Abstract
Metastatic squamous cell carcinoma (SCC) of the anal canal is rare with limited data regarding treatment. Epidermal growth factor receptor (EGFR) expression has been observed in SCC of the anal canal and Kristen rat sarcoma vial oncogene (KRAS) mutations are rare. EGFR monoclonal antibodies, cetuximab and panitumumab, represent a potential option in this patient population. We report a metastatic SCC of the anal canal patient treated with cetuximab in combination with cisplatin plus 5-Fluorouracil (5-FU) that had a dramatic response.
Keywords: Cetuximab, anal cancer, epidermal growth factor receptor (EGFR) inhibitors
Introduction
Anal cancer represents 0.4% of all cancer cases in the United States with 7,210 estimated new cases in 2014 (1). Although rare, the incidence of anal cancer has increased over the last several decades (2). Histologically, anal cancer is predominately squamous cell carcinoma (SCC) and majority of patients are diagnosed in the localized to locally advanced setting. Patients in this setting are treated with definitive chemoradiation with 5-Fluorouracil (5-FU) in combination with mitomycin C or cisplatin. An abdominoperineal resection is reserved for those with locoregional failure following chemoradiation.
Metastatic disease is reported in 10 to 20 percent of patients (2). Data regarding treatment in the metastatic setting is very limited due to the rarity of this setting. Currently only one prospective Phase II trial is underway dedicated to this population looking at the role of two platinum doublet options in the first-line setting (3). Currently, medical oncologists must rely on limited retrospective case series, case reports, and extrapolating from more prevalent SCC histology cancers (lung and head and neck) to aid in treatment decisions.
Epidermal growth factor receptor (EGFR) expression has been observed in SCC of the anal canal and Kristen rat sarcoma vial oncogene (KRAS) mutations are rare (4-7). Therefore, EGFR inhibitors, such as cetuximab or panitumumab, represent a potential option in the treatment of metastatic SCC of the anal canal. We report a case of a refractory metastatic SCC of the anal canal patient who had a dramatic response with cetuximab in combination with cisplatin and 5-FU. Our patient is currently still receiving this treatment with continued response.
Case report
A 53-year-old woman with metastatic SCC of the anal canal was first diagnosed in December 2011 with a T2N2 anal tumor, following initial presentation of bright red blood per rectum. Her past medical history was essentially unremarkable except for a history of migraines and generalized arthralgia. Her performance status was excellent. She presented to her primary care physician who attributed the rectal bleeding to a hemorrhoid and so she was referred for hemorrhoidectomy. Upon examination a mass was found and biopsies confirmed SCC. She received treatment at an outside institution with concurrent chemoradiation therapy with 5-FU plus mitomycin C and a total radiation dose of 50.4 Gy. Post-treatment positron emission tomography (PET) imaging demonstrated complete metabolic response and she continued to be followed under close surveillance. She did well until June 2013 when repeat computerized tomography (CT) imaging revealed interval development lung metastases. A biopsy of the lung on 07/24/2013 confirmed the presence of metastatic SCC.
She presented to MD Anderson Cancer Center in August of 2013 seeking a second opinion on treatment recommendations. Given phase I clinical trials available at that time for treatment of metastatic SCC, the patient opted for enrollment onto a phase I protocol. Baseline imaging was obtained on 08/13/2013 and showed bilateral lung metastases, as well as retroperitoneal lymphadenopathy. She received treatment from 09/10/2013 until 03/04/2014 with progression of disease noted on CT imaging done in 03/2014. Though the primary tumor site remained stable, she had worsening of mediastinal and hilar lymphadenopathy. She was placed on a second clinical trial and received treatment from 04/10/2014 to 07/04/2014. Imaging was obtained with a PET scan and she was again found to have disease progression with interval development of new faint fluorodeoxyglucose (FDG)-avid hypodense lesions in the liver, consistent with metastasis. Also noted, were several new foci of FDG-avid bone metastases involving the lumbar spine.
The patient returned to her primary GI Medical Oncology team for further assessment and recommendations. She had an ECOG performance status of 1 due to mild fatigue. Overall she was doing fairly well and was given recommendations for palliative chemotherapy with a combination of cetuximab (day 1), cisplatin (day 1), and 5-FU continuous infusion over 46 hours (starting day 1) given every 2 weeks. Following four cycles of therapy she underwent repeat PET imaging that demonstrated a dramatic response with resolution of previously seen FDG-avid lymphadenopathy, liver and bone metastasis (Figures 1,2,3,4,5).
Figure 1.
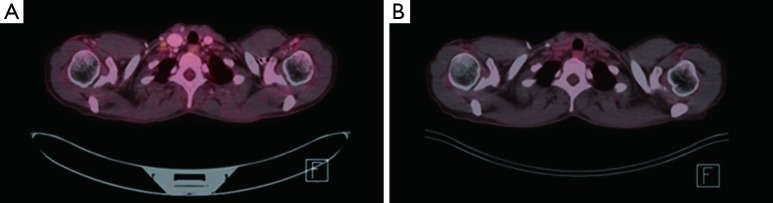
FDG PET/CT imaging. (A) Pretreatment, 9 mm right supraclavicular lymph node with an SUV of 2.4; (B) posttreatment, 9 mm right supraclavicular node with resolution of previously seen FDG avidity. FDG, fluorodeoxyglucose; PET, positron emission tomography; CT, computerized tomography; SUV, standardized uptake value.
Figure 2.
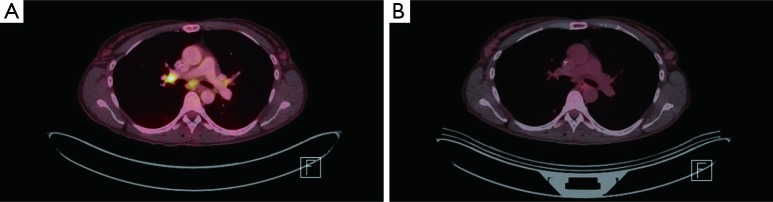
FDG PET/CT imaging. (A) Pretreatment, bilateral hilar FDG-avid hilar adenopathy. For example, right hilar node with SUV of 7.8; (B) posttreatment, interval resolution of FDG-avid bilateral hilar adenopathy. FDG, fluorodeoxyglucose; PET, positron emission tomography; CT, computerized tomography; SUV, standardized uptake value.
Figure 3.
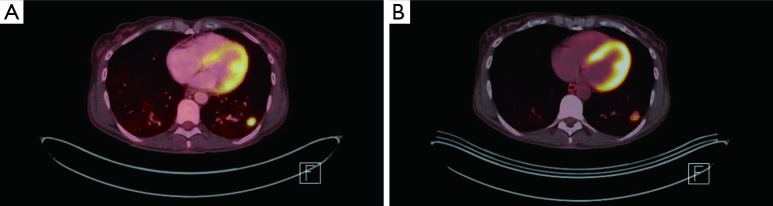
FDG PET/CT imaging. (A) Pretreatment, 1.6 cm × 1.9 cm left lower lung nodule with SUV of 8.4; (B) posttreatment, interval decrease in FDG avidity, SUV of 5.4. FDG, fluorodeoxyglucose; PET, positron emission tomography; CT, computerized tomography; SUV, standardized uptake value.
Figure 4.
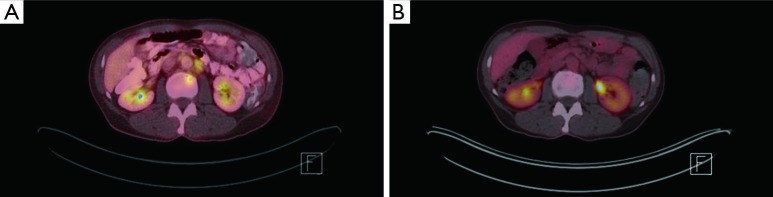
FDG PET/CT imaging. (A) Pretreatment, 8 mm lytic lesion at L2 with SUV of 4.7; (B) posttreatment, previously noted FDG avid focal skeletal lesion resolved. FDG, fluorodeoxyglucose; PET, positron emission tomography; CT, computerized tomography; SUV, standardized uptake value.
Figure 5.
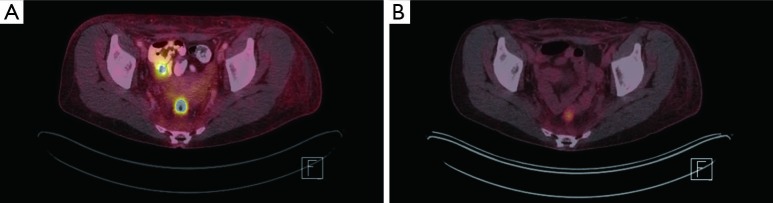
FDG PET/CT imaging. (A) Pretreatment, primary anorectal tumor with an SUV of 11.7; (B) posttreatment, improved FDG activity with an SUV of 11.4. FDG, fluorodeoxyglucose; PET, positron emission tomography; CT, computerized tomography; SUV, standardized uptake value.
Upon return she also reported a hospitalization that took place in August 2014 due to a partial small bowel obstruction attributed to adhesions that resulted in a diverting colostomy with significant improvement in her symptoms overall. She tolerated therapy very well despite mucositis that she developed with treatment. Recommendations were given to continue with this current regimen given her significant response and she is currently receiving the same treatment.
Discussion
Treatment for metastatic SCC of the anal canal as mentioned previously is limited. Currently, the National Comprehensive Cancer Network (NCCN) guidelines for this malignancy list one regimen (5-FU plus cisplatin) for this setting (2). This recommendation is based on small cohorts or case studies. A large retrospective evaluation in metastatic anal cancer patients has recently been published (8). This evaluation looked at systemic chemotherapy and the role of multidisciplinary management. Eng et al. identified 5-FU plus cisplatin and carboplatin plus taxol as two common regimens used in the metastatic first-line setting. Overall progression-free survival (PFS) and overall survival (OS) was found to be 7 and 22 months, respectively. Patients that had multidisciplinary management with the addition of localized treatment had longer PFS and OS. Therapy given after first-line in this evaluation was not reported and NCCN does not have recommendations beyond cisplatin based therapy failure (2,8). Therefore, it is imperative that other agents be evaluated in this setting to allow more therapeutic options in this population.
EGFR is overexpressed in SCC cancers of the head and neck and lung cancer (9,10). With this overexpression, cetuximab is a treatment option in these more prevalent SCC cancers. EGFR is reported to be overexpressed in 80 to 100 percent of SCC of the anal canal while KRAS mutations have been found to be uncommon in this malignancy (4-7). The lack of KRAS mutations decreases the likelihood of cetuximab resistance. Recent data in colorectal cancer has indicated that despite being KRAS wild-type, 15-18% of all patients have additional RAS mutations that may be identified with an expanded RAS panel analysis (11). These rare RAS mutations may negatively impact the benefit of EGFR inhibition regardless if initially KRAS wild-type. Cetuximab has been reported in case series and case reports in refractory metastatic SCC of the anal canal with majority of cases showing disease stability or response (12-14).
Conclusions
We report a metastatic SCC of the anal canal patient refractory to multiple lines of therapy that had a substantial response to cetuximab in combination with cisplatin plus 5-FU. Further data is needed to confirm the true benefit seen with cetuximab in this population and to determine its role in treatment. Our case, along with other case reports, poses the question that cetuximab or panitumumab may benefit metastatic SCC of the anal canal patients and should be investigated further.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN guidelines anal carcinoma. Version 1.2015. Available online: www.nccn.org, accessed 11/22/14.
- 3.Clinicaltrials.gov. Advanced anal cancer. Available online: www.clinicaltrials.gov, accessed 11/22/14.
- 4.Serup-Hansen E, Linnemann D, Høgdall E, et al. KRAS and BRAF mutations in anal carcinoma. APMIS 2015;123:53-9. [DOI] [PubMed] [Google Scholar]
- 5.Casadei Gardini A, Capelli L, Ulivi P, et al. KRAS, BRAF and PIK3CA status in squamous cell anal carcinoma (SCAC). PLoS One 2014;9:e92071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paliga A, Onerheim R, Gologan A, et al. EGFR and K-ras gene mutation status in squamous cell anal carcinoma: a role for concurrent radiation and EGFR inhibitors? Br J Cancer 2012;107:1864-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zampino MG, Magni E, Sonzogni A, et al. K-ras status in squamous cell anal carcinoma (SCC): it's time for target-oriented treatment? Cancer Chemother Pharmacol 2009;65:197-9. [DOI] [PubMed] [Google Scholar]
- 8.Eng C, Chang GJ, You YN, et al. The role of systemic chemotherapy and multidisciplinary management in improving the overall survival of patients with metastatic squamous cell carcinoma of the anal canal. Oncotarget 2014;5:11133-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivera F, García-Castaño A, Vega N, et al. Cetuximab in metastatic or recurrent head and neck cancer: the EXTREME trial. Expert Rev Anticancer Ther 2009;9:1421-8. [DOI] [PubMed] [Google Scholar]
- 10.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525-31. [DOI] [PubMed] [Google Scholar]
- 11.Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023-34. [DOI] [PubMed] [Google Scholar]
- 12.Lukan N, Ströbel P, Willer A, et al. Cetuximab-based treatment of metastatic anal cancer: correlation of response with KRAS mutational status. Oncology 2009;77:293-9. [DOI] [PubMed] [Google Scholar]
- 13.Rogers JE, Eng C. Cetuximab in refractory squamous cell carcinoma of the anal canal. J Gastrointest Cancer 2014;45 Suppl 1:198-200. [DOI] [PubMed] [Google Scholar]
- 14.Saif MW, Kontny E, Syrigos KN, et al. The Role of EGFR Inhibitors in the Treatment of Metastatic Anal Canal Carcinoma: A Case Series. J Oncol 2011;2011:125467. [DOI] [PMC free article] [PubMed]


