Abstract
Importance
Converging evidence suggests that Alzheimer’s disease (AD) involves insulin signaling impairment. AD patients and people at risk for AD show reduced glucose metabolism, as indexed by F18-fluorodeoxyglucose positron emission tomography ([F18]FDG-PET).
Objective
To determine if insulin resistance (IR) predicts AD-like global and regional glucose metabolism deficits in late middle-aged participants at risk for AD. A secondary objective was to examine if IR-predicted variation in regional glucose metabolism was associated with worse cognitive performance.
Setting
A general community sample enriched for AD family history.
Participants
Population-based, cross-sectional study of 150 cognitively normal, late middle-aged (mean=60.67 years) adults from the Wisconsin Registry for Alzheimer’s Prevention.
Design
Participants underwent cognitive testing, fasting blood draw, and an [F18]FDG-PET scan at baseline. The Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) was used to assess peripheral insulin resistance. Regression analysis tested the statistical effect of HOMA-IR on global glucose metabolism. A voxel-wise analysis was used to determine if HOMA-IR predicted regional glucose metabolism. Finally, predicted variation in regional glucose metabolism was regressed against cognitive factors. Covariates included age, sex, body mass index, Apolipoprotein E genotype, AD family history status, and a reference region used to normalize regional uptake.
Main Outcome Measures
Regional glucose uptake determined using [F18]FDG-PET, and neuropsychological factors.
Results
Higher HOMA-IR was associated with lower global glucose metabolism (β=−0.29, p<.01) and lower regional glucose metabolism across large portions of frontal, lateral parietal, lateral temporal, and medial temporal lobe (MTL; p<.05, family-wise error corrected). The association was especially robust in left MTL (R2=0.178). Lower left MTL glucose metabolism predicted by HOMA-IR was significantly related to worse immediate memory (β=0.317, p<.001) and delayed memory (β=0.305, p<.001) performance.
Conclusions
Our results show that IR, a prevalent and increasingly common condition in developed countries, is associated with significantly lower regional cerebral glucose metabolism, which in turn may predict worse memory performance. Midlife may be a critical period for initiating treatments to lower peripheral IR in order to maintain neural metabolism and cognitive function.
Keywords: Insulin resistance, fluorodeoxyglucose, memory
Introduction
Glucoregulatory impairment has reached epidemic proportions in the United States. According to the American Diabetes Association, 29.1 million Americans have diabetes, and more than half of adults over 64 years of age have “pre-diabetes”1. Type 2 diabetes increases the risk of Alzheimer’s disease (AD) and both clinical and pre-clinical hypergylcemia are characterized by insulin resistance. Insulin resistance is broadly defined as reduced tissue responsiveness to the action of insulin2. Insulin, a key hormone involved in carbohydrate metabolism, facilitates microvascular blood flow, glucose uptake, and glucose oxidation for adenosine-5′-triphosphate generation3.
In addition to its function in the periphery of the body, insulin has increasingly been recognized as playing an important role in the brain. Insulin resistance is related to higher AD risk,4 and several animal studies link central insulin resistance with the pathological features of AD, including atrophy, mitochondrial dysfunction, neuroinflammation, and progressive memory deficits [see de La Monte5 for review]. In humans, brain insulin resistance has been found in postmortem hippocampal tissue in patients with AD, and the degree of insulin signaling inhibition corresponds to the severity of antemortem cognitive dysfunction6. Several recent studies have shown a deleterious effect of insulin resistance on regional brain volume, both cross-sectionally7–9 and longitudinally10. Our group has also recently shown that participants with metabolic syndrome, a condition linked with insulin resistance, have markedly lower cerebral blood flow11, a presumed index of neural function. Finally, we have found that higher insulin resistance predicts temporal and frontal amyloid deposition in late-middle aged participants at risk for AD12.
Peripheral insulin resistance has also been linked with impaired cerebral metabolic rate of glucose in the brain13. Glucose metabolism is commonly assessed using [F18] Fluorodeoxyglucose Positron Emission Tomography ([F18]FDG-PET) uptake. Baker and colleagues showed that higher insulin resistance is associated with lower basal and task-based FDG uptake in older cognitively intact adults (mean age 74.4 years) with dysglycemia13. Willette et al.14 showed similar associations in AD patients. Patterns of lower glucose utilization included hypometabolism in posterior cingulate cortex and precuneus, as well as frontal and temporal cortices. Peripheral insulin resistance strongly corresponds to brain insulin resistance, either due to reduced insulin transport into brain or potentially similar changes in receptor sensitivity and activation6,15. The findings are intriguing given that lower glucose metabolism in these brain regions is also a feature characteristic of AD16–19. Lower glucose metabolism has also been observed in mild cognitive impairment (MCI)20 and in cognitively healthy carriers of the Apolipoprotein E ε4 allele (APOE-ε4), a genetic risk factor for AD21,22. However, the relationship between insulin resistance and brain glucose utilization in middle-age is unknown. Understanding the neural effects of midlife insulin resistance is important, given that the onset of type 2 diabetes is most common in middle age and increases risk for AD23.
In this study, we assessed the effect of insulin resistance on glucose utilization as indexed by [F18]FDG-PET uptake in a cognitively healthy, late middle-aged cohort of adults enriched for parental family history of AD. We hypothesized that participants with higher insulin resistance would show lower glucose utilization in brain regions that exhibit hypometabolism in early AD. Given that glucose utilization is tied to functional status, we also tested the extent to which variation in medial temporal lobe (MTL) glucose metabolism predicted by insulin resistance was associated with cognitive performance. Finally, based on prior work in this area21,24, we tested the main effects of both APOE-ε4 and parental family history of AD on glucose metabolism.
Research Design and Methods
Participants
Demographics are listed in Table 1. One hundred and fifty cognitively normal, older middle-aged adults (M = 60.67 ± 5.82 years) were recruited from the Wisconsin Registry for Alzheimer’s Prevention (WRAP) study25. This on-going study examines genetic, biological, and lifestyle factors that contribute to the development of dementia-related cognitive decline and neural dysfunction. Participants were originally recruited between the ages of 40 to 65 years of age and were classified as either having a positive or negative family history of AD25. Positive parental family history of AD classification was defined as having one or both parents with AD as determined by autopsy (13 cases), or by validated interview26, reviewed by a multidisciplinary diagnostic consensus panel, and as outlined by research criteria27,28. Detailed medical history and phone interviews were conducted to confirm AD negative participants. The inclusion criteria for this study consisted of: no clinical diagnosis of a memory disorder, no contraindication for brain imaging, a subsequent normal magnetic resonance imaging (MRI) scan, no current diagnosis of major psychiatric disease or other major medical conditions (e.g., myocardial infarction, or recent history of cancer), and no history of head trauma. All participants underwent MRI, FDG-PET, and neuropsychological testing. Participants were categorized as APOE-ε4 carriers (one or two ε4 alleles) or non-carriers (zero ε4 alleles). APOE extraction and isoform classification have been described previously29.
Table 1.
Demographics and other participant information
| M ± SD | Range | |
|---|---|---|
| Age | 60.7 ± 5.8 | 47.8 – 71.3 |
| Insulin (μU/mL) | 2.0 ± 7.0 | 2 – 48 |
| Glucose (mg/dL) | 94.6 ± 10.0 | 74 – 132 |
| HOMA-IR | 2.2 ±1.9 | 0.5 – 14.1 |
| BMI | 28.2 ± 5.3 | 18.5 – 47.3 |
| Speed & Flexibility | 0.1 ± 0.9 | −2.2 – 2.4 |
| Working Memory | 0.2 ± 1.1 | −2.4 – 3.2 |
| Verbal Learning | 0.2 ± 1.0 | −2.5 – 1.8 |
| Immediate Memory | 0.2 ± 1.1 | −2.5 – 2.9 |
|
| ||
| n (%) | ||
|
| ||
| Women | 108 (72.0%) | |
| Family History | 103 (68.7%) | |
| APOE ε 4 Genotype | 61 (40.7%) | |
| Type 2 Diabetes | 7 (4.7%) | |
APOE-ε4 = Apolipoprotein E ε4 genotype; BMI = Body Mass Index; HOMA-IR = Homeostatic Model Assessment of Insulin Resistance. The individual tests which loaded onto the identified factors were as follows: Rey Auditory Verbal Learning Test66 Trials 1 and 2 loaded onto Immediate Memory; Rey Auditory Verbal Learning Test66 Trials 3–5 and Delayed Recall Trial loaded onto Verbal Learning & Memory; Wechsler Adult Intelligence Scale – 3rd edition67, Digit Span forward and backward, and Letter-Numbering Sequencing subtests loaded onto Working Memory; and the interference trial from the Stroop Test68, and Trail Making Test A and B69 loaded onto Speed & Flexibility.
Neuropsychological Testing
WRAP participants undergo an extensive battery of neuropsychological tests. A previous factor analytic study of the WRAP cognitive battery within the larger cohort found that the tests map onto six cognitive factors30,31. The scores used in the current study were derived from tests administered at the participants’ most recent WRAP visit and represent cognitive domains known to change with age and AD include Immediate Memory, Verbal Learning & Memory, Working Memory, and Speed & Flexibility.
HOMA-IR, Diabetes Status, and Body Mass Index
Glucose and insulin were collected after a 12-hour fast during the clinical visit nearest in time to the FDG scan. Insulin resistance was indexed by HOMA-IR and calculated by taking the product of basal glucose (mg/dL) and basal insulin (μU/mL) and dividing by 40532. Matthews et al., by contrast, derived HOMA-IR using glucose in mmol/L32. While HOMA-IR was considered as a continuous variable, we also determined how many participants in our sample had type 2 diabetes using American Diabetes Association criteria, where participants with fasting blood glucose over 125 mg/dL were identified as having type 2 diabetes. No participants were currently or previously taking medication for glycemic control; however five participants had a self-reported history of diabetes. HOMA estimation of percentage beta cell function displayed normal variation (data not shown). Body mass index (BMI) was calculated based on height and weight.
[F18] FDG-PET
Images were acquired supine and head-first on one Siemens HR+ PET scanner in 3D mode after a 4 hour fast (where water was allowed). Blood glucose was closely monitored prior to the injection of [F18]FDG. Following injection with 5.0 ± 0.5 mCi of [F18]FDG, participants remained awake but relaxed in a quiet room. Imaging began 45 minutes post-injection. The scan was acquired as six 5-min. frames. A 5-min. transmission scan was acquired following the emission scan. The dynamic PET data was reconstructed using ECAT v7.2.2 software. A filtered back projection algorithm (DIFT) was used with brain mode sinogram trimming, zoom = 2.8, and a 4mm Gaussian filter to a reconstructed image of 128×128×63 voxel matrix (voxel size = 1.84mmx 1.84mm×2.43mm). The PET data were corrected for the attenuation of annihilation radiation (using segmented attenuation maps), scanner normalization, and scatter radiation.
In order to account for between- and within-subject noise, a reference cluster was used as a covariate in statistical analyses33,34. The reference cluster consisted of sixty-five 2 × 2 × 2 mm contiguous voxels centered in the right cuneus, a region where no significant relationship between [F18]FDG signal and HOMA-IR was found (controlling for age, sex, APOE-ε4 genotype, and family history status) (β = −0.09, t(148) = −1.1, p = 0.27). The region was derived via a data-driven method that identifies brain regions unaffected by the variable of interest and has previously been shown to improve detection of disease-related hypometabolism34–36. The raw values from the reference region were extracted with MarsBaR37.
Statistics
In order to test for effects of HOMA-IR, APOE-ε4, and family history status on global glucose metabolism (adjusted by the reference region), multiple regression analysis was implemented in SPSS (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp). In addition to testing for main effects, we assessed for interactions between HOMA-IR and APOE-ε4, as well as HOMA-IR and family history status. Both analyses of main effects and interactions were conducted using one design matrix model and controlled for age at time of scan, sex, BMI, and reference region. Voxel-wise statistical analysis used Statistical Parametric Mapping (SPM8) software (www.fil.ion.ucl.ac.uk/spm) to test for the regional effect of HOMA-IR on glucose metabolism. We controlled for covariates identical to the global analysis. A voxel-wise analysis was also conducted to test for the regional main effects of family history status and APOE-ε4 on glucose metabolism, again controlling for age, sex, BMI, and reference region. Models were also run that included either a HOMA-IR and APOE-ε4 or HOMA-IR and family history interaction term. Type 1 error was minimized by using voxel-level Family Wise Error (FWE) correction of p < .05. Multiple regression analysis was also used to regress HOMA-IR predicted variation in glucose metabolism from an a priori defined left MTL region against cognitive factors. These analyses covaried age at time of scan, sex, family history status, APOE-ε4, BMI, and reference region. Sample size was insufficient to conduct robust mediation analysis38. Logarithmic transformation of HOMA-IR was used in all analyses to optimize normality and reduce heteroscedasticity.
Results
Demographics and Cognition
Demographics, the four cognitive factors, and other summary data across participants are shown in Table 1. Additional metabolic risk factor characterization is provided in eTable 1.
Associations of insulin resistance, APOE-ε4 genotype, and family history status with global [F18]FDG-PET
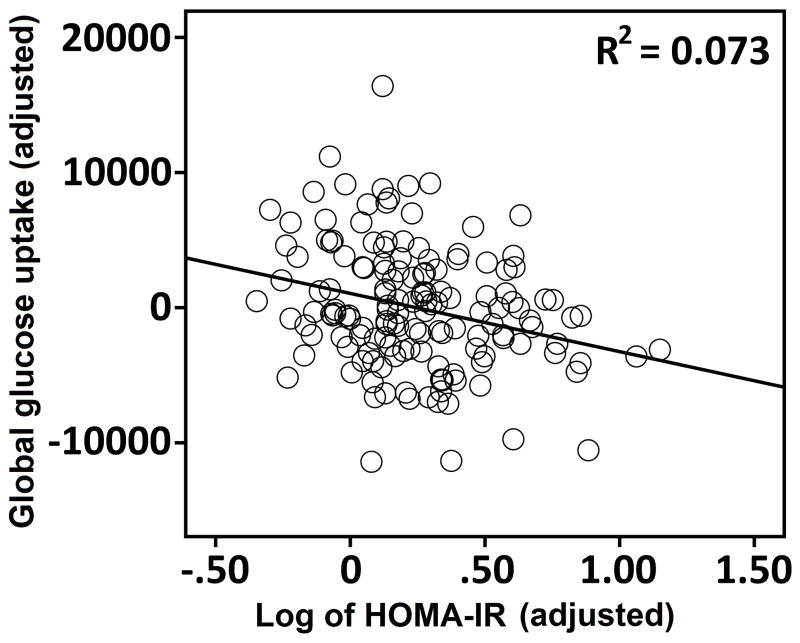
A single multiple regression model tested the statistical effects of HOMA-IR, APOE-ε4, and family history of AD on global glucose metabolism. Higher HOMA-IR was associated with lower global glucose metabolism, β = −0.29, t(143) = −3.10, p < .01. Figure 1 shows the degree of this insulin resistance association. The same regression model showed a significant effect of APOE-ε4, where carriers with one or two ε4 alleles had lower global glucose metabolism, β = −0.16, t(143) = −2.01, p < .05 compared to non-carriers. There was no significant effect of family history status on global glucose metabolism, nor did insulin resistance interact with APOE-ε4 or family history status to affect FDG-PET uptake.
Figure 1.
The association between HOMA-IR and global FDG-PET uptake in 150 late middle-aged adults. Uptake and HOMA-IR values were adjusted by the following covariates: age, sex, family history status, APOE-ε4 genotype, body mass index, and the FDG-PET reference region. * = p < .05.
Associations of insulin resistance, APOE-ε4 genotype, and family history status with regional [F18]FDG-PET
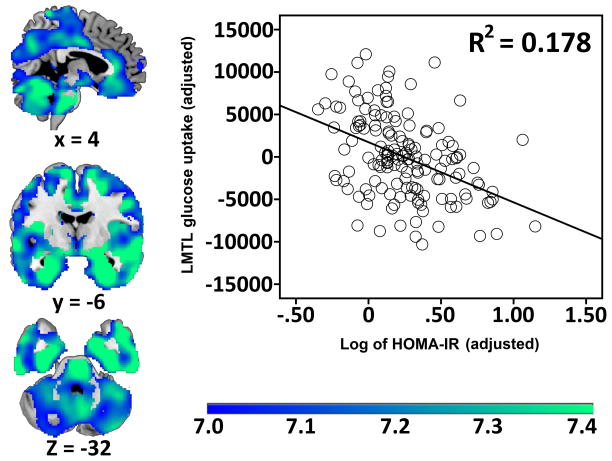
In a single voxel-wise regression model (p < .05 FWE at voxel level), HOMA-IR, APOE-ε4, and family history status were used to see if there were regional associations with glucose metabolism. Higher HOMA-IR was robustly associated with lower glucose metabolism in one contiguous cluster throughout the brain (k = 173612 voxels), with a maximum (Z score = 7.21) in left posterior MTL. The cluster spanned large portions of ventral prefrontal, cingulate, temporal, insula, and posteromedial cortices (Figure 2). Additionally, associations were seen in bilateral cerebellum. Coverage was sparse or absent in motor and pre-motor cortices, as well as dorsal prefrontal cortex. Thresholding revealed that associations were strongest in hippocampus and MTL, rostral and posterior cingulate, as well as precuneus and cuneus. The graph in Figure 2 shows the degree of this association between insulin resistance and mean signal in left MTL. There was no significant effect of APOE-ε4 or family history status on regional glucose metabolism. There was no interaction between APOE-ε4 and HOMA-IR or family history status and HOMA-IR.
Figure 2.
The association between higher HOMA-IR and lower regional FDG-PET uptake in 150 late middle-aged adults (p < .05 FWE). Results are displayed on representative cross-sections for temporal, parietal, and frontal regions. Uptake values were adjusted by the following covariates: age, sex, family history status, APOE-ε4 genotype, and glucose metabolism in the reference region. The color bar depicts t-values. Brains are oriented in neurological space. FWE = family-wise error corrected.
Relationships between insulin resistance, MTL [F18]FDG-PET, and cognitive function
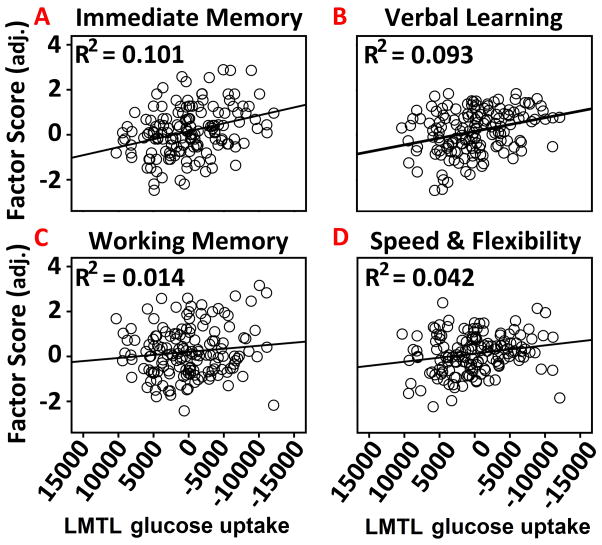
Mean predicted variation in glucose metabolism specific to HOMA-IR was extracted using the Eigenvariate tool in SPM8. The region of interest was left MTL, which was of a priori interest before voxel-wise analyses were conducted. Left medial temporal glucose metabolism was regressed against each of the four cognitive factors. Covariates were identical to the voxel-wise analysis. Adjusted glucose metabolism was associated with the Immediate Memory factor score, β = .317, t(148) = , p < .001, the Verbal Learning and Memory factor score, β = .305, t(148) = 3.895, p < .001), and weakly with the Speed and flexibility factor score, β = 0.204, t(148) = 2.537, p < .05 (Figure 3). There was no significant relationship with the working memory factor score, β = .118, t(148) = 1.448, p > .05 (Figure 3).
Figure 3.
The association between left medial temporal FDG-PET uptake predicted by the HOMA-IR analysis and cognitive function factors. (A–B) Higher FDG-PET in left medial temporal lobe (MTL), as a function of lower HOMA-IR, predicted better performance on the verbal learning and immediate memory factors. (C–D) Left MTL was not associated with the working memory factor, but was associated with the speed and flexibility factor. The FDG-PET signal was adjusted by the FDG reference region, age, sex, APOE-ε4 genotype, family history status, and HOMA-IR. * = p < .05; *** = p < .001. .
Discussion
Several studies suggest that insulin resistance is associated with brain changes that may contribute to AD pathology in the preclinical phase. This study assessed the extent to which insulin resistance may affect glucose metabolism as measured by [F18]FDG-PET uptake. We found that middle-aged adults with higher HOMA-IR showed lower glucose metabolism, and further, glucose metabolism was related to memory function.
Our results concur with findings in older adults indicating that insulin resistance13, hyperglycemia39 and diabetes40 are associated with hypometabolism on FDG-PET. Insulin resistance and hyperglycemia are related conditions, and hyperglycemia, even in the pre-diabetic range, is associated with significantly increased risk of later development of dementia41. However, fasting insulin and fasting glucose are not always correlated42 and insulin resistance may confer increased risk for AD independent of glycemic status within 3 years of assessment4. Our sample was on average 15 years younger than the sample studied in Baker et al.13, although the affected regions are similar. In particular, both studies show an association between higher HOMA-IR and less glucose metabolism in bilateral prefrontal cortex, temporal, and posteromedial parietal cortices. Willette et al. found14 similar associations in the same regions among AD patients. Interestingly, we also found bilateral hypometabolism in the cerebellar cortex, a region with appreciable insulin receptor density43,44. While not typically considered a region affected by AD, a few reports have found mild cerebellar hypometabolism in AD45,46. Post-mortem AD brains also show deficient insulin signaling in cerebellum6, while intranasal insulin may maintain glucose metabolism13.
A strong association between higher HOMA-IR and lower glucose metabolism was found in left MTL. It has been well established, at least in rodents, that piriform cortex, and adjoining cornu ammonis fields 1 and 2, have a high density of insulin receptors relative to moderate density in cerebral cortex44,47. Interestingly, when we examined mean glucose metabolism in left MTL, we found that lower glucose metabolism was associated with worse immediate and delayed memory performance factors. This result is in line with Wolk and Dickerson48, who found that entorhinal and perirhinal volumes in mild AD patients predicted later trials of the Rey Auditory Verbal Learning Test, on which the verbal learning and memory factor used in the current study is based. This finding provides a potential link between insulin resistance and cognitive decline.
There are several possible mechanisms that may underlie the association between higher insulin resistance and less glucose metabolism. For example, our group has found that higher peripheral insulin resistance in asymptomatic late middle-aged participants is linked with amyloid deposition measured in vivo12. In stable MCI participants enriched for amyloid status, Willette et al. observed that higher HOMA-IR predicted less prefrontal glucose metabolism only in the amyloid-positive group14. Loss of neuronal function due to mitochondrial damage is another possible mechanism. Both AD and type 2 diabetes are characterized by mitochondrial dysfunction49–51, providing a potential common link between the two diseases. Neurons rely heavily on mitochondria for the synthesis of adenosine triphosphate, and are therefore vulnerable to mitochondrial dysfunction. Indeed, lower expression of nuclear genes influencing mitochondrial energy metabolism co-localize with brain regions that show deficits in glucose utilization in AD patients. Insulin resistance may also facilitate several additional mechanisms that result in neurodegeneration including increased oxidative stress, neuroinflammation, and dysregulated lipid metabolism52.
Akin to our previous report examining HOMA-IR and brain atrophy in asymptomatic adults10, HOMA-IR associations with regional glucose metabolism in this study were robust, even with FWE correction, with moderate to moderate-strong relationships in most regions. Baker and colleagues13 found stronger relationships among cognitively normal aged adults with pre-diabetes or type 2 diabetes. Our finding that insulin resistance is associated with both increased amyloid burden and decreased glucose uptake in AD-centric brain regions, indicates that IR confers nontrivial risk for AD in midlife. It is also worth noting that HOMA-IR in this cohort appeared to predict glucose metabolism more strongly than APOE-ε4, which is an important predictor of glucose uptake deficits53 and AD in general. However, this study was cross-sectional in nature, and no causal inferences about insulin resistance may be inferred. While we found an effect of APOE-ε4 on global glucose metabolism, we did not observe any regional effects. Furthermore, HOMA-IR did not interact with APOE-ε4 status to affect global or regional glucose metabolism. Interactions between APOE-ε4 and metabolic dysfunction have been observed in previous studies, including effects on CSF biomarkers in preclinical AD54, post mortem plaque and tangle burden in patients with AD dementia55, development of amnestic MCI56, and response to intranasal insulin therapy57,58. Burns et al. have also found interactions between hyperglycemia and APOE-ε4 on FDG-PET39. While several studies do point toward a moderating effect of APOE-ε4 when considering the effects of insulin resistance on neural pathology, findings across the field are still mixed. In an existing report that shares similarities with our study, Roberts et al. examined diabetic individuals and did not find an interaction between diabetes and APOE-ε4 on FDG-PET 40. While our sample was enriched for parental family history of AD, HOMA-IR did not interact with family history status, nor did family history alone have an effect on glucose metabolism. Previous studies have found an effect of parental family history of AD on cerebral glucose metabolism, however, the findings are most robust when both parents are affected, or maternal family history is considered.59–61
In conclusion, this study provides evidence that insulin resistance is associated with brain glucose utilization in a late middle-age cohort enriched for AD risk factors. Several studies indicate that peripheral insulin resistance and related conditions such as metabolic syndrome and diabetes are risk factors for cognitive decline and AD, and are linked with increased risk of death from dementia62–65. The prevalence of AD continues to grow, and midlife may be a critical period for initiating treatments aimed at preventing or delaying the onset of AD. Accumulating evidence suggests that treatments targeting mechanisms involved in insulin signaling may impact central glucose utilization and should be investigated in the context of presymptomatic AD.
Supplementary Material
Acknowledgments
This project was supported by the National Institute on Aging [R01 AG037639 (BBB), R01 AG027161 (SCJ), ADRC P50 AG033514 (SA), ADRC sub-project #5977 (BBB), and R01 AG021155 (SCJ)], the University of Wisconsin Institute for Clinical and Translational Research, funded through a National Center for Research Resources/National Institutes of Health Clinical and Translational Science Award, 1UL1RR025011, a program of the National Center for Research Resources, United States National Institutes of Health, by P30 HD03352 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, as well as funding from the Department of Food Science and Human Nutrition at Iowa State University. The project was also facilitated by the facilities and resources at the Geriatric Research, Education, and Clinical Center (GRECC) of the William S. Middleton Memorial Veterans Hospital, Madison, WI. Funding organizations did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: BBB and AAW: Jointly designed, performed, and analyzed data, as well as wrote the paper. ACB and ES: Analyzed additional data. SCJ: Contributed analytic tools and edited the manuscript for intellectual content. BTC: Contributed reagents and analytic tools. ALR, BPH, RLK, EMJ, OCO, MAS, SA: Contributed reagents, analytic tools, and other resources, as well as edited the manuscript for intellectual content.
Conflicts of Interest: The authors have no relevant financial interests, activities, relationships, or affiliations with regard to this report.
References
- 1.Prevention CfDCa. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 2.Goldstein BJ. Insulin resistance as the core defect in type 2 diabetes mellitus. The American journal of cardiology. 2002;90(5A):3G–10G. doi: 10.1016/s0002-9149(02)02553-5. [DOI] [PubMed] [Google Scholar]
- 3.Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes/metabolism research and reviews. 2006;22(6):423–436. doi: 10.1002/dmrr.634. [DOI] [PubMed] [Google Scholar]
- 4.Schrijvers EM, Witteman JC, Sijbrands EJ, Hofman A, Koudstaal PJ, Breteler MM. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam Study. Neurology. 2010;75(22):1982–1987. doi: 10.1212/WNL.0b013e3181ffe4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Monte SM. Insulin resistance and Alzheimer’s disease. BMB reports. 2009;42(8):475–481. doi: 10.5483/bmbrep.2009.42.8.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. The Journal of clinical investigation. 2012;122(4):1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Convit A, Wolf OT, Tarshish C, de Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):2019–2022. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan ZS, Beiser AS, Fox CS, et al. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: the Framingham Offspring Study. Diabetes Care. 2011;34(8):1766–1770. doi: 10.2337/dc11-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasgon NL, Kenna HA, Wroolie TE, et al. Insulin resistance and hippocampal volume in women at risk for Alzheimer’s disease. Neurobiology of aging. 2011;32(11):1942–1948. doi: 10.1016/j.neurobiolaging.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willette AA, Xu G, Johnson SC, et al. Insulin resistance, brain atrophy, and cognitive performance in late middle-aged adults. Diabetes Care. 2013;36(2):443–449. doi: 10.2337/dc12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birdsill AC, Carlsson CM, Willette AA, et al. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity. 2013;21(7):1313–1320. doi: 10.1002/oby.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willette AA, Johnson SC, Birdsill AC, et al. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2014 doi: 10.1016/j.jalz.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68(1):51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willette AA, Modanlo N, Kapogiannis D. Insulin resistance predicts medial temporal hypermetabolism in MCI conversion to Alzheimer’s disease. Diabetes. 2015 doi: 10.2337/db14-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messier C, Teutenberg K. The role of insulin, insulin growth factor, and insulin-degrading enzyme in brain aging and Alzheimer’s disease. Neural Plast. 2005;12(4):311–328. doi: 10.1155/NP.2005.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabinovici GD, Rosen HJ, Alkalay A, et al. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology. 2011;77(23):2034–2042. doi: 10.1212/WNL.0b013e31823b9c5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET Evaluation of Cerebral Metabolic Decline in Dementia: A Potential Outcome Measure in Alzheimer’s Disease Treatment Studies. Am J Psychiatry. 2002;159(5):738–745. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- 18.Herholz K. FDG PET and differential diagnosis of dementia. Alzheimer Dis Assoc Disord. 1995;9(1):6–16. doi: 10.1097/00002093-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Herholz K, Salmon E, Perani D, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17(1):302–316. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- 20.Mosconi L, Tsui WH, De Santi S, et al. Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis. Neurology. 2005;64(11):1860–1867. doi: 10.1212/01.WNL.0000163856.13524.08. [DOI] [PubMed] [Google Scholar]
- 21.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reiman EM, Chen K, Alexander GE, et al. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A. 2005;102(23):8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes. 2009;58(1):71–77. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 25.Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. J Geriatr Psychiatry Neurol. 2005;18(4):245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- 26.Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. A validation study of the Dementia Questionnaire. Arch Neurol. 1994;51(9):901–906. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- 27.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 28.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson SC, La Rue A, Hermann BP, et al. The effect of TOMM40 poly-T length on gray matter volume and cognition in middle-aged persons with APOE epsilon3/epsilon3 genotype. Alzheimers Dement. 2011;7(4):456–465. doi: 10.1016/j.jalz.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowling NM, Hermann B, La Rue A, Sager MA. Latent structure and factorial invariance of a neuropsychological test battery for the study of preclinical Alzheimer’s disease. Neuropsychology. 2010;24(6):742–756. doi: 10.1037/a0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koscik RL, La Rue A, Jonaitis EM, et al. Emergence of mild cognitive impairment in late middle-aged adults in the wisconsin registry for Alzheimer’s prevention. Dement Geriatr Cogn Disord. 2014;38(1–2):16–30. doi: 10.1159/000355682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 33.Tosun D, Mojabi P, Weiner MW, Schuff N. Joint analysis of structural and perfusion MRI for cognitive assessment and classification of Alzheimer’s disease and normal aging. NeuroImage. 2010;52(1):186–197. doi: 10.1016/j.neuroimage.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yakushev I, Hammers A, Fellgiebel A, et al. SPM-based count normalization provides excellent discrimination of mild Alzheimer’s disease and amnestic mild cognitive impairment from healthy aging. Neuroimage. 2009;44(1):43–50. doi: 10.1016/j.neuroimage.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Dukart J, Perneczky R, Forster S, et al. Reference cluster normalization improves detection of frontotemporal lobar degeneration by means of FDG-PET. PloS one. 2013;8(2):e55415. doi: 10.1371/journal.pone.0055415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borghammer P, Aanerud J, Gjedde A. Data-driven intensity normalization of PET group comparison studies is superior to global mean normalization. Neuroimage. 2009;46(4):981–988. doi: 10.1016/j.neuroimage.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Brett M, AJ, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox (HBM Abstract) Neuroimage. 2002;16(2) [Google Scholar]
- 38.Mackinnon DP, Warsi G, Dwyer JH. A Simulation Study of Mediated Effect Measures. Multivariate behavioral research. 1995;30(1):41. doi: 10.1207/s15327906mbr3001_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burns CM, Chen K, Kaszniak AW, et al. Higher serum glucose levels are associated with cerebral hypometabolism in Alzheimer regions. Neurology. 2013;80(17):1557–1564. doi: 10.1212/WNL.0b013e31828f17de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts RO, Knopman DS, Cha RH, et al. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55(5):759–764. doi: 10.2967/jnumed.113.132647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crane PK, Walker R, Hubbard RA, et al. Glucose levels and risk of dementia. N Engl J Med. 2013;369(6):540–548. doi: 10.1056/NEJMoa1215740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibson TC, Horton ES, Whorton EB. Interrelationships of insulin, glucose, lipid and anthropometric data in a natural population. Am J Clin Nutr. 1975;28(12):1387–1394. doi: 10.1093/ajcn/28.12.1387. [DOI] [PubMed] [Google Scholar]
- 43.Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Mendelsohn FA. Localization and Characterization of Insulin-Like Growth Factor-I Receptors in Rat Brain and Pituitary Gland Using in vitro Autoradiography and Computerized Densitometry* A Distinct Distribution from Insulin Receptors. Journal of neuroendocrinology. 1989;1(5):369–377. doi: 10.1111/j.1365-2826.1989.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 44.Hill JM, Lesniak MA, Pert CB, Roth J. Autoradiographic localization of insulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience. 1986;17(4):1127–1138. doi: 10.1016/0306-4522(86)90082-5. [DOI] [PubMed] [Google Scholar]
- 45.Chen K, Ayutyanont N, Langbaum JB, et al. Characterizing Alzheimer’s disease using a hypometabolic convergence index. Neuroimage. 2011;56(1):52–60. doi: 10.1016/j.neuroimage.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kadir A, Marutle A, Gonzalez D, et al. Positron emission tomography imaging and clinical progression in relation to molecular pathology in the first Pittsburgh Compound B positron emission tomography patient with Alzheimer’s disease. Brain : a journal of neurology. 2011;134(Pt 1):301–317. doi: 10.1093/brain/awq349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Unger J, McNeill TH, Moxley RT, 3rd, White M, Moss A, Livingston JN. Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience. 1989;31(1):143–157. doi: 10.1016/0306-4522(89)90036-5. [DOI] [PubMed] [Google Scholar]
- 48.Wolk DA, Dickerson BC. Fractionating verbal episodic memory in Alzheimer’s disease. Neuroimage. 2011;54(2):1530–1539. doi: 10.1016/j.neuroimage.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim Biophys Acta. 2010;1802(1):2–10. doi: 10.1016/j.bbadis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses. 2004;63(1):8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 51.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102(4):401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Craft S, Cholerton B, Baker LD. Insulin and Alzheimer’s disease: untangling the web. Journal of Alzheimer’s disease : JAD. 2013;33(Suppl 1):S263–275. doi: 10.3233/JAD-2012-129042. [DOI] [PubMed] [Google Scholar]
- 53.Jagust WJ, Landau SM. Apolipoprotein E, not fibrillar beta-amyloid, reduces cerebral glucose metabolism in normal aging. J Neurosci. 2012;32(50):18227–18233. doi: 10.1523/JNEUROSCI.3266-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Starks EJ, O’Grady JP, Hoscheidt SM, et al. Insulin Resistance is Associated with Higher Cerebrospinal Fluid Tau Levels in Asymptomatic APOE epsilon4 Carriers. Journal of Alzheimer’s disease : JAD. 2015 doi: 10.3233/JAD-150072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malek-Ahmadi M, Beach T, Obradov A, et al. Increased Alzheimer’s disease neuropathology is associated with type 2 diabetes and ApoE epsilon.4 carrier status. Current Alzheimer research. 2013;10(6):654–659. doi: 10.2174/15672050113109990006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng L, Chong MS, Lim WS, et al. Metabolic syndrome and amnestic mild cognitive impairment: Singapore Longitudinal Ageing Study-2 findings. Journal of Alzheimer’s disease : JAD. 2013;34(3):649–657. doi: 10.3233/JAD-121885. [DOI] [PubMed] [Google Scholar]
- 57.Claxton A, Baker LD, Hanson A, et al. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis. 2015;44(3):897–906. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- 58.Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70(6):440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 59.Mosconi L, Brys M, Switalski R, et al. Maternal family history of Alzheimer’s disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A. 2007;104(48):19067–19072. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mosconi L, Mistur R, Switalski R, et al. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009;72(6):513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mosconi L, Murray J, Tsui WH, et al. Brain imaging of cognitively normal individuals with 2 parents affected by late-onset AD. Neurology. 2014;82(9):752–760. doi: 10.1212/WNL.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yaffe K. Metabolic syndrome and cognitive decline. Current Alzheimer research. 2007;4(2):123–126. doi: 10.2174/156720507780362191. [DOI] [PubMed] [Google Scholar]
- 63.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 64.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67(6):505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 65.Strand BH, Langballe EM, Hjellvik V, et al. Midlife vascular risk factors and their association with dementia deaths: results from a Norwegian prospective study followed up for 35 years. J Neurol Sci. 2013;324(1–2):124–130. doi: 10.1016/j.jns.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 66.Spreen O, Strauss E. A compendium of neuropsychological tests : administration, norms, and commentary. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 67.Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale. Psychological Corporation; 1997. [Google Scholar]
- 68.Trenerry MR. Stroop Neuropsychological Screening Test Manual. Psychological Assessment Resources; 1989. [Google Scholar]
- 69.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Press; 1993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.