Abstract
Background
Screening colonoscopy is a standard part of the liver transplant (LT) evaluation process. We aimed to evaluate the yield of screening colonoscopy and determine whether non-alcoholic fatty liver disease (NAFLD) was associated with an increased risk of colorectal neoplasia.
Methods
We retrospectively assessed all patients who completed LT evaluation at our center between 1/2008-12/2012. Patients <50 years old and those without records of screening colonoscopy, or with greater than average colon cancer risk were excluded.
Results
A total of 1,102 patients were evaluated, 591 met inclusion criteria and were analyzed. The mean age was 60 years, 67% were male, 12% had NAFLD and 88% had other forms of chronic liver disease. Overall, 42% of patients had a polyp found on colonoscopy: 23% with adenomas, 14% with hyperplastic polyps and with 1% inflammatory polyps. In the final multivariable model controlling for age, NAFLD [odds ratio (OR) 2.41, P=0.001] and a history of significant alcohol use (OR 1.69, P=0.004) were predictive of finding a polyp on colonoscopy. In addition, NAFLD (OR 1.95, P=0.02), significant alcohol use (OR 1.70, P=0.01) and CTP class C (OR 0.57, P=0.02) were associated with adenoma, controlling for age.
Conclusions
Screening colonoscopy in patients awaiting LT yields a high rate of polyp (43%) and adenoma (22%) detection, perhaps preventing the accelerated progression to carcinoma that can occur in immunosuppressed post-LT patients. Patients with NAFLD may be at a ~2 fold higher risk of adenomas and should be carefully evaluated prior to LT.
Keywords: Colon cancer, screening, cirrhosis, liver transplant (LT), non-alcoholic fatty liver disease (NAFLD)
Introduction
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer-related death in the United States (1). Risk-based screening, including in individuals over the age of 50 in the absence of other known risk factors, has been advocated (2-8). Obesity, and associated metabolic syndrome manifestations including diabetes have now been associated with increased CRC risk in epidemiologic studies, and increased risk of polyps by ~1.5-6.2-fold (9-17). While the mechanism of this association is uncertain, perturbations in cytokine and hormone levels (e.g., IGF and adipokines) have been implicated (9,18,19).
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of liver disease which includes steatosis, non-alcoholic steatohepatitis (NASH) and fibrosis/cirrhosis. It represents the hepatic manifestation of the metabolic syndrome. NAFLD is now estimated to impact 10-46% of the population in the United States (20-24) and is projected to become the leading indication for LT in the next decade (25-28). Extrahepatic complications of NAFLD (29) and in particular, an association between NAFLD and colorectal polyps have been suggested by a limited number of studies without histologic confirmation of NAFLD.
Given the increased risk of malignancy with post-LT immunosuppression (including CRC) (30) and the limited supply of organs, age-appropriate CRC screening with colonoscopy is a standard part of the evaluation for solid organ transplant, including liver transplant (LT) (31). Because the metabolic syndrome and its associated diseases (obesity, diabetes, hypertension and hyperlipidemia) are common in the transplant population, and the proportion of patients in transplant evaluation with NAFLD is projected to rise, we assessed the yield of screening colonoscopy in the transplant evaluation process and hypothesized that patients with NAFLD-related end stage liver disease would have a higher prevalence of colonic polyps than those in LT evaluation for other types of liver disease.
Patients and methods
Patients
This is a retrospective single center cohort study of all adult patients evaluated for LT between January 1, 2008 and December 31, 2012. Patients were excluded if they were less than 50 years old at LT evaluation, or had above average risk for CRC, including inflammatory bowel disease (IBD) (7,32), a history of multiple/recurrent adenomatous polyps (7,33), a significant family history of colon cancer (7,34), known cancer-predisposing gene alteration or a prior history of solid organ transplant (35,36). In addition, patients with HIV infection (37,38), a prior personal history of cancer (39,40), or with no colonoscopy report available for review were excluded.
Study design and definitions
All patients 50 years or older are required to have a screening colonoscopy within 5 years of LT evaluation, and records of this exam are included in the LT evaluation documentation for review. Records of prior colonoscopies and pathology reports were also reviewed when available. All lesions found on colonoscopy were recorded, including number, location (right, transverse or left colon) and size of each polyp. Polyps were classified as adenomatous (tubular, tubulovillous, villous, or serrated), hyperplastic or inflammatory based upon pathology reports. Dysplasia and evidence of invasive carcinoma were also noted when present.
The primary outcomes of our study were the presence of any polyp on screening colonoscopy and the presence of an adenomatous polyp. Only patients with histology available for review were included in the analysis of risk factors for adenoma.
The primary predictor evaluated was the diagnosis of NAFLD-related end stage liver disease. This was defined as biopsy proven [either with liver biopsy in the pre-LT period or on explant when available, (n=50), or clinically in patients with cryptogenic cirrhosis and NAFLD risk factors (n=18)]. We used clinical criteria for some patients because explants with cirrhosis often obscure underlying features of NAFLD. Sensitivity analyses were performed including only patients with biopsy-proven disease. Additional predictors tested included the presence of diabetes, hypertension, hyperlipidemia, the metabolic syndrome (defined as the presence of obesity and two associated comorbidities), age, toxic exposures (including significant alcohol or tobacco use), and characteristics of liver disease including etiology, MELD and Child-Turcotte-Pugh class. The study was approved by the Columbia University Institutional Review Board.
Statistical analysis
Baseline characteristics and outcomes of colonoscopy were compared between patients with and without NAFLD with student t-test or Rank sum (continuous variables), or chi-square (categorical variables) as appropriate. We used multiple logistic regression analysis to identify predictors of (I) any polyp on colonoscopy and (II) an adenomatous polyp. A backwards elimination approach was utilized in multivariable modeling, considering parameters with P<0.2 in the initial multivariable model and sequentially removing non-significant predictors. Age was included in all final models given the known significant impact of age on CRC risk. Sensitivity analyses were performed excluding those patients without biopsy-proven NAFLD (18/68, 26%) and those without colon polyp histology available for review (61/248, 24%).
Results
Patient characteristics
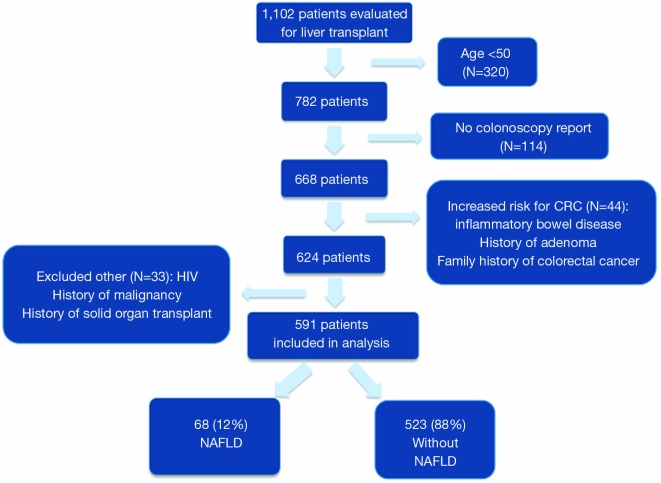
A total of 1,102 adult patients were evaluated for LT in the study period, 320 (29%) were excluded due to age less than 50, 44 (4%) due to higher than average risk of CRC, 21 (2%) due to HIV, 6 (0.5%) due to prior malignancy, 6 (0.5%) due to prior solid organ transplant and 114 (10%) for a lack of colonoscopy report available for review (Figure 1). Thus, 591 patients were included in the final analysis: 68 (12%) with NAFLD and 523 (88%) without NAFLD. Baseline characteristics including the degree of liver disease severity were generally similar between the NAFLD and non-NAFLD groups (Table 1). When compared to the non-NAFLD group, patients with NAFLD were older (mean age 62.5 vs. 59.5 years, P≤0.001), had a higher body mass index (BMI) (31.6 vs. 29.1, P≤0.001), were more likely to be obese (57% vs. 29%, P≤0.001), and to have comorbidities such as diabetes (71% vs. 28%, P≤0.001), hypertension (46% vs. 33%, P=0.05), coronary artery disease (31% vs. 9%, P≤0.001) and the metabolic syndrome (29% vs. 6%. P≤0.001). Any history of significant alcohol intake (9% vs. 37%, P≤0.001) and tobacco (32% vs. 49%, P=0.02) use were less common in the NASH patients.
Figure 1.
Flow diagram of patient population included in the final analysis. NAFLD, non-alcoholic fatty liver disease.
Table 1. Patient demographics.
| Variables | NAFLD (N=68) | Non-NAFLD (N=523) | P value |
|---|---|---|---|
| Age, mean | 62.5 | 59.5 | <0.001 |
| Male gender [%] | 42 [62] | 356 [68] | 0.30 |
| BMI, mean | 31.6 | 29.1 | <0.001 |
| Obese (BMI ≥30) | 39 [57] | 153 [29] | <0.001 |
| Race/ethnicity [%] | 0.12 | ||
| Non-Hispanic White | 46 [68] | 270 [52] | |
| Hispanic | 9 [13] | 105 [20] | |
| African American | 3 [4] | 35 [7] | |
| Asian | 4 [6] | 42 [8] | |
| Other/unknown | 6 [9] | 71 [14] | |
| Etiology of liver disease [%] | — | ||
| NAFLD | 68 [100] | — | |
| HCV | — | 266 [51] | |
| HBV | — | 37 [7] | |
| Alcohol | — | 113 [22] | |
| AIH/PSC/PBC | — | 57 [11] | |
| Other | — | 50 [10] | |
| HCC [%] | 19 [28] | 168 [32] | 0.49 |
| Medical comorbidities [%] | |||
| Metabolic syndrome | 20 [29] | 33 [6] | <0.001 |
| Diabetes mellitus | 48 [71] | 145 [28] | <0.001 |
| Hypertension | 31 [46] | 175 [33] | 0.05 |
| Coronary artery disease | 21 [31] | 48 [9] | <0.001 |
| Significant alcohol use [%] | <0.001 | ||
| Recent | 0 [0] | 9 [2] | |
| Former | 6 [9] | 184 [35] | |
| Never | 62 [91] | 330 [63] | |
| Tobacco use [%] | 0.02 | ||
| Current | 3 [4] | 56 [11] | |
| Former | 19 [28] | 202 [39] | |
| Never | 46 [68] | 265 [51] | |
| Intravenous drug use [%] | 0.01 | ||
| Current | 0 [0] | 1 [0.2] | |
| Former | 2 [3] | 86 [16] | |
| Never | 66 [97] | 436 [83] | |
| Listed for liver transplant [%] | 53 [78] | 420 [80] | 0.65 |
| Transplanted [%] | 37 [54] | 260 [50] | 0.47 |
| MELD, mean | 15.1 | 14.5 | 0.58 |
| CTP score, median | 8 | 8.1 | 0.89 |
| CTP class [%] | 0.29 | ||
| A | 18 [26] | 161 [31] | |
| B | 35 [51] | 217 [41] | |
| C | 15 [22] | 145 [28] | |
| Portal hypertension [%] | |||
| Esophageal varices | 45 [66] | 321 [61] | 0.44 |
| Hepatic encephalopathy | 36 [53] | 276 [53] | 0.98 |
| Ascites | 39 [57] | 295 [56] | 0.88 |
| Spontaneous bacterial peritonitis | 3 [4] | 16 [3] | 0.55 |
| Laboratory values*, mean | |||
| Albumin (g/dL) | 3.3 | 3.2 | 0.86 |
| Hemoglobin (g/dL) | 11.6 | 11.9 | 0.26 |
| INR | 1.4 | 1.4 | 0.54 |
| AFP (ng/mL) | 92.6 | 54 | 0.34 |
| Creatinine (mg/dL) | 1.3 | 1.2 | 0.4 |
| Bilirubin (mg/dL) | 3.6 | 3.7 | 0.96 |
| Platelets (103/uL) | 101.1 | 111.8 | 0.23 |
*, all laboratory values are at the time of presentation for listing; NAFLD, non-alcoholic fatty liver disease; BMI, body mass index; INR, international normalized ratio; AFP, alpha fetoprotein; MELD, model for end stage liver disease; CTP, child turcotte pugh score.
Colonoscopy findings
Overall, 248 (42%) patients had any polyp found on colonoscopy: 133 (23%) were adenomas, 81 (14%) were hyperplastic and 7 (1%) were inflammatory (Table 2). There were very few patients with dysplasia (2%) and no patients with invasive carcinoma. Other findings included hemorrhoids in 59%, diverticulosis in 29%, rectal varices in 7%, and vascular ectasia in 3%.
Table 2. Findings on colonoscopy.
| Variables | NAFLD (N=68) | Non-NAFLD (N=523) | P value |
|---|---|---|---|
| Any polyp [%] | 40 [59] | 208 [40] | 0.003 |
| Adenoma | 22 [32] | 111 [21] | 0.04 |
| Inflammatory | 3 [4] | 4 [1] | 0.01 |
| Hyperplastic | 11 [16] | 70 [13] | 0.53 |
| Unknown pathology | 9 [13] | 52 [10] | 0.4 |
| Location [%]* | |||
| Right | 20 [29] | 85 [16] | 0.01 |
| Left | 22 [32] | 126 [24] | 0.2 |
| Transverse | 12 [18] | 47 [9] | 0.03 |
| Adenoma location [%]* | |||
| Right | 9 [13] | 46 [9] | 0.25 |
| Left | 10 [15] | 54 [10] | 0.27 |
| Transverse | 7 [10] | 32 [6] | 0.21 |
| Polyp size | |||
| Largest polyp (mm), median [IQR] | 5.5 [5-8] | 5.0 [3-8] | 0.09 |
| No. >1 cm [%] | 6 [20] | 33 [23] | 0.7 |
| Adenoma size | |||
| Largest adenoma, median [IQR] | 5.0 [5-8] | 5.0 [3-10] | 0.27 |
| No. >1 cm [%] | 5 [20] | 24 [31] | 0.53 |
| Number [%] with | 0.001 | ||
| 4 polyps | 5 [7] | 12 [2] | |
| 3 polyps | 5 [7] | 27 [5] | |
| 2 polyps | 3 [4] | 52 [10] | |
| 1 polyp | 27 [40] | 117 [22] | |
| Dysplasia [%] | 1 [1] | 8 [2] | 0.97 |
| Invasive carcinoma [%] | 0 [0] | 0 [0] | 1.00 |
| Other findings [%] | |||
| Hemorrhoids | 42 [62] | 306 [59] | 0.61 |
| Diverticulosis | 26 [38] | 147 [28] | 0.08 |
| Rectal varices | 3 [4] | 37 [7] | 0.41 |
| Vascular ectasia | 1 [1] | 18 [3] | 0.39 |
*, patients may have more than one polyp or adenoma location if there is more than one encountered; NAFLD, non-alcoholic fatty liver disease.
Association between NAFLD and colorectal polyps
Patients with NAFLD were significantly more likely to have a polyp found on colonoscopy than those without NAFLD (59% vs. 40%, P=0.003) (Figure 2). In addition, NAFLD patients were more likely to have multiple polyps, with 15% vs. 8% having more than 2 polyps encountered (P=0.04, Table 2). In univariate analysis (Table 3), significant predictors of finding a polup included NALFD [odds ratio (OR) 2.16, P=0.003]. a significant history of alcohol use (OR 1.47, P=0.03), and the presence of diabetes (OR 1.46, P=0.03). The presence of the metabolic syndrome was not significantly predictive (OR 1.37, P=0.27). In multivariable analysis controlling for age, NAFLD (OR 2.42, P=0.001), and alcohol use (OR 1.69, P=0.004), remained the only significant predictors of encountering a polyp. In particular, polyps in the right (29% vs. 16%, P=0.01) and transverse (18% vs. 9%, P=0.03) segments of the colon were significantly more common in NAFLD patients (Figure 3). In sensitivity analysis excluding patients without histologically confirmed NAFLD (n=18) or those without available polyp histology (n=61), NAFLD remained a significant predictor of polyps in univariate (OR 2.44, P=0.003 and OR 1.97, P=0.009, respectively) and multivariable analyses (OR 2.68, P=0.002 and OR 2.08, P=0.007, respectively).
Figure 2.
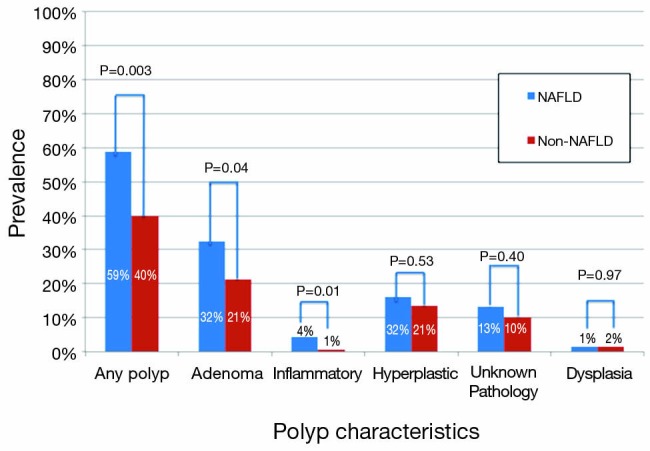
The prevalence of polyps by polyp type and liver disease etiology (NAFLD compared to non-NAFLD). NAFLD, non-alcoholic fatty liver disease.
Table 3. Unadjusted and adjusted predictors of a polyp or an adenoma on colonoscopy.
| Predictor | Polyp |
Adenoma |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
||||||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||||
| NAFLD | 2.16 (1.29-3.62) | 0.003 | 2.42 (1.42-4.11) | 0.001 | 1.95 (1.09-3.47) | 0.02 | 1.95 (1.09-3.48) | 0.02 | |||
| Age (per year) | 1.02 (0.99-1.05) | 0.18 | 1.02 (0.99-1.05) | 0.29 | 1.02 (0.99-1.06) | 0.2 | 1.02 (0.98-1.05) | 0.32 | |||
| Hispanic ethnicity | 1.12 (0.74-1.71) | 0.59 | 0.91 (0.56-1.48) | 0.72 | |||||||
| African American | 0.80 (0.40-1.57) | 0.51 | 0.63 (0.23-1.54) | 0.31 | |||||||
| BMI | 0.99 (0.97-1.02) | 0.60 | 0.99 (0.95-1.02) | 0.49 | |||||||
| Obesity (BMI ≥30) | 0.95 (0.67-1.35) | 0.78 | 0.95 (0.63-1.43) | 0.8 | |||||||
| Albumin | 1.26 (0.99-1.61) | 0.06 | 1.16 (0.87-1.54) | 0.33 | |||||||
| Hgb | 0.99 (0.92-1.07) | 0.84 | 1.01 (0.92-1.10) | 0.89 | |||||||
| INR | 0.97 (0.76-1.24) | 0.81 | 0.99 (0.73-1.33) | 0.93 | |||||||
| Creatinine | 1.04 (0.90-1.21) | 0.59 | 1.00 (0.84-1.19) | 0.97 | |||||||
| Total bilirubin | 0.98 (0.96-1.01) | 0.25 | 0.98 (0.94-1.02) | 0.25 | |||||||
| Platelets | 1.00 (0.998-1.00) | 0.31 | 1.00 (0.998-1.00) | 0.24 | |||||||
| Meld score | 0.98 (0.96-1.01) | 0.16 | 0.98 (0.95-1.00) | 0.1 | |||||||
| CTP score | 0.94 (0.88-1.01) | 0.10 | 0.91 (0.84-1.0) | 0.05 | |||||||
| CTP class | |||||||||||
| A | − (−) | — | − (−) | — | − (−) | — | |||||
| B | 0.87 (0.59-1.28) | 0.49 | 1.04 (0.67-1.63) | 0.85 | 0.98 (0.63-1.54) | 0.93 | |||||
| C | 0.69 (0.45-1.07) | 0.10 | 0.57 (0.35-0.92) | 0.04 | 0.54 (0.31-0.94) | 0.03 | |||||
| Diabetes | 1.46 (1.03-2.06) | 0.03 | 1.17 (0.78-1.75) | 0.45 | |||||||
| HTN | 1.11 (0.79-1.57) | 0.53 | 1.32 (0.89-1.97) | 0.17 | |||||||
| Coronary artery disease | 0.81 (0.49-1.37) | 0.44 | 0.95 (0.52-1.75) | 0.87 | |||||||
| Alcohol use | 1.47 (1.04-2.08) | 0.03 | 1.69 (1.19-2.42) | 0.004 | 1.70 (1.12-2.58) | 0.01 | 1.73 (1.14-2.62) | 0.01 | |||
| Tobacco | 1.04 (0.75-1.45) | 0.80 | 1.17 (0.79-1.72) | 0.43 | |||||||
| HCC | 0.98 (0.69-1.40) | 0.93 | 0.91 (0.60-1.38) | 0.66 | |||||||
| HCV | 0.81 (0.58-1.12) | 0.20 | 0.93 (0.63-1.37) | 0.71 | |||||||
| Portal hypertension | 1.35 (0.80-2.26) | 0.26 | 1.18 (0.64-2.20) | 0.59 | |||||||
NAFLD, non-alcoholic fatty liver disease; INR, international normalized ratio; Hgb, hemoglobin; BMI, body mass index; HTN, hypertension; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; CTP, child turcotte pugh score.
Figure 3.
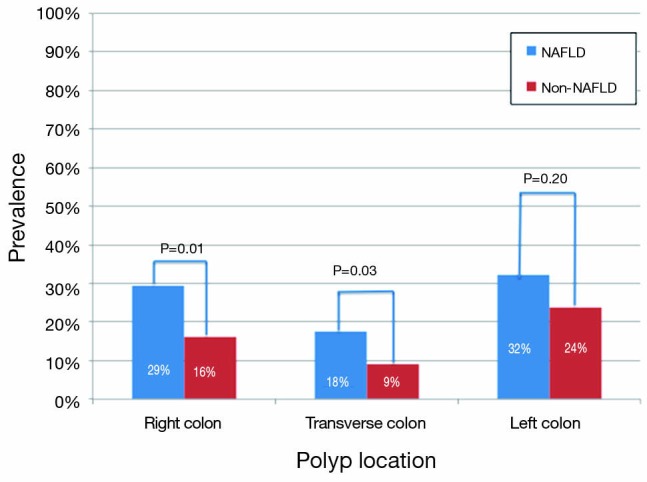
The prevalence of polyp by location and liver disease etiology (NAFLD compared to non-NAFLD). NAFLD, non-alcoholic fatty liver disease.
Association between NAFLD and colorectal adenoma
NAFLD patients were also more likely to have an adenomatous polyp than those without NAFLD (32% vs. 21%, P=0.04). In univariate analysis, significant predictors of finding an adenoma included NAFLD (OR 1.95, P=0.02), a significant history of alcohol use (OR 1.70, P=0.01), and CTP class C status (OR 0.57, P=0.04) (Table 3). The presence of diabetes (OR 1.15, P=0.45) or the metabolic syndrome (OR 1.4, P=0.29) were not predictive of adenoma. In multivariable analysis controlling for age, NAFLD (OR 1.95, P=0.02), and alcohol use (OR 1.73, P=0.01) were associated with a greater risk of adenomatous polyps and CTP class C status remained protective against adenoma (OR 0.54, P=0.03). In a sensitivity analysis excluding patients without biopsy confirmed NAFLD (n=18), NAFLD remained similarly predictive of adenomas in univariate (OR 1.71, P=0.10) and multivariable (OR 1.79, P=0.08) analyses though did not reach statistical significance possible due to decreased power.
Discussion
Our study demonstrates that polyps (42%), and specifically adenomatous polyps (23%), are common among average risk LT candidates undergoing screening colonoscopy, comparable to the average detection rates for adenomatous polyps in the general population (20-32%) (41-44). These rates of polyp and adenoma detection are also consistent with recent findings in the cirrhotic pre-LT population (49% and 29%, respectively) (41,45). In addition, polyps and adenomatous polyps are more common in patients with NAFLD than in those with other forms of end stage liver disease. In adjusted models, NAFLD patients had a ~2.5-fold higher risk of polyps and a ~2-fold higher risk of adenomatous polyps compared to non-NAFLD patients.
Previous literature suggests an association between colorectal neoplasia and the metabolic syndrome and its components, including diabetes mellitus and obesity (9-13,15-17,46-50). In one series, a 5 unit increase in BMI increased the risk of colorectal adenoma by as much as 19% (51). In our study, NAFLD was a much stronger predictor of polyps than the metabolic syndrome or diabetes. Several large retrospective studies and meta-analyses have recently demonstrated similar associations in adjusted analyses between NAFLD and polyps, adenomas and even CRC on screening colonoscopy (46-48,52-54), though these studies used predominantly imaging-based NAFLD diagnostic criteria. In one previous small series of younger patients in the US with histologically proven NAFLD, there was no difference in the prevalence of polyps, though NAFLD patients had a greater number of adenomas encountered (55).
Wong et al. performed perhaps the most detailed analysis with two cohorts, including patients with magnetic resonance spectroscopy evidence of steatosis, those with biopsy-proven NASH, and controls (46). Overall, in adjusted analysis, NAFLD was associated with a 3-fold higher risk of advanced colorectal neoplasms. Interestingly, this association was most strongly observed in patients with NASH as opposed to those with simple steatosis. Given that all the patients in our cohort had end stage liver disease requiring transplantation. It is likely that the vast majority of these patients did have NASH, rather than simple steatosis. In addition, as in our study, those with biopsy-proven NAFLD were more likely to have polyps or adenomas on colonoscopy.
We found the greatest difference in polyps was in right and transverse areas of the colon. This was also found in Wong et al. cohort of NASH patients, as well as in a recent large study of patients with cirrhosis undergoing pre-LT screening colonoscopy (46). A similar pattern has been well described in patients with primary sclerosing cholangitis (PSC) and IBD, thought possibly to be due to alterations in bile salt metabolism (56). The reasons for this association specifically with proximal lesions deserve additional investigation.
The mechanism of the observed association between NAFLD and colorectal polyps is not completely understood. It is possible that elevations in insulin and pro-inflammatory cytokines such as insulin-like growth factor in NAFLD patients may promote neoplastic growth of the colonic mucosa (57). In addition, reduced levels of adiponectin are seen in NAFLD patients (58,59), which has been associated with increased risk of colorectal adenomas (60), and may also contribute to this association. In addition, the postulated role of the intestinal microbiome in the pathogenesis of both NAFLD (61-65) and CRC (66-69) may suggest a common pathway putting patients at risk for both diseases, and additional investigation in this area is warranted.
We also found that patients with a history of significant alcohol exposure were at higher risk of polyps. In adjusted models, patients with prior heavy alcohol use were at ~1.7-fold higher risk of polyps or adenomatous polyps compared to those without a significant alcohol history. This finding is supported by previous literature, possibly due to an increase in reactive oxygen species and acetaldehyde contributing to mucosal hyper-regeneration (70). Those that do use alcohol tend to have earlier presentation of premalignant lesions in the colon (71). PSC was not associated with polyp risk in our cohort, despite previous reports of this association (72,73). The lack of association with PSC may be due to the exclusion of patients with a diagnosis of IBD, and the small number (10) of PSC patients included.
Unexpectedly, CTP class C status was associated with a significantly decreased risk of specifically adenomatous polyps in our cohort. It is uncertain whether this reflects a true decrease in rate of adenomas in patients with severe chronic liver disease, or whether it reflects a decreased detection rate due to poor prep or change in colonoscopic technique in this severely ill patient population. Additional studies are needed to confirm this finding.
Our study is limited by the retrospective study design and the lack of colonoscopy and pathology reports in a limited number of patients. However, even in adjusted analysis, the magnitude of association between NAFLD and polyps or adenomas was very similar in sensitivity analyses excluding those without pathology. In addition, given the variation in colonoscopy reports, we were unable to reliably extract data on prep quality, withdrawal times or overall adenoma detection rates for the individual colonoscopists.
In conclusion, screening colonoscopy in patients awaiting LT detects a high rate of polyps and adenomas. The presence of NAFLD and a history of significant alcohol use were highly predictive of encountering polyps and adenomas, even after controlling for traditional risk factors such as advanced age. Proximal lesions were particularly common in NAFLD patients, indicating that full colonoscopy may be the preferred screening strategy in this population and that deferral of the procedure until after transplant may not be advisable. It is uncertain whether these findings together the similar previously reported data warrant a change in the screening guidelines for NAFLD patients, or how NAFLD and the metabolic syndrome impacts post-LT rates of malignancy and therefore post-LT screening protocols.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. [DOI] [PubMed] [Google Scholar]
- 2.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993;328:1365-71. [DOI] [PubMed] [Google Scholar]
- 3.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348:1472-7. [DOI] [PubMed] [Google Scholar]
- 4.Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348:1467-71. [DOI] [PubMed] [Google Scholar]
- 5.Selby JV, Friedman GD, Quesenberry CP, Jr, et al. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med 1992;326:653-7. [DOI] [PubMed] [Google Scholar]
- 6.Newcomb PA, Norfleet RG, Storer BE, et al. Screening sigmoidoscopy and colorectal cancer mortality. J Natl Cancer Inst 1992;84:1572-5. [DOI] [PubMed] [Google Scholar]
- 7.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008;58:130-60. [DOI] [PubMed] [Google Scholar]
- 8.Whitlock EP, Lin JS, Liles E, et al. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2008;149:638-58. [DOI] [PubMed] [Google Scholar]
- 9.Eddi R, Karki A, Shah A, et al. Association of type 2 diabetes and colon adenomas. J Gastrointest Cancer 2012;43:87-92. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui AA, Maddur H, Naik S, et al. The association of elevated HbA1c on the behavior of adenomatous polyps in patients with type-II diabetes mellitus. Dig Dis Sci 2008;53:1042-7. [DOI] [PubMed] [Google Scholar]
- 11.Siddiqui A, Pena Sahdala HN, Nazario HE, et al. Obesity is associated with an increased prevalence of advanced adenomatous colon polyps in a male veteran population. Dig Dis Sci 2009;54:1560-4. [DOI] [PubMed] [Google Scholar]
- 12.Boutron-Ruault MC, Senesse P, Méance S, et al. Energy intake, body mass index, physical activity, and the colorectal adenoma-carcinoma sequence. Nutr Cancer 2001;39:50-7. [DOI] [PubMed] [Google Scholar]
- 13.Laiyemo AO. The risk of colonic adenomas and colonic cancer in obesity. Best Pract Res Clin Gastroenterol 2014;28:655-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almendingen K, Hofstad B, Vatn MH. Does high body fatness increase the risk of presence and growth of colorectal adenomas followed up in situ for 3 years? Am J Gastroenterol 2001;96:2238-46. [DOI] [PubMed] [Google Scholar]
- 15.Ashktorab H, Paydar M, Yazdi S, et al. BMI and the risk of colorectal adenoma in African-Americans. Obesity (Silver Spring) 2014;22:1387-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Summers RM, Liu J, Sussman DL, et al. Association between visceral adiposity and colorectal polyps on CT colonography. AJR Am J Roentgenol 2012;199:48-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben Q, An W, Jiang Y, et al. Body mass index increases risk for colorectal adenomas based on meta-analysis. Gastroenterology 2012;142:762-72. [DOI] [PubMed] [Google Scholar]
- 18.Comstock SS, Hortos K, Kovan B, et al. Adipokines and obesity are associated with colorectal polyps in adult males: a cross-sectional study. PLoS One 2014;9:e85939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmqvist R, Hallmans G, Rinaldi S, et al. Plasma insulin-like growth factor 1, insulin-like growth factor binding protein 3, and risk of colorectal cancer: a prospective study in northern Sweden. Gut 2002;50:642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beltrán-Sánchez H, Harhay MO, Harhay MM, et al. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999-2010. J Am Coll Cardiol 2013;62:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124-31. [DOI] [PubMed] [Google Scholar]
- 22.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006;43:S99-S112. [DOI] [PubMed] [Google Scholar]
- 23.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274-85. [DOI] [PubMed] [Google Scholar]
- 24.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005-23. [DOI] [PubMed] [Google Scholar]
- 25.Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol 2004;2:1048-58. [DOI] [PubMed] [Google Scholar]
- 26.Angulo P. Nonalcoholic fatty liver disease and liver transplantation. Liver Transpl 2006;12:523-34. [DOI] [PubMed] [Google Scholar]
- 27.Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011;141:1249-53. [DOI] [PubMed] [Google Scholar]
- 28.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59:2188-95. [DOI] [PubMed]
- 29.Armstrong MJ, Adams LA, Canbay A, et al. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 2014;59:1174-97. [DOI] [PubMed] [Google Scholar]
- 30.Sint Nicolaas J, de Jonge V, Steyerberg EW, et al. Risk of colorectal carcinoma in post-liver transplant patients: a systematic review and meta-analysis. Am J Transplant 2010;10:868-76. [DOI] [PubMed] [Google Scholar]
- 31.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011;306:1891-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jussila A, Virta LJ, Pukkala E, et al. Malignancies in patients with inflammatory bowel disease: a nationwide register study in Finland. Scand J Gastroenterol 2013;48:1405-13. [DOI] [PubMed] [Google Scholar]
- 33.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992;326:658-62. [DOI] [PubMed] [Google Scholar]
- 34.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol 2001;96:2992-3003. [DOI] [PubMed] [Google Scholar]
- 35.Phelan CM, Iqbal J, Lynch HT, et al. Incidence of colorectal cancer in BRCA1 and BRCA2 mutation carriers: results from a follow-up study. Br J Cancer 2014;110:530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JM, Choi MG, Kim SW, et al. Increased incidence of colorectal malignancies in renal transplant recipients: a case control study. Am J Transplant 2010;10:2043-50. [DOI] [PubMed] [Google Scholar]
- 37.Bini EJ, Park J, Francois F. Use of flexible sigmoidoscopy to screen for colorectal cancer in HIV-infected patients 50 years of age and older. Arch Intern Med 2006;166:1626-31. [DOI] [PubMed] [Google Scholar]
- 38.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med 2008;148:728-36. [DOI] [PubMed] [Google Scholar]
- 39.Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol 2007;25:1489-97. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez AM, Kuo YF, Goodwin JS. Risk of colorectal cancer among long-term cervical cancer survivors. Med Oncol 2014;31:943. [DOI] [PubMed] [Google Scholar]
- 41.Lloyd SC, Harvey NR, Hebert JR, et al. Racial disparities in colon cancer. Primary care endoscopy as a tool to increase screening rates among minority patients. Cancer 2007;109:378-85. [DOI] [PubMed] [Google Scholar]
- 42.Giacosa A, Frascio F, Munizzi F. Epidemiology of colorectal polyps. Tech Coloproctol 2004;8:s243-7. [DOI] [PubMed] [Google Scholar]
- 43.Qumseya BJ, Wallace MB. Advanced colorectal polyp detection techniques. Curr Gastroenterol Rep 2012;14:414-20. [DOI] [PubMed] [Google Scholar]
- 44.Heitman SJ, Ronksley PE, Hilsden RJ, et al. Prevalence of adenomas and colorectal cancer in average risk individuals: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2009;7:1272-8. [DOI] [PubMed] [Google Scholar]
- 45.Jeschek P, Ferlitsch A, Salzl P, et al. A greater proportion of liver transplant candidates have colorectal neoplasia than in the healthy screening population. Clin Gastroenterol Hepatol 2015;13:956-62. [DOI] [PubMed] [Google Scholar]
- 46.Wong VW, Wong GL, Tsang SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut 2011;60:829-36. [DOI] [PubMed] [Google Scholar]
- 47.Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol 2012;27:91-5. [DOI] [PubMed] [Google Scholar]
- 48.Hwang ST, Cho YK, Park JH, et al. Relationship of non-alcoholic fatty liver disease to colorectal adenomatous polyps. J Gastroenterol Hepatol 2010;25:562-7. [DOI] [PubMed] [Google Scholar]
- 49.Morita T, Tabata S, Mineshita M, et al. The metabolic syndrome is associated with increased risk of colorectal adenoma development: the Self-Defense Forces health study. Asian Pac J Cancer Prev 2005;6:485-9. [PubMed] [Google Scholar]
- 50.Ahmed RL, Schmitz KH, Anderson KE, et al. The metabolic syndrome and risk of incident colorectal cancer. Cancer 2006;107:28-36. [DOI] [PubMed] [Google Scholar]
- 51.Ben Q, An W, Jiang Y, et al. Body mass index increases risk for colorectal adenomas based on meta-analysis. Gastroenterology 2012;142:762-72. [DOI] [PubMed] [Google Scholar]
- 52.Armstrong MJ, Adams LA, Canbay A, et al. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 2014;59:1174-97. [DOI] [PubMed] [Google Scholar]
- 53.Stadlmayr A, Aigner E, Steger B, et al. Nonalcoholic fatty liver disease: an independent risk factor for colorectal neoplasia. J Intern Med 2011;270:41-9. [DOI] [PubMed] [Google Scholar]
- 54.Shen H, Lipka S, Kumar A, et al. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systemic review and meta-analysis. J Gastrointest Oncol 2014;5:440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Touzin NT, Bush KN, Williams CD, et al. Prevalence of colonic adenomas in patients with nonalcoholic fatty liver disease. Therap Adv Gastroenterol 2011;4:169-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shetty K, Rybicki L, Brzezinski A, et al. The risk for cancer or dysplasia in ulcerative colitis patients with primary sclerosing cholangitis. Am J Gastroenterol 1999;94:1643-9. [DOI] [PubMed] [Google Scholar]
- 57.Paterson AC, Leeding KS, Bach LA, et al. More about: prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst 2000;92:1947-50. [DOI] [PubMed] [Google Scholar]
- 58.Agrawal RP, Sheroan V, Ola V, et al. Hepatocyte growth factor, adiponectin and hepatic histopathology in non-alcoholic steatohepatitis. J Assoc Physicians India 2013;61:789-92. [PubMed] [Google Scholar]
- 59.Turer AT, Browning JD, Ayers CR, et al. Adiponectin as an independent predictor of the presence and degree of hepatic steatosis in the Dallas Heart Study. J Clin Endocrinol Metab 2012;97:E982-6. [DOI] [PubMed] [Google Scholar]
- 60.Kumor A, Daniel P, Pietruczuk M, et al. Serum leptin, adiponectin, and resistin concentration in colorectal adenoma and carcinoma (CC) patients. Int J Colorectal Dis 2009;24:275-81. [DOI] [PubMed] [Google Scholar]
- 61.Frasinariu OE, Ceccarelli S, Alisi A, et al. Gut-liver axis and fibrosis in nonalcoholic fatty liver disease: an input for novel therapies. Dig Liver Dis 2013;45:543-51. [DOI] [PubMed] [Google Scholar]
- 62.Vajro P, Paolella G, Fasano A. Microbiota and gut-liver axis: their influences on obesity and obesity-related liver disease. J Pediatr Gastroenterol Nutr 2013;56:461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012;482:179-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014;146:1513-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014;513:59-64. [DOI] [PubMed] [Google Scholar]
- 66.Lochhead P, Chan AT, Giovannucci E, et al. Progress and opportunities in molecular pathological epidemiology of colorectal premalignant lesions. Am J Gastroenterol 2014;109:1205-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer 2013;13:800-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hullar MA, Burnett-Hartman AN, Lampe JW. Gut microbes, diet, and cancer. Cancer Treat Res 2014;159:377-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Irrazábal T, Belcheva A, Girardin SE, et al. The multifaceted role of the intestinal microbiota in colon cancer. Mol Cell 2014;54:309-20. [DOI] [PubMed] [Google Scholar]
- 70.Seitz HK, Maurer B, Stickel F. Alcohol consumption and cancer of the gastrointestinal tract. Dig Dis 2005;23:297-303. [DOI] [PubMed] [Google Scholar]
- 71.Rueda M, Robertson Y, Acott A, et al. Association of tobacco and alcohol use with earlier development of colorectal pathology: should screening guidelines be modified to include these risk factors? Am J Surg 2012;204:963-7; discussion 967-8. [DOI] [PubMed] [Google Scholar]
- 72.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010;51:660-78. [DOI] [PubMed] [Google Scholar]
- 73.Claessen MM, Vleggaar FP, Tytgat KM, et al. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol 2009;50:158-64. [DOI] [PubMed] [Google Scholar]