., 2012a, 2012b); however, the evenness of its uptake among all men with prostate cancer remains unknown. Slower uptake of this more effective radiation technology among AA men with prostate cancer may explain, in part, the racial disparity in prostate cancer outcomes. In this study, data from the Surveillance, Epidemiology, and End Results (SEER)—Medicare linked database were analyzed to examine diffusion of IMRT in CA and AA patients with prostate cancer. Given the disparities in access to care and treatment for AA patients as reviewed above, this study hypothesized that there was slower diffusion of IMRT among AA patients.
Method
Data Source
A population-based retrospective cohort was created from SEER-Medicare data. The linkage of the SEER and Medicare data is the result of the collaborative effort of the National Cancer Institute (NCI), the SEER registries, and the Center for Medicare and Medicaid Services (Warren, Klabunde, Schrag, Bach, & Riley, 2002). The SEER-Medicare database is composed of 16 population-based cancer registries that represent approximately 26% of the U.S. population, linked to health care utilization data from Medicare, which provides benefits to 97% of the U.S. population ≥65 (Sheets et al., 2013).
Study Cohort
The study cohort included AA and CA men 66 years and older (to allow at least 1 year of Medicare claims before diagnosis for calculation of a comorbidity score; Klabunde, Legler, Warren, Baldwin, & Schrag, 2007; Stokes et al., 2013), who received radiation treatment for prostate cancer between 2002 and 2006, the early years of IMRT diffusion (Sheets et al., 2012; Sheets et al., 2013; Stokes et al., 2013). Men with additional cancer diagnoses, metastatic disease, disease diagnosed at autopsy, and those missing month of diagnosis were excluded. Additionally, men enrolled in a health maintenance organization and those who were not enrolled in both Medicare Part A and Part B, from 1 year before to 1 year after diagnosis were excluded, to ensure complete capture of health services. The final analytic cohort included 10,975 AA and CA patients.
Outcomes
The primary outcome of this study was receipt of IMRT, which was determined using the Current Procedural Terminology/Healthcare Common Procedure Coding System procedure codes, and International Classification of Disease–Ninth Revision codes:
IMRT: G0174, G0178, 77418, 0073T
Non-IMRT: 77305, 77310, 77315, 77321, 77371, 77372, 77373, 77402, 77403, 77404, 77406, 77407, 77408, 77409, 77411, 77412, 77413, 77414, 77416, 77422, 77423, 92.24, 92.26, 77301, 77418, 0073T, 77380, 77381, 77520, 77522, 77523, and 77525
Covariates
Demographic characteristics, including race, age, year of radiation treatment, martial status, census tract–level education/income, NCI combined comorbidity score, and population density were obtained from SEER. SEER regions were grouped into Northeast (Connecticut, New Jersey), South (Atlanta, rural Georgia, Kentucky, Louisiana), Central (Detroit, Iowa, New Mexico, Utah), and West (San Francisco, Hawaii, Seattle, San Jose, Los Angeles, greater California). Medicare claims data from 12 months preceding prostate cancer diagnosis were used to obtain a comorbidity score validated specifically for claims data (Stokes et al., 2013). Clinical stage were group T1/T2 (well and moderately differentiated) and T3/T4 (poorly differentiated and undifferentiated).
Statistical Analysis
Pearson chi-square tests were used to evaluate differences in baseline demographics and treatment by race. Logistic regression was used to examine potential difference in receipt of IMRT in AA and CA patients, while adjusting for age of diagnosis (66-69, 70-74, ≥75 years), year of radiation treatment, marital status, census-tract educational attainment, population density (urban or rural), and SEER region. Potential interaction terms race * year of radiation treatment, race * region, race * urban–rural, race * age, and age * marital status were examined. Statistical significance was set at p < .05; all tests were two-tailed. Analyses were performed using SAS Version 9.3 (SAS Institute, Cary, NC). Approval from the University of North Carolina at Chapel Hill Institutional Review Board was waived.
Results
Table 1 summarizes the demographic and clinical characteristics of the analytic cohort, which included 947 AA and 10,028 CA patients. Compared with AA patients, a greater proportion of CA patients received IMRT (53% vs. 45%, p < .0001). More AA men than CA men were not married, lived in areas with lower income/educational attainment, and had higher comorbidity scores.
Table 1.
Demographic and Clinical Characteristics by Race, SEER-Medicare 2002-2006.
| Characteristics | AA (N = 947); n (%) | CA (N = 10,028); n (%) | p |
|---|---|---|---|
| Age at diagnosis (years) | <.0001 | ||
| 66-69 | 256 (27) | 1,907 (19) | |
| 70-74 | 362 (38) | 3,651 (36) | |
| ≥75 | 329 (35) | 4,470 (45) | |
| Year of radiation | .977 | ||
| 2002 | 212 (22) | 2,198 (22) | |
| 2003 | 191 (20) | 2,109 (20) | |
| 2004 | 178 (19) | 1,863 (19) | |
| 2005 | 180 (19) | 1,876 (19) | |
| 2006 | 186 (20) | 1,982 (20) | |
| Marital status | <.0001 | ||
| Married | 535 (56) | 7,295 (73) | |
| Not married/unknown | 412 (44) | 2,733 (37) | |
| NCI combined comorbidity score | <.0001 | ||
| 0 | 478 (50) | 6,327 (63) | |
| >0 | 469 (50) | 3,701 (37) | |
| % census income | <.0001 | ||
| 0%-25% (low income) | 566 (60) | 2,256 (22) | |
| 26%-50% (low–medium income) | 209 (22) | 2,638 (26) | |
| 51%-75% (medium–high income) | 122 (13) | 2,567 (26) | |
| >75% (High income) | 50 (5) | 2,567 (26) | |
| % non–high school graduate in census tract | <.0001 | ||
| 0%-25% (low education) | 550 (58) | 2,157 (22) | |
| 26%-50% (low–medium education) | 218 (23) | 2,459 (25) | |
| 51%-75% (medium–high education) | 129 (14) | 2,756 (27) | |
| >75% (high education) | 50 (5) | 2,656 (26) | |
| Population density | .038 | ||
| Urban | 898 (95) | 8,845 (88) | |
| Rural | 49 (5) | 1,183 (12) | |
| Tumor grade | |||
| I | 478 (50) | 4,507 (45) | .002 |
| II | 443 (47) | 5,127 (51) | |
| III/IV | 26 (3) | 394 (4) | |
| Clinical stage | .399 | ||
| T1/T2 | 516 (54) | 5,607 (56) | |
| T3/T4 | 431 (45) | 4,421 (44) | |
| Treatment modality | <.0001 | ||
| IMRT | 423 (45) | 5,282 (53) | |
| CRT | 524 (55) | 4,746 (47) | |
| Geographic region | <.0001 | ||
| South | 252 (26) | 1,604 (16) | |
| Northeast | 280 (30) | 2,871 (29) | |
| Central | 272 (29) | 2,108 (21) | |
| West | 143 (15) | 3,445 (34) |
Note. SEER = Surveillance, Epidemiology, and End Results; AA = African American; CA = Caucasian; NCI = National Cancer Institute; IMRT = intensity-modulated radiation therapy; CRT = conformal radiation therapy. SEER sites were grouped into four geographic regions for analysis.
a. Determined using the chi-square test.
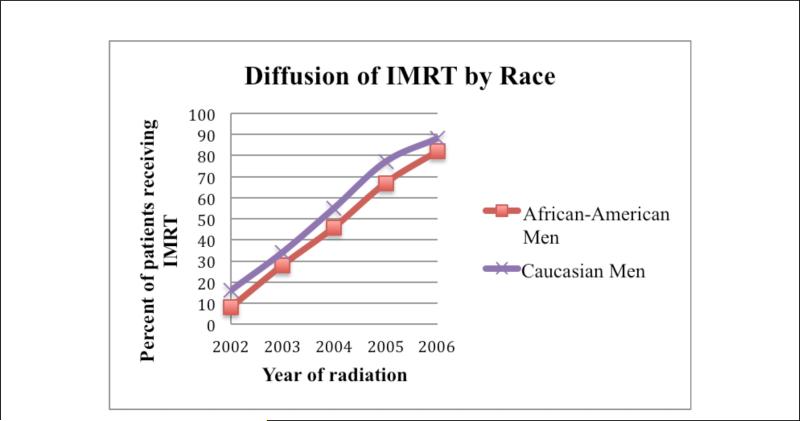
Table 2 examines receipt of IMRT by patient characteristics, stratified by race. In both AA and CA men, IMRT use in creased from 2002 to 2006: AA from 8% to 82% (p < .0001) and CA from 16% to 88% (p < .0001). There was also significant regional variation in IMRT use for both AA and CA patients, with highest use in the West and Northeast, and higher IMRT use associated with areas with higher income.
Table 2.
Demographic and Clinical Characteristics by Race and Treatment Modality, SEER-Medicare 2002-2006.
| AA (N = 947) |
CA (N = 10,028) |
|||||
|---|---|---|---|---|---|---|
| CRT (n = 524) | IMRT (n = 423) | CRT (n = 4,746) | IMRT (n = 5,282) | |||
| Characteristics | No. (%)a | No. (%)a | P | No. (%)a | No. (%)a | P |
| Age at diagnosis (years) | .530 | .781 | ||||
| 66-69 | 134 (52) | 122 (48) | 889 (47) | 1,018 (53) | ||
| 70-74 | 204 (56) | 158 (44) | 1,737 (48) | 1,914 (52) | ||
| ≥75 | 186 (56) | 143 (44) | 2,120 (47) | 2,350 (53) | ||
| Year of radiation | <.0001 | <.0001 | ||||
| 2002 | 196 (92) | 16 (8) | 1,845 (84) | 353 (16) | ||
| 2003 | 138 (72) | 53 (28) | 1,399 (66) | 710 (34) | ||
| 2004 | 97 (54) | 81 (46) | 837 (45) | 1,026 (55) | ||
| 2005 | 59 (33) | 121 (67) | 432 (23) | 1,444 (77) | ||
| 2006 | 34 (18) | 152 (82) | 233 (12) | 1,749 (88) | ||
| Marital status | .146 | .876 | ||||
| Married | 285 (53) | 250 (47) | 3,456 (47) | 3,839 (53) | ||
| Not married/unknown | 239 (58) | 173 (42) | 1,290 (47) | 1,443 (53) | ||
| NCI combined comorbidity score | .215 | .0003 | ||||
| 0 | 255 (53) | 223 (47) | 2,908 (46) | 3,419 (54) | ||
| >0 | 269 (57) | 200 (43) | 1,838 (50) | 1,863 (50) | ||
| % census income | .023 | <.0001 | ||||
| 0%-25% (low income) | 328 (58) | 238 (42) | 1,232 (55) | 1,024 (45) | ||
| 26%-50% (low–medium income) | 119 (57) | 90 (43) | 1,332 (51) | 1,306 (49) | ||
| 51%-75% (medium–high income) | 55 (45) | 67 (55) | 1,161 (45) | 1,406 (55) | ||
| >75% (high income) | 22 (44) | 28 (56) | 1,021 (40) | 1,546 (60) | ||
| % non–high school graduate in census tract | .127 | <.0001 | ||||
| 0%-25% (low education) | 312 (57) | 238 (43) | 1,230 (57) | 927 (43) | ||
| 26%-50% (low–medium education) | 127 (58) | 91 (42) | 1,232 (50) | 1,227 (50) | ||
| 51%-75% (medium–high education) | 62 (48) | 67 (52) | 1,247 (45) | 1,509 (55) | ||
| >75% (high education) | 23 (46) | 27 ( 54) | 1,037 (39) | 1,619 (61) | ||
| Population density | .007 | <.0001 | ||||
| Urban | 506 (56) | 392 (44) | 4,011 (45) | 4,834 (55) | ||
| Rural | 18 (37) | 31 (63) | 735 (62) | 448 (38) | ||
| Tumor grade | <.0001 | <.0001 | ||||
| I | 237 (50) | 241 (50) | 1,850 (41) | 2,657 (59) | ||
| II | 274 (62) | 169 (38) | 2,685 (52) | 2,442 (48) | ||
| III/IV | 13 (50) | 13 (50) | 211 (54) | 183 (46) | ||
| Clinical stage | .0003 | <.0001 | ||||
| T1/T2 | 313 (60) | 203 (39) | 2,883 (61) | 3,240 (52) | ||
| T3/T4 | 211 (49) | 220 (51) | 1,863 (39) | 2,989 (48) | ||
| Geographic region | <.0001 | <.0001 | ||||
| South | 133 (53) | 119 (47) | 900 (56) | 704 (44) | ||
| Northeast | 133 (48) | 147 (52) | 1,086 (38) | 1,785 (62) | ||
| Central | 193 (71) | 79 (29) | 1,263 (60) | 845 (40) | ||
| West | 65 (45) | 78 (55) | 1,497 (43) | 1,948 (57) | ||
Note. SEER = Surveillance, Epidemiology, and End Results; AA = African American; CA = Caucasian; NCI = National Cancer Institute; IMRT = intensity-modulated radiation therapy; CRT = conformal radiation therapy. SEER sites were grouped into four geographic regions for analysis.
Determined using the chi-square test.
On multivariate analysis (Table 3), a lower comorbidity score was associated with receipt of IMRT. There was significant geographic variation in diffusion of IMRT, including differential use by SEER region, race, urban/rural residence, living in areas with different regional educational attainment, and age at diagnosis.
Table 3.
Multivariate Logistic Regression Models for Evaluating Predictors of IMRT Diffusion, SEER-Medicare 2002-2006.
| Adjusted predictors for IMRT diffusiona |
|||
|---|---|---|---|
| Characteristics | OR | 95% CI | P |
| Race | |||
| CA | 1.00 | Reference | — |
| AA (urban only) | 0.20 | [0.09, 0.42] | <.0001 |
| Age at diagnosis, (years) | |||
| 66-69 | 1.00 | Reference | — |
| 70-74 | 0.99 | [0.87, 1.13] | .921 |
| ≥75 | 0.93 | [0.82, 1.05] | .0.252 |
| Year of radiation | |||
| 2002 | 1.00 | Reference | — |
| 2003 | 2.93 | [2.53, 3.39] | <.0001 |
| 2004 | 7.69 | [6.62, 8.94] | <.0001 |
| 2005 | 22.40 | [19.03, 26.37] | <.0001 |
| 2006 | 52.00 | [43.31, 62.43] | <.0001 |
| Marital status | |||
| Married | 1.00 | Reference | — |
| Not married/unknown | 1.00 | [0.95, 1.05] | .0987 |
| NCI combined comorbidity score | |||
| 0 | 1.00 | Reference | — |
| >0 | 0.82 | [0.74, 0.90] | <.0001 |
| Income (census tract) | |||
| Quartile 1 (0%-25%) | 1.00 | Reference | — |
| Quartile 2 (26%-50%) | 1.04 | [0.90, 1.21] | .575 |
| Quartile 3 (51%-75%) | 1.18 | [1.00, 1.38] | .0.0496 |
| Quartile 4 (76%-100%) | 1.21 | [0.99, 1.47] | .061 |
| Education (census tract) | |||
| Quartile 1 (0-%25%) | 1.00 | Reference | — |
| Quartile 2 (26%-50%) | 1.30 | [1.12, 1.50] | .0006 |
| Quartile 3 (51%-75%) | 1.69 | [1.43, 2.00] | <.0001 |
| Quartile 4 (76%-100%) | 2.28 | [1.87, 2.77] | <.0001 |
| Population density | |||
| Urban | 1.00 | Reference | — |
| Rural | 0.17 | [0.08, 0.37] | <.0001 |
| Tumor grade | |||
| I | 1.00 | Reference | — |
| II | 0.95 | [0.87, 1.05] | .374 |
| III/IV | 0.81 | [0.63, 1.04] | .099 |
| Clinical stage | |||
| T1/T2 | 1.00 | Reference | — |
| T3/T4 | 0.91 | [0.87-0.96] | .0003 |
| Geographic region | |||
| South | 1.00 | Reference | — |
| Northeast | 2.38 | [2.05, 2.76] | <.0001 |
| Central | 0.90 | [0.77, 1.04] | .159 |
| West | 1.77 | [1.53, 2.04] | <.0001 |
| Interaction terms | |||
| Race (AA) * Urban | 4.39 | 2.17-8.89 | <.0001 |
Note. NCI = National Cancer Institute; SEER = Surveillance, Epidemiology, and End Results; 95% CI = confidence interval; OR = odds radio; AA = African American; CA = Caucasian; IMRT = intensity-modulated radiation therapy. SEER sites were grouped into four geographic regions for analysis.
Logistic regression adjusted for race, age (3 categories), year of radiation, marital status, Charlson comorbidity index, income, education, population density, tumor grade, clinical stage, geographic region, and race (AA) * urban.
Overall, AA race was associated with less use of IMRT (crude OR = 0.73, p < 0.0001). Racial differences were similar in multivariable analysis though did not achieve statistical significance (OR = 0.95, p < 0.54); data in supplementary analysis). In analytic models testing varying degrees of specification, the preponderance of variance in use of IMRT was explained by time and socioeconomic, geographic, and tumor-related factors (Table 3). Of the tested interaction terms, only Race(AA)*Urban residence was significant and therefore retained in the final model. Examining the interaction effects, the OR for AA vs. CA use of IMRT was smaller for urban areas (OR = 0.2) than in rural areas (OR = 0.88, derived from 0.2 * 4.4), suggesting that racial disparities may differ between urban and rural areas
Discussion
The use of IMRT was less common in AA compared with CA patients (Figure 1, Table 2). Multivariable analysis indicated that this disparity is likely more a function of factors that research has shown to be associated with AA race rather than race alone. Specifically, there was significant geographic variation in diffusion of IMRT, including differential use by SEER region, race, urban/rural residence, and living in areas with different regional educational attainment. Although racial disparity in prostate cancer treatment and outcomes is well described (Cohen et al., 2006; Du et al., 2006; Ellis et al., 2013; Godley et al., 2003; Hayn et al., 2011; Shavers et al., 2004; Tyson & Castle, 2014), this is the first population-based study to examine whether such disparity exists in diffusion of innovative treatment technology.
Figure I.
Diffusion of intensity-modulated radiation therapy (IMRT) by race, 2002-2006 (n = 5,705).
The geographic variation in IMRT diffusion is not surprising. Compared with older radiation technology (three-dimensional conformal radiation), IMRT is considerably more expensive, and upgrading equipment to allow IMRT can cost more than a million dollars (Ellis et al., 2013; Nguyen et al., 2011; Roberts et al., 2013). Because of the significant capital required to purchase the infrastructure to deliver IMRT, this technology may not be evenly adopted in clinical settings (Jacobs et al., 2012a, 2012b). The descriptive statistics (Table 2) and regression results (Table 3) suggest that AAs live in lower socioeconomic areas with significantly lower incomes. It may be that this corresponds to lower reimbursement for radiation therapy providers, which therefore have fewer financial resources and are not able to reinvest in capital improvements—namely, new IMRT technology—as rapidly as areas of greater wealth and greater health care reimbursement. In the higher socioeconomic regions, health care providers may simultaneously experience greater competition for lucrative payer contracts, and may be even more likely to rapidly adopt innovative technologies as they compete on innovation and state-of-the-art care to win those contracts. This corresponds to research by Jacobs et al. (2012b) who examined the association between managed care penetration in health care markets and diffusion of IMRT. The authors reported that markets with the highest managed care penetration delivered IMRT more rapidly than those without penetration. This may explain the greater proportion of IMRT diffusion observed in the current study for AA and CA men in the West.
In so far as AA men were more likely than CA men to live in areas such as the South and urban areas that were slower to adopt IMRT, they were also less likely to receive this newer treatment. In addition, the racial differences in IMRT use by urban/rural location (Table 2, Table 3) merit close examination in future research to differentiate whether these are a function of factors amenable to intervention, or other more challenging factors including inherent biases.
Whether there is a differential diffusion of innovative cancer treatment by patient subgroups including race has not been well studied. The current findings are similar with those reported by Reeder-Hayes et al. (2011), who identified significant disparities in the receipt of sentinel lymph node biopsy, an innovative and morbidity-sparing procedure for early-stage breast cancer, among vulnerable populations, including AA women. In another study, Meyer et al. (2013), examined whether organizational research and teaching affiliation were associated with accelerated diffusion of sentinel lymph node biopsy, and reported that women receiving surgery at institutions affiliated with NCI cooperative groups were more likely to receive sentinel lymph node biopsy compared with women treated at nonaffiliated organizations.
The current results and those reported by Meyer et al. (2013), suggest that one way to reduce disparities in cancer treatment, specifically related to receipt of innovative treatments and technologies, may be to improve access to these treatments for minority patients living in the South, areas that have a high proportion of minority patients, or receiving treatment in organizations that were found to slowly adopt new treatment technology. Operationalizing this concept has always been challenging. Opportunities may include enhanced partnerships between academic and tertiary care centers—historically sites of innovation discovery and early adoption—and historically slower adopting centers. Such partnerships may mirror those embraced by the NCI's Community Clinical Oncology Program, which has promoted the two-way communication and collaboration between academic centers and community-based practices for the purpose of communicating research-based innovations, informing the development of research that is not only innovative but also practical, and developing mutually agreeable referral relationships both for clinical research and clinical care (Minasian et al., 2010). Such affiliation relationships may promote critical injections of necessary capital to acquire the innovative technology, and medical education to facilitate medical training in its appropriate use. It may also help identify alternative routes for acquisition of resources, such as through grants and government subsidies, that may have been unknown previously by the community center. This study only sought to identify whether there was a racial disparity in receipt of this innovative technology, and to gain preliminary insight into its causes. Future research should seek to more qualitatively identify the causal factors for this disparity, and in doing so identify tractable solutions for resolving it.
There are several limitations of this study. The study was a population-based retrospective cohort using SEER-Medicare, and therefore the results may not be generalizable to younger populations (Warren et al., 2002). However, because the median age at the time of prostate cancer diagnosis is 68 years (American Cancer Society, 2010), this study is applicable to the vast majority of patients who receive radiation therapy for prostate cancer. SEER-Medicare data also have limited person-level data on socioeconomic variables, and instead rely on Census measures of income and education at the local level. In addition, small cell sizes for some measures challenge our ability to conduct more nuanced examinations of the details or underpinnings of the observed associations, including the apparent associations between race, region, and urban/rural residence. Accordingly, future should seek to examine these factors at the person-level, which may clarify which are the key components of the often-conflated measures of race, education, and income, which are all associated with differential access to care (Stokes et al., 2013). The strength of using SEER-Medicare data for this current study is the population-based design and the large number of patients included, allowing findings of this study to be reflective of practice patterns across the United States.
Conclusions
A greater proportion of CA patients, compared with AA patients received IMRT during the early years of diffusion for this innovative radiation technology. There was significant geographic variation in diffusion of IMRT, including differential use by SEER region, race, urban/rural residence, living in areas with different regional educational attainment, and age at diagnosis. Further research is needed to directly examine whether efforts targeting specific areas to improving access to innovative cancer treatment and technologies can reduce or eliminate the racial disparities in cancer care.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health (T32 No. 5T32CA128582-043 to E.C.); (T32 No. 5T32CA128590-04 to D.P.); contract HHSN-261200800726P; 5R01CA124402; and the Integrated Cancer Information and Surveillance System, UNC Lineberger Comprehensive Cancer Center with funding provided by the University Cancer Research Fund via the State of North Carolina; by the National Center for Research Resources and the National Center for Advancing Translational Sciences, Grant No. UL1TR000083. Portions of this work were supported by the Carolina Community Network through a grant from the National Cancer Institute, Grant No. U01CA114629, and P01CA142538.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement # U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
This study has been presented at the International Society for Pharmacoeconomics and Outcomes Research 16th Annual European Congress, Dublin, Ireland, November 2013.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- American Cancer Society . Cancer facts & figures 2010. Author; Atlanta, GA: 2010. [Google Scholar]
- American Cancer Society . Cancer facts & figures for African Americans 2013-2014. Author; Atlanta, GA: 2013a. [Google Scholar]
- American Cancer Society . Cancer facts & figures 2013. Author; Atlanta, GA: 2013b. [Google Scholar]
- Cahlon O, Hunt M, Zelefsky MJ. Intensity-modulated radiation therapy: Supportive data for prostate cancer. Seminars in Radiation Oncology. 2008;18:48–57. doi: 10.1016/j.semradonc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Cohen JH, Schoenbach VJ, Kaufman JS, Talcott JA, Schenck AP, Peacock S, Godley PA. Racial differences in clinical progression among Medicare recipients after treatment for localized prostate cancer (United States). Cancer Causes & Control. 2006;17:803–811. doi: 10.1007/s10552-006-0017-7. [DOI] [PubMed] [Google Scholar]
- De Meerleer GO, Vakaet LA, De Gersem WR, De Wagter C, De Naeyer B, De Neve W. Radiotherapy of prostate cancer with or without intensity modulated beams: A planning comparison. International Journal of Radiation Oncology, Biology, Physics. 2000;47:639–648. doi: 10.1016/s0360-3016(00)00419-3. [DOI] [PubMed] [Google Scholar]
- Du XL, Fang S, Coker AL, Sanderson M, Aragaki C, Cornier JN, Chan W. Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma: Findings from a large community-based cohort. Cancer. 2006;106:1276–1285. doi: 10.1002/cncr.21732. [DOI] [PubMed] [Google Scholar]
- Ellis SD, Blackard B, Carpenter WR, Mishel M, Chen RC, Godley PA, Bensen JT. Receipt of National Comprehensive Cancer Network guideline–concordant prostate cancer care among African American and Caucasian American men in North Carolina. Cancer. 2013;119:2282–2290. doi: 10.1002/cncr.28004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe K, Blacksburg S, Stone N, Stock RG. Intensity-modulated radiotherapy causes fewer side effects than three-dimensional conformal radiotherapy when used in combination with brachytherapy for the treatment of prostate cancer. International Journal of Radiation Oncology, Biology, Physics. 2012;83:630–635. doi: 10.1016/j.ijrobp.2011.06.2013. [DOI] [PubMed] [Google Scholar]
- Godley PA, Schenck AP, Amamoo MA, Schoenbach VJ, Peacock S, Manning M, Talcott JA. Racial differences in mortality among Medicare recipients after treatment for localized prostate cancer. Journal of the National Cancer Institute. 2003;95:1702–1710. doi: 10.1093/jnci/djg094. [DOI] [PubMed] [Google Scholar]
- Gross CP, Smith BD, Wolf E, Andersen M. Racial disparities in cancer therapy: Did the gap narrow between 1992 and 2002? Cancer. 2008;112:900–908. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayn MH, Orom H, Shavers VL, Sanda MG, Glasgow M, Mohler JL, Underwood W. Racial/ethnic differences in receipt of pelvic lymph node dissection among men with localized/regional prostate cancer. Cancer. 2011;117:4651–4658. doi: 10.1002/cncr.26103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RM, Gilliland J, Eley W, Harlan LC, Stephenson RA, Stanford JL, Potosky AL. Racial and ethnic differences in advanced-stage prostate cancer: The prostate cancer outcomes study. Journal of the National Cancer Institute. 2001;93:388–395. doi: 10.1093/jnci/93.5.388. [DOI] [PubMed] [Google Scholar]
- Holmes L, Jr., Chan W, Jiang Z, Ward D, Essien EJ, Du XL. Impact of androgen deprivation therapy on racial/ethnic disparities in the survival of older men treated for locoregional prostate cancer. Cancer Control: Journal of the Moffitt Cancer Center. 2009;16:176–185. doi: 10.1177/107327480901600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Zhang Y, Skolarus TA, Wei JT, Montie JE, Schroeck FR, Hollenbeck BK. Certificate of need regulations and the diffusion of intensity-modulated radiotherapy. Urology. 2012a;80:1015–1020. doi: 10.1016/j.urology.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Zhang Y, Skolarus TA, Wei JT, Montie JE, Schroeck FR, Hollenbeck BK. Managed care and the diffusion of intensity-modulated radiotherapy for prostate cancer. Urology. 2012b;80:1236–1242. doi: 10.1016/j.urology.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde C, Legler J, Warren J, Baldwin L, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Annals of Epidemiology. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Ling CC, Burman C, Chui CS, Kutcher GJ, Leibel SA, LoSasso T, Fuks Z. Conformal radiation treatment of prostate cancer using inversely-planned intensity-modulated photon beams produced with dynamic multileaf collimation. International Journal of Radiation Oncology, Biology, Physics. 1996;35:721–730. doi: 10.1016/0360-3016(96)00174-5. [DOI] [PubMed] [Google Scholar]
- Meyer AM, Reeder-Hayes KE, Liu H, Wheeler SB, Penn D, Weiner BJ, Carpenter WR. Differential receipt of sentinel lymph node biopsy within practice-based research networks. Medical Care. 2013;51:812–818. doi: 10.1097/MLR.0b013e31829c8ca4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minasian LM, Carpenter WR, Weiner BJ, Anderson DE, McCaskill-Stevens W, Nelson S, Kaluzny AD. Translating research into evidence-based practice: The National Cancer Institute Community Clinical Oncology Program. Cancer. 2010;116:4440–4449. doi: 10.1002/cncr.25248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins CD, Onukwugha E, Bikov K, Seal B, Hassain A. Health disparities in staging of SEER-Medicare prostate cancer patients in the United States. Urology. 2010;76:566–573. doi: 10.1016/j.urology.2009.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PL, Gu X, Lipsitz SR, Choueiri TK, Choi WW, Lei Y, Hu JC. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. Journal of Clinical Oncology. 2011;29:1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisu M, Oliver JS, Kim YI, Elder K, Martin M, Richardson LC. Treatment for older prostate cancer patients: Disparities in a southern state. Medical Care. 2010;48:915–922. doi: 10.1097/MLR.0b013e3181eb31a8. [DOI] [PubMed] [Google Scholar]
- Reeder-Hayes KE, Bainbridge J, Meyer AM, Amos KD, Weiner BJ, Godley PA, Carpenter WR. Race and age disparities in receipt of sentinel lymph node biopsy for early-stage breast cancer. Breast Cancer Research and Treatment. 2011;128:863–871. doi: 10.1007/s10549-011-1398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KB, Soulos PR, Herrin J, Yu JB, Long JB, Dostaler E, Gross CP. The adoption of new adjuvant radiation therapy modalities among Medicare beneficiaries with breast cancer: Clinical correlates and cost implications. International Journal of Radiation Oncology, Biology, Physics. 2013;85:1186–1192. doi: 10.1016/j.ijrobp.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavers VL, Brown ML, Potosky AL, Klabunde CN, Davis WW, Moul J, Fahey A. Race/ethnicity and the receipt of watchful waiting for the initial management of prostate cancer. Journal of General Internal Medicine. 2004;19:146–155. doi: 10.1111/j.1525-1497.2004.30209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets NC, Goldin GH, Meyer A-M, Wu Y, Chang Y, Sturmer T, R. C. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA: The Journal of the American Medical Association. 2012;307:1611–1620. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets NC, Hendrix LH, Allen IM, Chen RC. Trends in the use of postprostatectomy therapies for patients with prostate cancer: A surveillance, epidemiology, and end results Medicare analysis. Cancer. 2013;119:3295–3301. doi: 10.1002/cncr.28222. [DOI] [PubMed] [Google Scholar]
- Stokes WA, Hendrix LH, Royce TJ, Allen IM, Godley PA, Wang AZ, Chen RC. Racial differences in time from prostate cancer diagnosis to treatment initiation: A population-based study. Cancer. 2013;119:2486–2493. doi: 10.1002/cncr.27975. [DOI] [PubMed] [Google Scholar]
- Tyson MD, II, Castle EP. Racial disparities in survival for patients with clinically localized prostate cancer adjusted for treatment effects. Mayo Clinic Proceedings. 2014;89:300–307. doi: 10.1016/j.mayocp.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Medical Care. 2002;40(8 Suppl.):IV3–IV18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]