Abstract
Background
Hepatocellular carcinoma (HCC) is aggressive primary malignancy of the liver that most commonly presents late in the disease course. As a result, the majority of patients are not candidates for curative therapies. Locoregional therapies including Yttrium-90 (Y-90) radioembolization play an important role in management of the vast majority of patients with HCC.
Methods
Patients with unnresectable HCC (n=17) treated with Y-90 radioembolization from 2005 to 2014 were evaluated retrospectively. Data was abstracted from medical records including patient charts, laboratory data, and imaging. Toxicities were recorded using Common Terminology Criteria 3.0. Response was recorded according to modified RECIST (mRECIST) criteria.
Results
Seventeen patients received 33 treatments with Y-90 radioembolization. A majority (65%) received TheraSphere with a minority (35%) receiving SIR-Spheres. The median treatment activity delivered was 1.725 gBq (range, 1.4-2.5 gBq). The median treatment dose delivered was 100 Gy (range, 90-120 Gy). The median lung shunt fraction was 2.02% (range, 1.5-4.1%). The most common clinical toxicity among all patients was nausea and vomiting (59%), primarily grade 1 and 2. Other post-treatment findings included abdominal pain (29%), fatigue (53%), and weight loss (18%). One patient developed a grade 5 gastric ulcer after the treatment. A clinical benefit, defined as patients achieving complete response (CR), partial response (PR) or stable disease (SD), was seen in 48% of patients. PR was seen in 24% of cases; progressive disease (PD) was noted in 35%. Patients survived for a median of 8.4 months (range, 1.3 to 21.1 months) after the first radioembolization treatment. Median survival after Y-90 treatment was 8.4 months among patients treated TheraSphere as compared with 7.8 months in patients treated with SIR-Spheres. The mean overall survival from the time of diagnosis was 11.7 months (range, 3.4 to 43.2 months).
Conclusions
For patients with unresectable HCC, Y-90 radioembolization is a safe and well-tolerated procedure. Our experience suggests that a significant percentage of patients achieve clinical benefit including many with PR. Survival after treatment from this single-center, transplant center is in line with prior reports. Prospective, randomized data is required to compare radioembolization with other therapies including chemoembolization and systemic therapy with sorafenib.
Keywords: Unresectable hepatocellular carcinoma (HCC), radioembolization, Yttrium-90 (Y-90) microspheres, TheraSphere, SIR-spheres
Introduction
Hepatocellular carcinoma (HCC) is a primary liver tumor most often associated with chronic liver disease and cirrhosis. It is an aggressive malignancy with the annual incidence nearly equaling the mortality rate (1). HCC is typically identified late in the disease course with a median survival of 6 to 20 months (2). While multiple therapeutic modalities available, tumor resection and liver transplantation is considered by most to be the only potentially curative treatment options. Important considerations in determining an initial treatment approach include underlying liver function, tumor size, involvement of portal and hepatic veins, as well as the presence of metastatic disease. Unfortunately, only 10-20% of patients are eligible for curative therapy (3,4). As a result, locoregional therapies have a critical role in the management of the vast majority of patients with HCC, as primary palliative treatment and in the neoadjuvant setting prior to curative surgical treatment. Transarterial chemoembolization (TACE) and radiofrequency ablation are two therapeutic modalities currently available (5,6).
In patients with advanced, unresectable disease who are not candidates for locoregional therapy, systemic therapy is an appropriate option. While conventional cytotoxic chemotherapy has a limited role, molecularly targeted agents, particularly sorafenib, offer a survival benefit when compared with standard care (7). Although HCC is a radiosensitive tumor, the use of external beam radiation therapy is limited by hepatic toxicity (8). A typical radiation dose of 30 Gy has been applied to whole liver external beam radiation in order avoid organ damage (9). However, this dose is likely insufficient to provide significant response to therapy (10).
Radioembolization is a directed technique that utilizes microspheres embedded with Yttrium-90 (Y-90) into branches of the hepatic artery. Y-90 is a pure beta-emitter with an average energy is of 0.9 MeV. The mean penetration range is 2.5 mm, which corresponds to approximately 1,000 cell diameters. Y-90 has a physical half-life of 64.2 hours and decays to stable Zirconium-90. Microspheres, which vary in size ranging from 20 to 60 microns, are embedded with Y-90. Two commercially available forms of Y-90 are available in the United States including Y-90 tagged glass (TheraSphere) and resin (SIR-Spheres) microspheres.
Intrahepatic malignancies derive the vast majority of their blood supply from the hepatic artery rather than the portal circulation. As a result, selective, catheter-based administration of Y-90 microspheres into the hepatic artery is thought to preferentially deliver therapy to tumor, sparing normal liver parenchyma. This technique allows delivery of higher radiation doses up to 50 to 150 Gy as compared with external beam radiation (11,12). The mechanism of action of Y-90 radioembolization differs from TACE in several key aspects. TACE particles are much larger than Y-90 microspheres (200-500 microns versus 20-30 microns). As a result, TACE exhibits a more significant embolic effect compared to radioembolization. The primary mechanism of action of Y-90 is related to the radiation effect with a minor role of embolic occlusion of the tumor blood supply.
Here we report our experience at a tertiary-care, liver transplant center with intrahepatic arterial Y-90 microspheres in patients with unresectable HCC. In particular, we describe the proportion and duration of response as well as the safety of profile of this intervention.
Methods
Patient cohort
Between January 1, 2005 and May 16, 2014, seventeen patients with HCC were treated with Y-90 microspheres at our institution. A comprehensive review of each subject’s medical record was performed including baseline patient characteristics, toxicities, radiographic imaging, and survival outcomes. Data were collected retrospectively. Our institutional review board approved the study and patient data was de-identified compliant with the Health Insurance Portability and Accountability Act.
Patient selection for Yttrium-90 (Y-90) radioembolization
The patients were evaluated by a multi-disciplinary team including hepatologists, surgeons, medical oncologists, interventional radiologists, and radiation oncologists. Eligible patients were diagnosed with HCC based on tumor biopsy, radiographic imaging, or a combination thereof. Inclusion criteria to undergo radioembolization required the following: patients with unresectable HCC who either failed or had disease not amenable to alternative locoregional therapies; age equal to or over 18 years; ECOG performance status 0-2; serum total bilirubin less than 2 mg/dL; and ability to undergo angiography. Exclusion criteria included the following: uncorrectable flow to the GI tract, significant extrahepatic disease, applied lung dose greater than 30 Gy in a single fraction, and Child Turcotte-Pugh class C cirrhosis.
Pretreatment evaluation and staging
Pretreatment evaluation included comprehensive history and physical, routine laboratory tests and baseline imaging studies. Tumor staging was accomplished by American Joint Committee on Cancer (AJCC) TNM staging system (13). In addition, patients were classified according to Barcelona Clinic Liver Cancer (BCLC) staging system based upon the extent of the primary lesion, performance status, presence of constitutional symptoms, vascular invasion, metastasis, and Okuda stage (14).
Intervention
All patients underwent pretreatment computed tomography (CT) scan and/or MRI of the abdomen in order to determine both liver and tumor volumes. Planning celiac and hepatic angiography was performed to define hepatic and tumor vascular anatomy. In particular, the hepatic artery and its branches supplying the tumors were identified. Collateral arteries branching from the hepatic artery supplying the gallbladder, stomach, or intestine underwent coil embolization in order to avoid secondary complications. The presence of collateral arteries not amenable to coil embolization was considered an absolute contraindication to Y-90 therapy.
Additionally, patients received Tc-99-macro-aggregated albumin via the hepatic artery, followed by a whole-body nuclear scan to determine the estimate of body distribution and the percent of lung shunting. Macro-aggregated albumin was chosen due to its similar physical size to Y-90 microspheres (20-50 micron). This pre-treatment procedure was done in order to prevent accidental delivery of microspheres to the lung in order to avoid radiation pneumonitis.
The prescribed activity of Y-90 for resin microsphere was determined according to body surface area (BSA) method outlined in the User’s Manual and Package Insert provided by the manufacturer (15,16). The method varies the prescribed activity based upon the size of the patient as well as the proportion of tumor involvement of the liver. Activity delivered was reduced if there was evidence of increased lung shunt. Glass microspheres activity was determined utilizing conventional Medical Internal Radiation Dose (MIRD) Committee technique adjusted according to the calculated shunt of lung particles (17,18). The dose calculation was performed by a medical physicist and a radiation oncologist, and confirmed by an interventional radiologist.
Post-treatment evaluation
Tumor response was assessed by sonography, CT scanning, or MRI scanning as determined by the treating clinician. Response was classified according to guidelines from modified RECIST (mRECIST) (19). Complete response (CR) was defined as the disappearance of any intratumoral arterial enhancement in all target lesions. Partial response (PR) was defined as greater than 30% decrease in the sum of the diameters of viable target lesions. Progressive disease (PD) was defined as greater than 20% increase in diameters of enhancing target lesions or evidence of new lesion. Stable disease (SD) was defined as any tumor response between PR and PD. If a patient expired before repeat imaging could be performed, they were considered to have PD.
Clinical and laboratory toxicities
Patients were followed up with visits at regular intervals as determined by the treating physicians. Clinical and laboratory adverse events were abstracted from clinical notes following the National Cancer Institute Common Terminology Criteria (NCI CTC) version 3.0. Toxicities were recorded if present at any time during post-follow-up period. If patients had evidence of an abnormal laboratory value before treatment, NCI CTC were not applied. Rather, grade 1 toxicity (mild) was defined as a 1-50% increase from baseline; grade 2 toxicity (moderate) as a 51-200% increase from baseline, and a grade 3 toxicity (severe) as a >200% increase from baseline (20).
Statistical analyses
Overall survival was the primary endpoint used in this study defined as the time between the date of first treatment and date of death. Secondary endpoints included survival since diagnosis, as well as proportion of patients with CR, PR, SD, and PD. Data was analyzed using descriptive methods. Survival curves were generated using Kaplan-Meier modeling. Statistical analysis was performed using the computing environment R version 3.1.2 [2014].
Results
Patient population
Demographics and clinical characteristics of the patients included in this study are presented in Table 1. The median age was 67.7 years with a range 50.4 to 82.6 years. Twenty-four percent were 75 years of age or older. All patients (100%) were male with the majority being Caucasian (76%). The most common etiologies of liver disease were alcoholic liver disease and hepatitis C cirrhosis. The vast majority of patients (82%) had Karnofsky performance status (KPS) scores of 80 or greater.
Table 1. Baseline characteristics.
| Baseline characteristics | N [%] |
|---|---|
| Age (y) | |
| Younger than 65 | 4 [24] |
| 65-75 | 9 [53] |
| 75 or older | 4 [24] |
| Sex | |
| Male | 17 [100] |
| Female | 0 [0] |
| Ethnicity | |
| White | 13 [76] |
| Asian | 1 [6] |
| Hispanic | 1 [6] |
| Other | 1 [6] |
| Unknown | 1 [6] |
| Etiology | |
| HCV | 1 [6] |
| Alcohol | 2 [12] |
| HBV | 2 [12] |
| HCV and alcohol | 4 [24] |
| NASH | 1 [6] |
| Primary biliary cirrhosis | 3 [18] |
| Unknown | 4 [24] |
| KPS | |
| 100 | 2 [12] |
| 90 | 5 [29] |
| 80 | 7 [41] |
| 70 | 2 [12] |
| Unknown | 1 [6] |
| Prior therapy | |
| None | 8 [47] |
| Resection | 1 [6] |
| Radiofrequency ablation | 1 [6] |
| Chemoembolization | 2 [12] |
| Sorafenib | 7 [41] |
| Chemotherapy | 1 [6] |
| External beam radiation | 1 [6] |
| Method of diagnosis | |
| Biopsy | 11 [65] |
| Imaging | 8 [47] |
| Imaging findings | |
| Cirrhosis | 3 [18] |
| Ascites | 8 [47] |
| Portal hypertension | 6 [35] |
| Tumor characteristics | |
| Solitary | 3 [18] |
| Multifocal | 14 [82] |
| Bilobar | 8 [47] |
| Unilobar | 9 [53] |
| PVT | |
| None | 13 [76] |
| Branch | 4 [24] |
| Main | 0 [0] |
| Child-Pugh staging | |
| A | 9 [53] |
| B | 7 [41] |
| C | 1 [6] |
| BCLC staging | |
| A | 1 [6] |
| B | 11 [65] |
| C | 4 [24] |
| D | 1 [6] |
| Y-90 treatment modality | |
| TheraSpheres | 11 [65] |
| SIR-Spheres | 6 [35] |
| Number of treatments | |
| One | 11 [65] |
| Two | 6 [35] |
HCV, hepatitis C virus; HBV, hepatitis B virus; NASH, nonalcoholic steatohepatitis; KPS, Karnofsky performance status; PVT, portal vein thrombosis; BCLC staging, Barcelona Clinic Liver Cancer staging; Y-90, Yttrium-90.
HCC was diagnosed by biopsy in 65% of cases. Forty-seven percent had no therapy in addition to radioembolization; forty-one percent received sorafenib either before or after radioembolization. Most patients (82%) had evidence of multi-focal disease. The study population was split evenly between patients with uni-lobar (53%) and bi-lobar (47%) disease. Branch portal vein thrombosis was noted in a minority (24%) of subjects; no patients in our study had portal vein thrombosis involving the main portal vein. Metastatic disease was present in 13%. A total of 53% were Child-Pugh class A and 65% were BCLC class B.
Treatment
Seventeen patients underwent treatment with Y-90 radioembolization. A total of 33 treatments were administered, with 65% of patients receiving one treatment and 35% receiving two treatments; all were performed on an outpatient basis. A majority (65%) received TheraSphere with a minority (35%) receiving SIR-Spheres. The median treatment activity delivered was 1.725 gBq (range, 1.4-2.5 gBq). The median treatment dose delivered was 100 Gy (range, 90-120 Gy). The median lung shunt fraction was 2.02% (range, 1.5-4.1%).
Clinical and laboratory toxicities
Toxicities are listed in Table 2. The most common clinical toxicity among all patients was nausea and vomiting (59%). Other post-treatment findings included abdominal pain (29%), fatigue (53%), and weight loss (18%). There were no patients who experienced pulmonary toxicity. The most common laboratory toxicities were mild elevations (grade 1/2) of aspartate aminotransferase (AST) (53%) and alanine aminotransferase (ALT) (41%). Elevations in serum bilirubin occurred in 30% of all subjects. One patient developed a biopsy-proven, Y90 induced bleeding gastric ulcer several months after Y-90 treatment requiring care in the ICU. His hospital course was complicated by sepsis which led to death. The ulcer was thought to be due to a collateral vessel off of the proper hepatic artery supplying the stomach.
Table 2. Summary of clinical and laboratory toxicities.
| Laboratory toxicities | Total (n=17) | TheraSpheres (n=11) | SIR-Spheres (n=6) |
|---|---|---|---|
| Bilirubin | |||
| Grade 1/2 | 3 [18] | 3 [27] | 0 [0] |
| Grade 3/4 | 2 [12] | 2 [18] | 0 [0] |
| Albumin | |||
| Grade 1/2 | 8 [47] | 6 [55] | 2 [33] |
| Grade 3/4 | 1 [6] | 1 [11] | 0 [0] |
| Aspartate aminotransferase | |||
| Grade 1/2 | 9 [53] | 5 [45] | 4 [66] |
| Grade 3/4 | 0 [0] | 0 [0] | 0 [0] |
| Alanine aminotransferase | |||
| Grade 1/2 | 7 [41] | 5 [45] | 2 [33] |
| Grade 3/4 | 0 [0] | 0 [0] | 0 [0] |
| Alkaline phosphatase | |||
| Grade 1/2 | 10 [59] | 7 [64] | 3 [50] |
| Grade 3/4 | 1 [6] | 0 [0] | 1 [17] |
| Clinical toxicities | |||
| Fatigue | 9 [53] | 5 [45] | 4 [67] |
| Abdominal pain | 5 [29] | 3 [27] | 2 [33] |
| Nausea/vomiting | 10 [59] | 6 [55] | 4 [67] |
| Anorexia/weight loss | 3 [18] | 1 [9] | 2 [33] |
| Diarrhea | 1 [6] | 0 [0] | 1 [17] |
| Gastric ulcer | 1 [6] | 0 [0] | 1 [6] |
Outcomes
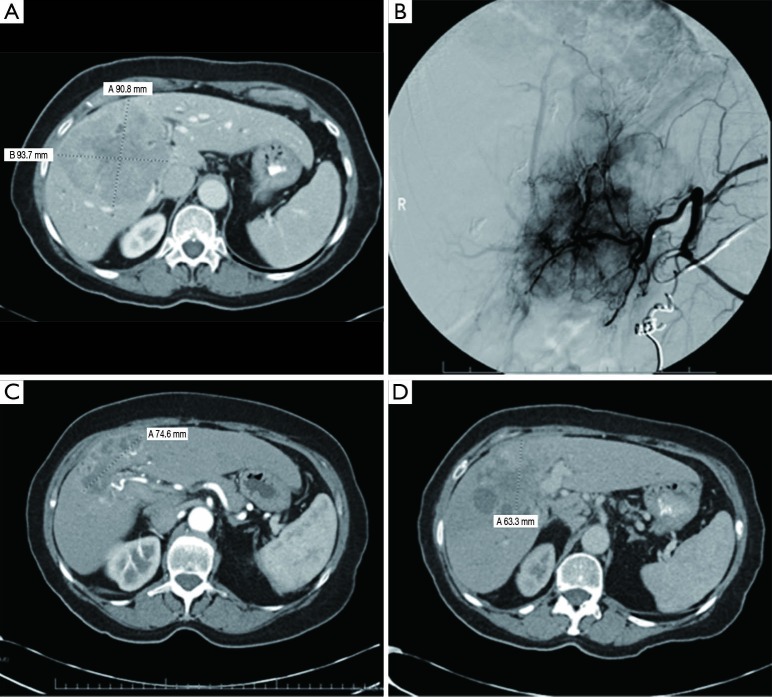
A total of 17 patients underwent Y-90 radioembolization for treatment of HCC with palliative intent. Two subjects are still alive and were therefore excluded from survival analysis. Table 3 reports outcome data. A clinical benefit response rate, defined as the proportion of patients achieving PR or SD, was noted to be 48%. PR was present in 24% of cases. PD was noted in 35% of cases (Table 4). Partial treatment response from a patient is represented in Figure 1.
Table 3. Summary of survival.
| Summary of survival | Survival since Y-90 treatment, months (n=15) |
Survival since diagnosis, months (n=15) |
|||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Overall | 8.4 | 1.3-21.1 | 11.7 | 3.4-43.2 | |
| Treatment modality | |||||
| TheraSpheres | 8.4 | 1.3-21.1 | 11.7 | 3.4-43.2 | |
| SIR-Spheres | 7.8 | 2.3-12.5 | 9.9 | 3.8-19.4 | |
| Child-Pugh | |||||
| A (n=7) | 8.8 | 2.27-21.1 | 11.7 | 5.2-43.2 | |
| B (n=7) | 8.4 | 3.0-19.2 | 13.7 | 3.8-36.1 | |
| C (n=1) | 1.3 | 3.4 | |||
| BCLC staging | |||||
| A (n=1) | 12.5 | 14.5 | |||
| B (n=10) | 9.7 | 2.3-21.1 | 15.1 | 5.2-43.2 | |
| C (n=3) | 3 | 2.3-5.7 | 7.1 | 3.8-11.4 | |
| D (n=1) | 1.3 | 3.4 | |||
Y-90, Yttrium-90; BCLC staging, Barcelona Clinic Liver Cancer staging.
Table 4. Tumor response.
| Tumor response | N=17 |
|---|---|
| Progressive disease | 6 [35] |
| Stable disease | 4 [24] |
| Partial response | 4 [24] |
| Complete response | 0 [0] |
| No data | 3 [18] |
Figure 1.
Large 9 cm liver tumor with corresponding hepatic angiogram showing a highly vascular tumor (A) and coiling of a collateral blood vessel (B). CT scan patient 6 and 12 months after Y-90 radioembolization (C,D). This patient was considered to have a partial response, and later developed progression of disease. CT, computed tomography; Y-90, Yttrium-90.
Patients survived for a median of 8.4 months (range, 1.3 to 21.1 months) after their first radioembolization treatment. Median survival from Y-90 treatment was 8.4 months among patients treated with TheraSphere as compared with 7.8 months in patients treated with SIR-Spheres. The median survival since diagnosis in the BCLC stage B group, our largest subgroup, was 15.1 months. The mean overall survival from the time of diagnosis was 11.7 months (range, 3.4 to 43.2 months). Figure 2 displays a Kaplan-Meier curve for survival since radioembolization treatment (Figure 2A) and survival since diagnosis (Figure 2B).
Figure 2.
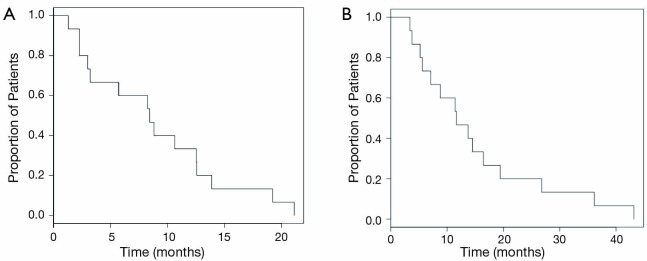
Survival (all patients) in months from time of (A) first Y-90 radio embolization (B) diagnosis. Y-90, Yttrium-90.
Discussion
The goal of radioembolization is to deliver tumoricidal doses of radiation within the tumor capillary bed, sparing uninvolved liver tissue. Conventional external beam radiation is limited by the extreme radiosensitivity of liver parenchyma (21). Radioembolization takes advantage of the unique vascular supply of the liver and hepatic solid tumors. Liver tumors, in contrast to normal liver parenchyma, derive 80% to 100% of their blood supply from the hepatic artery (22,23). Microspheres embedded with Y-90, when installed through the hepatic artery, concentrate in liver tumors in a 3:1 to 20:1 ratio in comparison to normal liver parenchyma. Radioembolization with Y-90 microspheres has been found to deliver highly effective radiation doses (100 to 1,000+ Gy) to tumor tissue (24).
Our experience adds to the growing body of evidence suggesting that intra-arterial radioembolization is a safe and effective palliative intervention in patients with unresectable HCC. Clinical benefit defined as the presence of CR, PR, or SD has been previously reported in 60-90% of cases (25). A clinical benefit by mRECIST criteria was noted in 62% of our cases with 29% achieving a PR. CR in advanced HCC is exceedingly uncommon as demonstrated in our series, which is consistent with prior reports (25). This is likely a consequence of the aggressive natural history of advanced HCC.
In patients with HCC, response by imaging to locoregional interventions is associated with a survival advantage (26). Measuring tumor response by conventional imaging techniques (CT, MRI) alone may underestimate actual clinical response. In particular, peritumoral edema and ring enhancement may confound accurate characterization of residual tumor burden in patients treated with locoregional therapies (27). Although not used in this study, end points such as decrement in tumor size and degree of tumor enhancement may better characterize tumor response in patients treated with Y-90 (28).
We report a mean survival of 8.2 months after first treatment with Y-90 radioembolization. This is consistent with reported survival durations of 7 to 12 months in prior series (29,30). Overall survival from diagnosis was 15 months, consistent with previous reports ranging from 9 to 24 months (31,32). Survival since diagnosis in the BCLC stage B subgroup, our largest subgroup, was 15.1 months with a range of 5.2 to 43.2 months; median overall survival rates in patients with intermediate to advanced HCC based on BCLC criteria have reported survival rates ranging from 6 to 16 months without any intervention (33). A survival benefit was not evident based on our results.
Our series of patients is unique in that patients from a single-center were treated with both commercially available Y-90 microspheres (TheraSphere and SIR-Spheres). TheraSphere (MDS Nordion, Ottawa, Ontario, Canada) was approved by the Food and Drug Administration (FDA) in 1999 under the Humanitarian Device Exemption Guidelines for unresectable HCC. TheraSphere particles have an average diameter of 20-30 µ and are composed of glass microspheres. SIR-Spheres (SIRTeX Medical Ltd., Sydney, Australia) were approved in 2002 for colorectal cancer with liver metastases in conjunction with floxuridine. SIR-Spheres have an average size of 35 µ and are composed of resin microspheres. Both of these modalities have a half-life of 64.1 hours (2.67 days) and emit beta radiation with a mean tissue penetration of 2.5 mm and a maximum of 1 cm.
Our data suggest that both clinical and laboratory toxicity profiles were similar between the TheraSphere and Sir-Sphere groups, with the exception of bilirubin level which was noted to be a more relevant marker of toxicity in the TheraSphere group. Median overall survival after diagnosis and after treatment with Y-90 were both noted to be longer in the TheraSphere group versus the SIR-sphere group, however it is important to note that almost twice as many patients received TheraSphere versus SIR-spheres in our study. Although no randomized data is available comparing the two modalities, a meta-analysis by Vente et al., suggested a response advantage of SIR-Spheres compared with TheraSphere. An absolute response rate of 89% was seen in patients treated with resin microspheres (SIR-Spheres) compared with 78% in patients treated with glass microspheres (P=0.02) (25). An important distinction between these two therapies is the number of microspheres infused in a treatment. A single dose of TheraSphere contains four million microspheres compared with 50 million microspheres in a dose of SIR-Spheres (34). It is unclear whether or not the number of microspheres confers a significant advantage. However, one theoretical consideration is that a greater distribution of radiation dose may be achieved with a higher number of microspheres. This may be particularly important in HCC, which often has a heterogeneous distribution (25).
This cohort of patients reaffirms that radioembolization with Y-90 is a safe and well-tolerated procedure except for one patient who developed a grade 5 gastric ulcer. The most common symptoms reported included nausea, vomiting, fatigue, and mild abdominal pain. The vast majority of laboratory toxicities were low-grade and included derangements of alkaline phosphatase, albumin, and transaminases. Postembolization syndrome (PES) is well-described complication reported after Y-90 treatment. Symptoms include nausea, fevers, right upper quadrant pain, and vomiting and likely accounts for the reported clinical toxicities in our patient cohort (35). Other serious complications have been reported, including gastrointestinal ulceration/bleeding, cholecystitis, pancreatitis, and radiation pneumonitis (25). Patient selection and embolization of gastrointestinal tract arteries are important pre-treatment considerations in order to prevent serious complications (36).
The role of Y-90 radioembolization within the context of alternative locoregional and systemic therapies is evolving. Carr et al. demonstrated a modest but statistically significant survival benefit of patients treated with Y-90 radioembolization compared to TACE (11.5 and 8.5 months respectively). This finding occurred within the background of milder disease in the Y-90 arm, limiting its generalizability (37). Kooby et al. reviewed 71 patients treated with either Y-90 or TACE and found no significant differences in terms of treatment efficacy or toxicity (38). Furthermore, one prospective study demonstrated improved quality of life in patients who underwent radioembolization compared to chemoembolization (39). The patients who clearly benefit from radioembolization as compared to chemoembolization are those with branch or lobar portal venous thrombus (40,41).
Whether or not there is an advantage of systemic therapy with sorafenib versus radioembolization is similarly unclear. Gramenzi et al. retrospectively reviewed 137 patients with locally advanced HCC treated with either Y-90 radioembolization or sorafenib. No difference in survival was found (42). Recent data suggests that there may be a complementary role for sorafenib in combination with radioembolization although prospective data are lacking (43). Several prospective studies are currently underway-comparing available treatment modalities which will likely influence our current management of unresectable HCC in the near future.
The results from the present study support the growing body of evidence that radioembolization with Y-90 microspheres for the treatment of HCC is a generally well-tolerated procedure. A clinical response was achieved in almost half of our patient sample, which is comparable to prior reports. The safety profile of this treatment modality was acceptable, although severe complications may occur despite appropriate preventative measures.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [DOI] [PubMed] [Google Scholar]
- 2.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28:751-5. [DOI] [PubMed] [Google Scholar]
- 3.McDermott WV, Cady B, Georgi B, et al. Primary cancer of the liver. Evaluation, treatment, and prognosis. Arch Surg 1989;124:552-4; discussion 554-5. [DOI] [PubMed] [Google Scholar]
- 4.Johnson PJ. Why can't we cure primary liver cancer? Eur J Cancer 1995;31A:1562-4. [DOI] [PubMed] [Google Scholar]
- 5.Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005;234:961-7. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M, Practice Guidelines Committee, et al . Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [DOI] [PubMed] [Google Scholar]
- 8.Ingold JA, Reed GB, Kaplan HS, et al. Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med 1965;93:200-8. [PubMed] [Google Scholar]
- 9.Lewandowski RJ, Salem R. Yttrium-90 radioembolization of hepatocellular carcinoma and metastatic disease to the liver. Semin Intervent Radiol 2006;23:64-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goin JE, Salem R, Carr BI, et al. Treatment of unresectable hepatocellular carcinoma with intrahepatic yttrium 90 microspheres: factors associated with liver toxicities. J Vasc Interv Radiol 2005;16:205-13. [DOI] [PubMed] [Google Scholar]
- 11.Andrews JC, Walker SC, Ackermann RJ, et al. Hepatic radioembolization with yttrium-90 containing glass microspheres: preliminary results and clinical follow-up. J Nucl Med 1994;35:1637-44. [PubMed] [Google Scholar]
- 12.Dancey JE, Shepherd FA, Paul K, et al. Treatment of nonresectable hepatocellular carcinoma with intrahepatic 90Y-microspheres. J Nucl Med 2000;41:1673-81. [PubMed] [Google Scholar]
- 13.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [DOI] [PubMed] [Google Scholar]
- 15.SIR-Spheres User's Manual. Sirtex Medical Limited. 2004;202:38-42. Available online: http://www.westernesse.com/portfoliolks/projects/sirtex/site/pdfs/sir-spheres_user_manual.pdf.
- 16.SIR-Spheres Package Insert. Sirtex Medical Limited, 2004. Available online: http://www.sirtex.com/media/29845/ssl-us-10.pdf
- 17.TheraSphere Package Insert. MDS Nordion, 2004. Available online: http://www.therasphere.com/physicians-package-insert/package-insert-eu-en.pdf
- 18.Kennedy A, Coldwell D, Sangro B, et al. Radioembolization for the treatment of liver tumors general principles. Am J Clin Oncol 2012;35:91-9. [DOI] [PubMed] [Google Scholar]
- 19.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dancey JE, Shepherd FA, Paul K, et al. Treatment of nonresectable hepatocellular carcinoma with intrahepatic 90Y-microspheres. J Nucl Med 2000;41:1673-81. [PubMed] [Google Scholar]
- 21.Fox RA, Klemp PF, Egan G, et al. Dose distribution following selective internal radiation therapy. Int J Radiat Oncol Biol Phys 1991;21:463-7. [DOI] [PubMed] [Google Scholar]
- 22.Ackerman NB, Lien WM, Kondi ES, et al. The blood supply of experimental liver metastases. I. The distribution of hepatic artery and portal vein blood to "small" and "large" tumors. Surgery 1969;66:1067-72. [PubMed] [Google Scholar]
- 23.Archer SG, Gray BN. Vascularization of small liver metastases. Br J Surg 1989;76:545-8. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy AS, Nutting C, Coldwell D, et al. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys 2004;60:1552-63. [DOI] [PubMed] [Google Scholar]
- 25.Vente MA, Wondergem M, van der Tweel I, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol 2009;19:951-9. [DOI] [PubMed] [Google Scholar]
- 26.Sala M, Llovet JM, Vilana R, et al. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology 2004;40:1352-60. [DOI] [PubMed] [Google Scholar]
- 27.Atassi B, Bangash AK, Bahrani A, et al. Multimodality imaging following 90Y radioembolization: a comprehensive review and pictorial essay. Radiographics 2008;28:81-99. [DOI] [PubMed] [Google Scholar]
- 28.Keppke AL, Salem R, Reddy D, et al. Imaging of hepatocellular carcinoma after treatment with yttrium-90 microspheres. AJR Am J Roentgenol 2007;188:768-75. [DOI] [PubMed] [Google Scholar]
- 29.Sangro B, Bilbao JI, Boan J, et al. Radioembolization using 90Y-resin microspheres for patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2006;66:792-800. [DOI] [PubMed] [Google Scholar]
- 30.Dancey JE, Shepherd FA, Paul K, et al. Treatment of nonresectable hepatocellular carcinoma with intrahepatic 90Y-microspheres. J Nucl Med 2000;41:1673-81. [PubMed] [Google Scholar]
- 31.Lau WY, Ho S, Leung TW, et al. Selective internal radiation therapy for nonresectable hepatocellular carcinoma with intraarterial infusion of 90yttrium microspheres. Int J Radiat Oncol Biol Phys 1998;40:583-92. [DOI] [PubMed] [Google Scholar]
- 32.Salem R, Lewandowski RJ, Atassi B, et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol 2005;16:1627-39. [DOI] [PubMed] [Google Scholar]
- 33.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 2008;48:S20-37. [DOI] [PubMed] [Google Scholar]
- 34.Murthy R, Nunez R, Szklaruk J, et al. Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radiographics 2005;25:S41-55. [DOI] [PubMed] [Google Scholar]
- 35.Sato K, Lewandowski RJ, Bui JT, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol 2006;29:522-9. [DOI] [PubMed] [Google Scholar]
- 36.Murthy R, Kamat P, Nuñez R, et al. Radioembolization of yttrium-90 microspheres for hepatic malignancy. Semin Intervent Radiol 2008;25:48-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carr BI, Kondragunta V, Buch SC, et al. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer 2010;116:1305-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kooby DA, Egnatashvili V, Srinivasan S, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2010;21:224-30. [DOI] [PubMed] [Google Scholar]
- 39.Salem R, Gilbertsen M, Butt Z, et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol 2013;11:1358-65.e1. [DOI] [PubMed]
- 40.Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 2008;47:71-81. [DOI] [PubMed] [Google Scholar]
- 41.Tsai AL, Burke CT, Kennedy AS, et al. Use of yttrium-90 microspheres in patients with advanced hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol 2010;21:1377-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gramenzi A, Golfieri R, Mosconi C, et al. Yttrium-90 radioembolization vs sorafenib for intermediate-locally advanced hepatocellular carcinoma: a cohort study with propensity score analysis. Liver Int 2015;35:1036-47. [DOI] [PubMed] [Google Scholar]
- 43.Gadani S, Mahvash A, Avritscher R, et al. Yttirum-90 resin microspheres as an adjunct to sorafenib in patients with unresectable HCC: a retrospective study for evaluation of survival benefit and adverse events. JVIR 2013;24:S35. [Google Scholar]