Abstract
Gastric and esophageal cancers represent a major global cancer burden and novel approaches are needed. Despite recent improvements in outcomes with trastuzumab and ramucirumab the prognosis for advanced disease remains poor, with a median overall survival of 1 year. Comprehensive genomic characterization has defined molecular subgroups and potentially actionable genomic alterations, but the majority of patients do not yet benefit from molecularly directed therapies. Breakthroughs in immune checkpoint blockade have provided new therapeutic avenues in melanoma, and continue to expand into other tumor types, with ongoing investigations in gastrointestinal (GI) malignancies. The frequency of programmed death ligand 1 (PD-L1) overexpression, a putative response biomarker, approaches forty percent in gastric cancers. Translational studies and molecular classification suggest gastric and esophageal cancers are candidate malignancies for immune checkpoint inhibition trials and early clinical data is promising. Here we review the mechanisms, preclinical, and early clinical data supporting the role for immune checkpoint blockade in gastric and esophageal cancer.
Keywords: Immunotherapy, gastric, esophageal, cancer, programmed death ligand 1 (PD-L1), checkpoint, programmed cell death protein 1 (PD-1)
Introduction
Despite therapeutic advances in oncology, the prognosis of late stage gastric and esophageal carcinoma remains exceedingly poor. Gastric cancer is the second leading cause of global cancer-related death, with an estimated 723,000 deaths in 2012 (1). Nearly 1 million new gastric cancers are diagnosed annually making this the fifth most common malignancy overall (1). Esophageal cancer affected an additional 456,000 people in 2012 and caused approximately 400,000 deaths, making it the sixth most common cause of cancer-related death and eighth most common cancer globally (1). While the overall incidence gastric cancer is on the decline, the prevalence of esophageal cancer is rising (2-4).
The majority of gastric and esophageal cancer patients present with advanced disease and evidence-based therapeutic options are limited. First line systemic therapy for metastatic disease is largely based on a platinum/5-fluoropyrimidine backbone, which produces moderate survival benefits in patients with good performance status (5). The addition of an anthracycline or taxane to platinum/5-fluoropyrimidine regimens may provide additional survival benefit in select patients (5-7). In Her2 amplified adenocarcinoma incorporation of the anti-Her2 monoclonal antibody, trastuzumab, significantly improves survival, and is the first molecularly targeted agent to improve outcomes in advanced gastric and esophageal cancers (8). The recently approved vascular endothelial growth factor receptor 2 (VEGFR-2) antibody ramucirumab has also been shown to improve survival in patients with gastric and gastroesophageal junction (GEJ) adenocarcinoma who progressed on first line therapy (9). While ramucirumab and trastuzumab are meaningful additions to the gastroesophageal armamentarium, overall survival outcomes remain poor and novel approaches are needed.
Immunotherapy has caused a paradigm shift in the treatment of melanoma and its use continues to expand to include other tumor types (10-12). With increasing clinical experience, biomarker analyses, and improvements in preclinical models, the potential role for immunotherapy in gastric and esophageal cancers is emerging. The major approaches to harnessing immunotherapeutic anticancer effects have come from the development of inhibitory antibodies which modulate immune check points, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed death ligand 1 (PD-L1). Here we review the basic immunotherapeutic mechanisms of CTLA-4 and PD-L1, existing preclinical data, and available clinical results incorporating immunotherapy into the treatment of advanced gastric and esophageal cancers.
Immunotherapeutic mechanisms
Numerous co-stimulatory and inhibitory molecules interact to form a network of activating and inhibitory pathway “checkpoints” which serve to regulate the human immune system. This molecular interplay allows for uninterrupted pathogen-fighting capabilities while simultaneously preventing autoimmunity and persistent immune response (13). Many of these pathways converge on T lymphocytes, which play a central role in triggering adaptive immune responses to both foreign pathogens as well as neoplastic cells. However, in cases of malignancy, tumor cells frequently escape immune detection by hijacking elements of these checkpoint pathways thereby inhibiting T cell effector function. Ultimately this results in reduced tumor surveillance and tumor recognition (14). The development of antibodies to immune checkpoints, known collectively as immune checkpoint inhibitors, has now translated to improved patient outcomes in several malignancies (11,15).
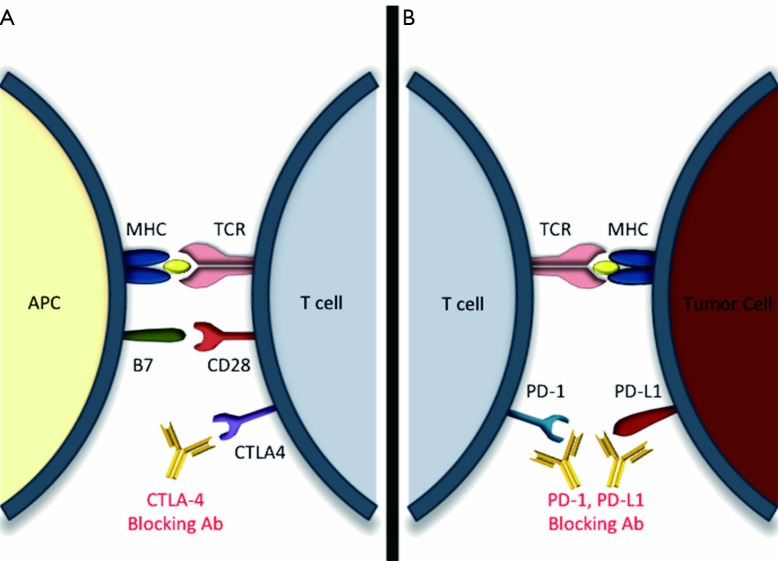
CTLA-4 is a ubiquitous T-cell receptor belonging to the immunoglobin superfamily. CTLA-4 shares many similarities with the T-cell co-stimulatory protein CD28, and like CD28, is activated upon binding with CD80 (B7-1) or CD86 (B7-2) (16). In fact, CTLA-4 has been shown to compete with CD28 for CD80 and CD86 binding (17). However, unlike CD28, which stimulates T cells, the effects of CTLA-4 activation differ between T-cell subsets. In CD4+ helper T cells activated CTLA-4 down modulates activity, whereas in CD4+ T regulatory cells (TReg) CTLA-4 up-regulates function (18). The net effect of endogenous CTLA4 activation is immune tolerance (19) (Figure 1).
Figure 1.
Immune checkpoint blockade in central and peripheral immune compartments. (A) Expression of CTLA-4 is up regulated on T cells in lymphoid tissues following activation via MHC/TCR and M7/CD28 mediated signaling. Once activated, CTLA-4 inhibits T cell function leading to immune tolerance. In the presence of blocking antibodies this tolerance can be broken, allowing for enhanced antitumor response; (B) PD-1, also expressed on T lymphocytes, inhibits the action of T lymphocytes upon binding to its ligands PD-L1/2; this process likely occurs in the tumor microenvironment, between PD-L1/2 expressing tumor cells and PD-1 expressing T lymphocytes; (A,B) blocking antibodies to either PD-1 or its ligands allows for T cell activation, enhancing anti-tumor effects peripherally. CTLA-4, cytotoxic T-lymphocyte antigen 4; PD-1, programmed death 1; PD-L1, programmed death ligand 1; APC, antigen presenting cell; MHC, major histocompatibility complex; TCR, T cell receptor.
Similarly, the T-cell surface receptor PD-1, also a member of the immunoglobin superfamily, inhibits T cell function upon binding to its ligands PD-L1 (B7-H1) and PD-L2 (B7-DC) (20) (Figure 1). The PD-1 ligands are also members of the B7 family, although the inhibitory pathway that PD-1 participates in is thought to be mutually exclusive to that of CTLA-4 (21). PD-L1 is expressed on T cells, B cells, NK cells, dendritic cells, monocytes/macrophages, mast cells, and various tumor types where it is thought to play a role in tumor immune escape (22) (Figure 1). It has been suggested that while CTLA-4 may play a significant role in early immune response, primarily occurring in lymphoid tissues, PD-1 whose expression is up regulated after T cell activation in peripheral tissues may be more involved in late immune response (23). Although CTLA-4 inhibition highlighted the power of immune checkpoint modulation, therapeutic focus is shifting towards the use of PD-1 and PD-L1 blockade, which offer benefits of potentially fewer side effects and perhaps improved outcome data.
Preclinical observations in gastric and esophageal cancers
Distribution of PD-L1/PD-L2
PD-L1 is broadly expressed in many human tissues and organs. In addition to immune cells PD-L1 has been identified on endothelial cells, mesenchymal stem cells, cells of the eye and placenta (22). In contrast, PD-L2 expression is restricted to lymphoid tissues and has only been observed on macrophages and dendritic cells, suggesting non-redundant roles for these two ligands (24). Varying levels of PD-L1 and PD-L2 are expressed on a majority of human cancer cells including: melanoma, renal cell carcinoma (RCC), multiple myeloma, breast, bladder, colon, and lung cancer (22,25,26). Melanoma, RCC, and non-small cell lung cancer (NSCLC) tumor series have shown high levels of PD-L1 expression by both immunohistochemical and RNA analysis, ranging from 66-100% (27-29).
Until recently, few studies had attempted to quantify PD-L1 and PD-L2 expression in gastric and esophageal caners. Work by Ohigashi et al. using immunohistochemical and RT-PCR approaches to examine expression from 41 esophageal squamous cell cancer (ESCC) patients found that 43.9% of samples had either PD-L1 or PD-L2 overexpressing tumor cells (30) (Table 1). Similarly, PD-L1 expression was detected in 42.2% of gastric adenocarcinoma samples (n=102) and was undetectable in normal gastric tissue controls and only weakly detectible in gastric adenomas using an IHC approach (31). A recent Chinese series (n=111) suggested PD-L1 positivity in 63% (70/111) of gastric adenocarcinoma resection specimens (32) (Table 1). Data from the phase Ib KEYNOTE-012 trial corroborated the above results and found a 40% rate of PD-L1 overexpression in advanced gastric adenocarcinomas (33). Few studies have yet to specifically address rates of PD-1 and PD-L1 positivity in GEJ adenocarcinomas, the predominant location and histology in US patients. Although more studies will be necessary to substantiate these findings in gastric and esophageal cancers, PD-L1 expression levels are comparable to cancers in which anti-PD-L1 directed therapies have demonstrated early success.
Table 1. Frequency of PD-L1 expression and correlation with clinical outcomes in gastric and esophageal cancer.
| Investigational compound | Target | Phase | ClinTrials identifier | Primary endpoint | Secondary endpoints |
|---|---|---|---|---|---|
| Ipilimumab | CTLA-4 | II | NCT01585987 | irPFS | PFS, OS, irBORR |
| Nivolumab | PD-1 | I-II | NCT01928394 | ORR | AE |
| PD-1 | I | NCT00836888 | Safety, PK | PD, RR | |
| Lirilumab + nivolumab | KIR, PD-L1 | I | NCT01714739 | Safety | BOR, irRECIST, PK, PD |
| MSB0010718C | PD-L1 | I | NCT01943461 | DLT | irBORR, PD-L1 expression, irPFS, OS |
| PD-L1 | I | NCT01772004 | DLT | irBORR, PD-L1 expression, irPFS, OS | |
| MPDL3280A | PD-L1 | I | NCT01375842 | DLT | AE |
| Pembrolizumab | PD-L1 | I (KEYNOTE-012) | NCT01848834 | ORR, AE | Cohort RR |
| PD-L1 | II (KEYNOTE-059) | NCT02335411 | ORR, AE | PFS, discontinuation | |
| MEDI4736 | PD-L1 | I-II | NCT01693562 | ORR, AE | OS, PFS, DCR, PK |
PD-L1, programmed death ligand 1; irPFS, immune related progression free survival; PFS, progression free survival; OS, overall survival; irBORR, immune related best overall response rate; PD-1, programmed cell death protein 1; ORR, overall response rate; AE, adverse events; PK, pharmacokinetics; PD, pharmacodynamics; RR, response rate; irRECIST, immune related response evaluation criteria in solid tumors; DCR, disease control rate.
PD-1 expression and tumor infiltrating lymphocytes (TILs)
The presence of lymphocytes in close tumor proximity has been used as a crude surrogate for immune responsiveness to tumor presence. Multiple large studies in melanoma, colorectal, ovarian, and breast have shown a correlation between increased immune infiltrates and favorable outcomes (34-37). Previous work has also correlated a higher density of TILs with improved outcomes in GI malignancies (38). Recently, work by Turcotte et al. defined the presence of endogenous CD8+ tumor infiltrating T-cells in a small series of patients with advanced gastrointestinal (GI) malignancies, including gastric cancer. They were able to demonstrate that naturally occurring CD8+ TILs can recognize specific autologous tumor-derived cell lines (39). However, despite the presence of TILs in the tumor microenvironment, tumor regression of late stage gastric and esophageal cancers is rarely seen suggesting endogenous mechanisms are likely inadequate. Preclinical models have suggested that there are greater TIL numbers in earlier stage disease, and that advanced GI malignancies are less immunogenic due to selection of the least immunogenic cancer cell clones during disease progression (40,41). Several studies have identified up regulation of PD-1 on TILs in both RCC and hepatocellular carcinoma and correlated increased PD-1 expression with worse prognosis (42,43). In gastric cancer, PD-1 expression on CD8+ lymphocytes is significantly higher than that of normal gastric mucosa and peripheral blood (44). Further studying the relationship of TIL density to stage and immunotherapy response may help refine the optimal disease setting in which to pursue immune checkpoint inhibition in gastric and esophageal cancer.
PD-L1/PD-L2 expression and patient outcomes
In many cancers increased PD-L1 and PD-L2 expression correlate with worse prognosis, and ongoing investigation is needed to determine the prognostic power of PD-L1 expression in gastric and esophageal cancers (45-50). Increased PD-L1 expression in both gastric and esophageal cancer is associated with nodal metastases, advanced stage, and worse outcomes (31,32). Jiang et al. demonstrated a positive correlation between expression of B7-H4, another B7 family member, and gastric cancer invasiveness and metastasis. The median overall survival is significantly reduced in gastric cancer patients with higher B7-H4 expression (51). Similarly, higher levels of PD-L1 and PD-L2 expression have been shown to be negative prognostic markers in esophageal cancer, especially in cases in which both ligands are expressed (30). Higher tumor B7-H4 levels, detected by IHC, were associated with worse prognosis and inversely correlated with CD3+ and CD8+ T-cells in 112 ESCC samples (52). PD-L1 overexpression, particularly at higher levels, may also serve as a predictive response biomarker in gastric cancer. Updated analysis from the KEYNOTE-012 phase I study suggests a trend toward improved overall response rate (ORR), progression free survival (PFS) with higher levels of PD-L1 overexpression (33). Further support for the predictive power has come from lambrolizumab melanoma and NSCLC cohorts suggesting increased tumor PD-L1 expression correlates with response rate (53,54).
Previous gastroesophageal immunotherapies
The role for immune modulating therapies in gastric cancer has been a subject of multiple prior investigations, largely in Asian patients. Non-specific immune potentiators such as polysaccharide-K, OK-432, and BCG have been previously investigated dating back to 1975 (55-60). The pleiotropic immune modulator protein-bound polysaccharide (PSK), derived from the CM-101 strain of the fungus Coriolus versicolor, has been shown to increase leukocyte activation, shift the Th1:Th2 balance and inhibit tumor growth in several cancer models (61-63). In Japanese gastric cancer patients undergoing gastrectomy the addition of PSK to mitomycin/5-FU adjuvant therapy improved the five year disease free survival (DFS) (70.7% vs. 59.4%) and 5-year OS (73% vs. 60%) (57). The sclerosant OK-432 (penicillin-killed lyophilized Streptococcus pyrogenes) induces IL-12, stimulates NK and T-cells favoring a Th1 response, and may improve the function of antigen presenting dendritic cells (64-68). In a small Japanese trial the combination of OK-432 with 5-FU/leucovirin and cisplatin was safe an produced a response rate of 40%, however, a larger adjuvant trial comparing S-1 vs. S-1 and OK-432 failed to demonstrate a survival difference (58,69). Similarly, the non-specific immune upregulation following BCG has translated to some anti-tumor responses without a reliable improvement in overall survival in combination studies (55,70). More recently, a small Chinese trial investigating cytokine-induced natural killer cells given after adjuvant 5-FU based chemotherapy for resected gastric cancer demonstrated a trend toward improved OS and a 6-month improvement in median DFS (34.1 vs. 40.4 months) (71). Retrospective analysis of this data suggested that benefits might be restricted to intestinal type histology (71). The combination of cytotoxic chemotherapy with non-specific immune modulators (chemoimmunotherapy) has largely been restricted to Asian patients and the lack of reproducible survival improvements has limited clinical adoption.
Early checkpoint inhibitor clinical experience
The first clinical success with immune checkpoint blockade was observed in patients with metastatic melanoma treated with the anti-CTLA-4 monoclonal antibody (mAb) ipilimumab (15). Subsequently, ipilimumab, and another anti-CTLA-4 mAb, tremelimumab, have shown promising results in phase I-III clinical trials in several cancer types including, gastric/GEJ carcinomas (72). Several anti-PD-1 mAbs including nivolumab, pembrolizumab (MK-3475), and pidilizumab have been developed and early data with these agents has shown significant response rates in melanoma, NSCLC, RCC, and diffuse large B-cell lymphoma (73-75). PD-L1 blocking antibodies have also demonstrated favorable outcomes in early trials (12).
Gastric and esophageal cancers have represented a small minority of patients in early phase immune checkpoint inhibitor trials. In the multicenter phase I trial of the anti-PD-L1 mAb BMS-936559 only 7 of 207 enrolled patients had gastric cancer. The gastric cancer cohort were assigned to the safety arm as opposed to the efficacy arm, and limited efficacy data in gastric cancer is available (12). In a second line gastroesophageal-specific phase II trial (n=18) with tremelimumab (anti-CTLA4 mAb) the observed response rate (RR) was 5%, below the observed response rate to second-line cytotoxic chemotherapy (76). Although this trial failed to meet its pre-specified RR endpoint several patients achieved stable disease (SD) and one patient achieved a partial response (PR), which is quite impressive given the aggressive natural history of advanced gastric and esophageal cancer. Further support comes from the interim analysis of the anti-PD-L1 mAbs MPDL3280A and MEDI4736 (77,78). In the MEDI4736 gastroesophageal cohort (n=16) two heavily pretreated patients remained on study over 24 weeks in the early reporting, beyond the median PFS for second line gastric and esophageal cancer therapies (78). In the most recent ESMO conference preliminary data from the phase IB anti-PD-1 antibody pembrolizumab trial (KEYNOTE-012) in advanced gastric cancer was presented. Patients with PD-L1 positive advanced gastric adenocarcinoma (IHC positive in >1% cells) received pembrolizumab 10 mg/kg every 2 weeks until progression or toxicity. A total of 39 patients were enrolled after screening 162 samples for PD-L1 (65 positive samples, 40% IHC+) (33). An updated analysis of this trial has suggested an ORR of 22% and a median response duration of 24 weeks in this heavily pre-treated population (33). There was a positive correlation with PD-L1 positivity and PFS (P=0.032). Results of this trial have prompted the planned KEYNOTE-059 phase II trial of cisplatin/5-FU in combination with pembrolizumab (33). The toxicity profile and early efficacy signals have prompted expansion of immune checkpoint inhibitors in advanced gastric and esophageal cancers (Table 2).
Table 2. Ongoing clinical investigations targeting immune checkpoint blockade in gastric and esophageal cancer.
| Study population | Histology | Number of samples | PD-L1 positive (%) | Outcome | Reference |
|---|---|---|---|---|---|
| Esophageal | Squamous | 41 | 44 | Worse outcomes | (30) |
| Gastric | Adenocarcinoma | 102 | 42.2 | Nodal mets, advanced stage | (31) |
| Adenocarcinoma | 111 | 63 | Advanced stage, worse outcome | (32) | |
| Adenocarcinoma | 243 | 43.6 | Improved DFS, lower stage | (49) |
PD-L1, programmed death ligand 1; DFS, disease free survival.
Conclusions and future directions
Advanced gastric and esophageal cancers carry a poor prognosis with limited therapeutic options, and few major therapeutic advances. While improving molecular characterization will continue to identify subsets of patients who may benefit from genotype-directed targeted therapies, a majority of patients do not yet benefit and therefore further therapies are needed.
The recently published Cancer Genome Atlas (TCGA) gastric cancer analysis has provided molecular rationale for division of gastric adenocarcinoma into four distinct molecular subtypes (79). Interestingly, the EBV-positive gastric cancer subgroup demonstrated high levels of PD-L1/L2 overexpression highlighting a molecularly defined patient population possibly most likely to derive benefit from immune checkpoint blockade (79). Early translational efforts have suggested comparable rates of PD-1 and PD-L1 expression in gastric and esophageal cancers, strengthening the argument that immune checkpoint inhibitors warrant further clinical investigation. Development and validation of predictive biomarkers for response to immune checkpoint blockade will help to refine the optimal location for immunotherapy in gastric and esophageal cancers. Some recent biomarker analyses suggest that PD-L1 directed therapy is most effective in patients with higher pre-treatment CTLA4 expression, absence of fractalkine (CX3CL1) in pre-treatment biopsies, and T-helper type 1 gene expression patterns (80). Interesting preclinical work continues to expand immunotherapy combination approaches including low dose chemosensitization with alkylating agents (81). Irradiation is known to induce antigen presentation and upregulate PD-L1 expression (82-84). The frequent use of adjuvant chemoradiation and high recurrence rates despite adjuvant therapy may make the use of anti-PD-L1 therapies an interesting adjunct to adjuvant therapy, a concept currently under investigation in NSCLC. Here we have presented a review of the current landscape of immunotherapy in gastric and esophageal cancer with attention to translational studies and early clinical investigations.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available online: http://globocan.iarc.fr
- 2.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [DOI] [PubMed] [Google Scholar]
- 3.Edgren G, Adami HO, Weiderpass E, et al. A global assessment of the oesophageal adenocarcinoma epidemic. Gut 2013;62:1406-14. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [DOI] [PubMed] [Google Scholar]
- 5.Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006;24:2903-9. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [DOI] [PubMed] [Google Scholar]
- 7.Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2010;(3):CD004064. [DOI] [PubMed] [Google Scholar]
- 8.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [DOI] [PubMed] [Google Scholar]
- 10.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [DOI] [PubMed] [Google Scholar]
- 11.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol 2006;90:297-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linsley PS, Greene JL, Brady W, et al. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1994;1:793-801. [DOI] [PubMed] [Google Scholar]
- 17.Linsley PS, Bradshaw J, Greene J, et al. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 1996;4:535-43. [DOI] [PubMed] [Google Scholar]
- 18.Peggs KS, Quezada SA, Chambers CA, et al. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med 2009;206:1717-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider H, Downey J, Smith A, et al. Reversal of the TCR stop signal by CTLA-4. Science 2006;313:1972-5. [DOI] [PubMed] [Google Scholar]
- 20.Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005;25:9543-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008;8:467-77. [DOI] [PubMed] [Google Scholar]
- 23.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med 2012;366:2517-9. [DOI] [PubMed] [Google Scholar]
- 24.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med 2001;193:839-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rozali EN, Hato SV, Robinson BW, et al. Programmed death ligand 2 in cancer-induced immune suppression. Clin Dev Immunol 2012;2012:656340. [DOI] [PMC free article] [PubMed]
- 26.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res 2013;19:1021-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. [DOI] [PubMed] [Google Scholar]
- 28.Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res 2007;13:709s-15s. [DOI] [PubMed] [Google Scholar]
- 29.Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 2004;10:5094-100. [DOI] [PubMed] [Google Scholar]
- 30.Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005;11:2947-53. [DOI] [PubMed] [Google Scholar]
- 31.Wu C, Zhu Y, Jiang J, et al. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 2006;108:19-24. [DOI] [PubMed] [Google Scholar]
- 32.Hou J, Yu Z, Xiang R, et al. Correlation between infiltration of FOXP3 regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Exp Mol Pathol 2014;96:284-91. [DOI] [PubMed] [Google Scholar]
- 33.Muro K, Bang YJ, Shankaran V, et al. Relationship between PD-L1 expression and clinical outcomes in patients with advanced gastric cancer treated with the anti-PD-1 monoclonal antibody pembrolizumab in KEYNOTE-012. J Clin Oncol 2015;33:abstr 3.
- 34.Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol 2010;222:350-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003;348:203-13. [DOI] [PubMed] [Google Scholar]
- 36.Lee HJ, Seo JY, Ahn JH, et al. Tumor-Associated Lymphocytes Predict Response to Neoadjuvant Chemotherapy in Breast Cancer Patients. J Breast Cancer 2013;16:32-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahir G, Moser M. Tumor microenvironment and lymphocyte infiltration. Cancer Immunol Immunother 2012;61:751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fridman WH, Pages F, Sautes-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298-306. [DOI] [PubMed] [Google Scholar]
- 39.Turcotte S, Gros A, Tran E, et al. Tumor-Reactive CD8+ T Cells in Metastatic Gastrointestinal Cancer Refractory to Chemotherapy. Clin Cancer Res 2014;20:331-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DuPage M, Mazumdar C, Schmidt LM, et al. Expression of tumour-specific antigens underlies cancer immunoediting. Nature 2012;482:405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsushita H, Vesely MD, Koboldt DC, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 2012;482:400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson RH, Dong H, Lohse CM, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res 2007;13:1757-61. [DOI] [PubMed] [Google Scholar]
- 43.Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer 2011;128:887-96. [DOI] [PubMed] [Google Scholar]
- 44.Saito H, Kuroda H, Matsunaga T, et al. Increased PD-1 expression on CD4+ and CD8+ T cells is involved in immune evasion in gastric cancer. J Surg Oncol 2013;107:517-22. [DOI] [PubMed] [Google Scholar]
- 45.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A 2004;101:17174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006;66:3381-5. [DOI] [PubMed] [Google Scholar]
- 47.Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010;116:1757-66. [DOI] [PubMed] [Google Scholar]
- 48.Gadiot J, Hooijkaas AI, Kaiser AD, et al. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer 2011;117:2192-201. [DOI] [PubMed] [Google Scholar]
- 49.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra37. [DOI] [PMC free article] [PubMed]
- 50.Kim JW, Nam KH, Ahn SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 51.Jiang J, Zhu Y, Wu C, et al. Tumor expression of B7-H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol Immunother 2010;59:1707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen LJ, Sun J, Wu HY, et al. B7-H4 expression associates with cancer progression and predicts patient's survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother 2011;60:1047-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daud A, Hamid O, Ribas A, et al. Antitumor activity of the anti-PD-1 monoclonal antibody MK-3475 in melanoma(MEL): Correlation of tumor PD-L1 expression with outcome. Proc Ann Meeting AACR 2014:abstr CT104. [Google Scholar]
- 54.Gandhi L, Balmanoukian A, Hui R, et al. MK-3475 (anti-PD-1 monoclonal antibody) for non-small cell lung cancer (NSCLC): Antitumor activity and association with tumor PD-L1 expression. Proc Ann Meeting AACR 2014:abstr CT105. [Google Scholar]
- 55.Popiela T, Kulig J, Czupryna A, et al. Efficiency of adjuvant immunochemotherapy following curative resection in patients with locally advanced gastric cancer. Gastric Cancer 2004;7:240-5. [DOI] [PubMed] [Google Scholar]
- 56.Oba K, Teramukai S, Kobayashi M, et al. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curative resections of gastric cancer. Cancer Immunol Immunother 2007;56:905-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakazato H, Koike A, Saji S, et al. Efficacy of immunochemotherapy as adjuvant treatment after curative resection of gastric cancer. Study Group of Immunochemotherapy with PSK for Gastric Cancer. Lancet 1994;343:1122-6. [DOI] [PubMed] [Google Scholar]
- 58.Yoshikawa T, Tsuburaya A, Kobayashi O, et al. A combination immunochemotherapy of 5-fluorouracil, cisplatin, leucovorin, and OK-432 for advanced and recurrent gastric carcinoma. Hepatogastroenterology 2003;50:2259-63. [PubMed] [Google Scholar]
- 59.Sakamoto J, Teramukai S, Nakazato H, et al. Efficacy of adjuvant immunochemotherapy with OK-432 for patients with curatively resected gastric cancer: a meta-analysis of centrally randomized controlled clinical trials. J Immunother 2002;25:405-12. [DOI] [PubMed] [Google Scholar]
- 60.Jiang J, Xu N, Wu C, et al. Treatment of advanced gastric cancer by chemotherapy combined with autologous cytokine-induced killer cells. Anticancer Res 2006;26:2237-42. [PubMed] [Google Scholar]
- 61.Fisher M, Yang LX. Anticancer effects and mechanisms of polysaccharide-K (PSK): implications of cancer immunotherapy. Anticancer Res 2002;22:1737-54. [PubMed] [Google Scholar]
- 62.Kanazawa M, Yoshihara K, Abe H, et al. Effects of PSK on T and dendritic cells differentiation in gastric or colorectal cancer patients. Anticancer Res 2005;25:443-9. [PubMed] [Google Scholar]
- 63.Jiménez-Medina E, Berruguilla E, Romero I, et al. The immunomodulator PSK induces in vitro cytotoxic activity in tumour cell lines via arrest of cell cycle and induction of apoptosis. BMC Cancer 2008;8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okamoto M, Oshikawa T, Tano T, et al. Mechanism of anticancer host response induced by OK-432, a streptococcal preparation, mediated by phagocytosis and Toll-like receptor 4 signaling. J Immunother 2006;29:78-86. [DOI] [PubMed] [Google Scholar]
- 65.Oshimi K, Kano S, Takaku F, et al. Augmentation of mouse natural killer cell activity by a streptococcal preparation, OK-432. J Natl Cancer Inst 1980;65:1265-9. [PubMed] [Google Scholar]
- 66.Fujimoto T, Duda RB, Szilvasi A, et al. Streptococcal preparation OK-432 is a potent inducer of IL-12 and a T helper cell 1 dominant state. J Immunol 1997;158:5619-26. [PubMed] [Google Scholar]
- 67.Itoh T, Ueda Y, Okugawa K, et al. Streptococcal preparation OK432 promotes functional maturation of human monocyte-derived dendritic cells. Cancer Immunol Immunother 2003;52:207-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuroki H, Morisaki T, Matsumoto K, et al. Streptococcal preparation OK-432: a new maturation factor of monocyte-derived dendritic cells for clinical use. Cancer Immunol Immunother 2003;52:561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sato Y, Kondo M, Kohashi S, et al. A randomized controlled study of immunochemotherapy with OK-432 after curative surgery for gastric cancer. J Immunother 2004;27:394-7. [DOI] [PubMed] [Google Scholar]
- 70.Popiela T, Zembala M, Oszacki J, et al. A follow-up study on chemoimmunotherapy (5-fluorouracil and BCG) in advanced gastric cancer. Cancer Immunol Immunother 1982;13:182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi L, Zhou Q, Wu J, et al. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother 2012;61:2251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett 2014;588:368-76. [DOI] [PubMed] [Google Scholar]
- 73.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol 2013;31:4199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ralph C, Elkord E, Burt DJ, et al. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res 2010;16:1662-72. [DOI] [PubMed] [Google Scholar]
- 77.Herbst RS, Gordon MS, Fine GD, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. J Clin Oncol 2013;31:abstr 3000.
- 78.Segal NH, Antonia SJ, Brahmer JR, et al. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. J Clin Oncol 2014;32:abstr 3002.
- 79.Cancer Genome Atlas Research Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pallasch CP, Leskov I, Braun CJ, et al. Sensitizing protective tumor microenvironments to antibody-mediated therapy. Cell 2014;156:590-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med 2007;204:49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [DOI] [PMC free article] [PubMed] [Google Scholar]