Abstract
Although surgical resection remains the only potentially curative treatment for gastric cancer (GC), poor long-term outcomes with resection alone compel a multimodality approach to this disease. Multimodality strategies vary widely; while adjuvant approaches are typically favored in Asia and the United States (USA), a growing body of evidence supports neoadjuvant and/or perioperative strategies in locally advanced tumors. Neoadjuvant approaches are particularly attractive given the morbidity associated with surgical management of GC and the substantial risk of omission of adjuvant therapy. The specific advantages of chemoradiotherapy (CRT) compared to chemotherapy have not been well defined, particularly in the preoperative setting and trials aimed at determining the optimal elements and sequencing of therapy are underway. Future studies will also define the role of targeted and biologic therapies.
Keywords: Gastric cancer (GC), neoadjuvant therapy, multimodality therapy, surgery, chemotherapy, radiotherapy, chemoradiotherapy (CRT)
Introduction
In 2012, there were an estimated 951,000 new diagnoses of gastric cancer (GC) and 723,000 GC deaths worldwide, accounting for 6.8% of all cancer diagnoses and 8.8% of all cancer related deaths (1). In the United States (USA) in 2014, an estimated 22,220 new diagnoses and 10,990 deaths were attributable to GC (2). GC is more prevalent in Eastern Asian countries; China accounts for the largest number of cases. In certain countries (e.g., Japan), established GC screening programs enable cancer detection at earlier stages; consequently, 5-year survival for affected patients in Japan is nearly 52%, comparing favorably to survival rates in other parts of the world. Notably, 5-year survival in the USA, Europe, and China remain poor at 20-25% (3).
Surgical resection is the only potentially curative treatment for GC. Adjuvant and neoadjuvant perioperative approaches, including chemotherapy and/or radiotherapy are now increasingly used in conjunction with surgery for locally advanced disease and even early stage disease; however, little consensus exists regarding optimal treatment sequencing. Indeed, competing approaches are supported by randomized data of variable quality. Compared with surgery alone, a survival advantage has been demonstrated with adjuvant chemotherapy in Asian trials, adjuvant chemoradiotherapy (CRT) in the largest North American study, and perioperative chemotherapy in the most cited European trial. In this review, we summarize the existing strategies and future directions in multimodality therapy for GC treatment, with a particular focus on the emerging role of, and rationale for, neoadjuvant approaches.
Adjuvant therapy for GC
In Eastern Asia, adjuvant chemotherapy alone is the standard of care, with multiple trials demonstrating benefit for this approach. Sakuramoto et al. (i.e., ACTS-GC trial) demonstrated superior overall survival (OS) with surgery followed by S-1 therapy compared with surgery alone. In this randomized controlled trial (RCT), 529 patients were randomized to D2 gastrectomy followed by S-1 beginning within 6 weeks of surgery and continuing for one year. Five hundred and thirty patients were randomized to D2 gastrectomy alone (4). At five years, disease-free survival (DFS) (65.4% vs. 53.1%) and OS (71.7% vs. 61.1%) were improved with adjuvant S-1 compared with surgery alone (5).In a smaller Japanese study of 190 patients with T2N1 or N2 GC, OS was significantly improved (86% vs. 73%) with surgery followed by 16 months of oral uracil-tegafur compared to surgery alone (6). Results of the CLASSIC trial were similar, although outcomes were reported after only three years. In this study, 1,035 patients were randomized to D2 gastrectomy followed by eight 3-week cycles of adjuvant capecitabine and oxaliplatin (XELOX) or D2 gastrectomy alone. At three years, adjuvant XELOX significantly improved DFS (74% vs. 59%, P<0.0001) and OS (83% vs. 78%, P=0.0493) compared with surgery alone (7).
With the exception of one small trial in Spain (8), Western studies have failed to reproduce results from Asia demonstrating a survival benefit with adjuvant chemotherapy alone (9-20). Although a large meta-analysis of RCTs investigating the impact of postoperative chemotherapy versus surgery alone in GC reinforced the survival impact of adjuvant chemotherapy (21), these results were largely driven by Japanese trials.
The disparate outcomes with adjuvant chemotherapy in Eastern and Western studies may be explained by differences in the genetic underpinnings of the disease and/or surgical approach in the two regions. Data from the USA suggests that Japanese-Americans have superior stage-matched survival compared to all other Americans. Notably, GC in Japanese-Americans is characterized by fewer proximal tumors, a lower male to female ratio, and less frequent adjacent organ resection (22). A different biology hypothesis is further supported by results from the multinational Avastin in Gastric Cancer (AVAGAST) trial, which examined the addition of bevacizumab to first-line capecitabine and cisplatin (XP) chemotherapy in unresectable patients. While Asian patients benefited least from the addition of bevacizumab, their median OS in the XP chemotherapy-only arm was markedly longer than pan-American patients—12.1 vs. 6.8 months, respectively (23).
In addition, more standardized surgery with uniform D2 gastrectomy and increased extirpation of regional lymph nodes (LNs) in Asia may account for reduction in residual tumor burden, and consequent lower rates of systemic failure following chemotherapy. We recently demonstrated that, even in patients receiving adjuvant therapy in the USA, inadequate surgical LN staging (<15 nodes examined) was associated with worse risk-adjusted OS compared with adequate staging, supporting the notion that standardized surgery may translate to better outcomes and allow more rational selection of adjuvant therapy (24).
While support for adjuvant chemotherapy alone in North American and European patients is lacking, the USA Intergroup-0116 trial indicated improved DFS and OS with adjuvant CRT compared with surgery alone. Patients were randomized to surgery alone or adjuvant therapy with 5 weeks of fluorouracil and leucovorin followed by 5 weeks of radiotherapy with an additional 5-day fluorouracil cycle one and two months following completion of radiotherapy. Median OS was significantly longer in the CRT arm compared with surgery alone (36 vs. 27 months, P=0.005) (25). There was sustained improvement in DFS and OS at 10-year follow-up [hazard ratio (HR) for OS 1.32 (95% CI: 1.10-1.60; P=0.0046); HR for RFS 1.51 (95% CI: 1.25-1.83; P<0.001)] (26). However, this study has been criticized for lack of surgical standardization—only 10% of patients underwent D2 resections, whereas over 50% underwent D0 resection—which may have led to overestimation of the effect of CRT.
While the additional contribution of adjuvant radiotherapy to chemotherapy has not been examined in a randomized fashion in the USA or Europe, the 2013 Korean phase III ARTIST trial demonstrated no incremental survival benefit with the addition of radiotherapy to postoperative XP chemotherapy following D2 gastrectomy. Interestingly, subgroup analysis suggested improved DFS in patients with LN metastasis in the CRT arm compared to the chemotherapy alone arm (27). While these data lend further credence to the hypothesis that radiotherapy may have compensated for inadequate surgery in the Intergroup-0116 trial, the applicability of evidence from Asia—where standardized surgery is the norm—regarding dispensability of adjuvant radiotherapy to USA/European patients is uncertain.
Rationale for a neoadjuvant approach in gastrointestinal malignancies
There are numerous purported, and some proven, advantages of a neoadjuvant approach—particularly chemotherapy—in the treatment of aggressive solid tumor malignancies. Early treatment of distant microscopic disease, the ability to gauge in vivo response to therapy, and the potential for tumor downstaging to enhance resectability are frequently invoked and may translate to better outcomes (28). A neoadjuvant strategy may increase the likelihood of completing multimodality therapy, particularly when surgical management is associated with significant morbidity and complications may preclude timely adjuvant therapy (29,30).
Application of radiotherapy in the neoadjuvant setting has several additional and distinct advantages. The presence of intact tumor and preserved normal anatomy facilitates treatment planning and may limit toxicity to adjacent organs. Conversely, adjuvant radiotherapy mandates higher dosing and larger treatment fields with the potential for increased toxicity. Such advantages have translated into better outcomes in rectal and esophageal cancer. In rectal cancer, preoperative CRT decreases locoregional recurrence compared to postoperative CRT (31,32). Preoperative, compared with postoperative, CRT also decreases the incidence of grade 3 and 4 adverse events and long-term toxic effects (31), and improves sphincter preservation (33,34). In esophageal cancer, neoadjuvant CRT improves DFS and OS compared with surgery alone (35). The success of preoperative radiotherapy in esophageal and rectal cancer may be related to the anatomic location of the esophagus and rectum in enclosed spaces where the ability to achieve a negative radial margin may be challenging and treatment can be administered with less risk of toxicity to adjacent organs.
The perception that a neoadjuvant approach may compromise curative therapy in a subset of patients that progress prior to surgery is largely unfounded. Conversely, identification of patients who can be spared a potentially morbid non-curative resection (i.e., those that would recur distantly at an early time point) is an additional advantage of a neoadjuvant approach. There is a potential for treatment-related toxicity that may preclude surgical therapy in patients with curable disease. Notwithstanding, the poor outcomes associated with surgery alone for all but early stage gastrointestinal malignancies mitigate such concerns.
National, regional, institutional and disease site-specific trends influence approach. Generally, a nuanced approach whereby patients with clinical early stage disease are treated with surgery first and those with locally advanced disease receive neoadjuvant therapy is increasingly advocated. The former group undergoes potentially curative surgery without delay, and decisions regarding adjuvant therapy are predicated on more accurate pathologic staging. Neoadjuvant therapy is used to select a subset of patients from the latter group for whom surgical resection is most appropriate.
Many of the aforementioned advantages of neoadjuvant therapy in general are applicable to the treatment of GC. First, even with an R0 resection, local and systemic recurrence is common (36), suggesting that early treatment of occult microscopic disease could decrease recurrence. Second, gastrectomy for GC is associated with substantial morbidity (29). Neoadjuvant therapy may increase the likelihood of multimodality therapy completion. Third, the ability to gauge treatment response is relevant as treatment response to neoadjuvant therapy predicts long-term outcome (37); a favorable response may, therefore, justify an aggressive surgical approach.
Neoadjuvant and perioperative chemotherapy in GC
One of the early RCTs investigating the impact of neoadjuvant chemotherapy in GC was the Dutch FAMTX trial. This trial included 59 patients—29 were randomized to receive four cycles of 5-fluorouracil, leucovorin, and methotrexate (FAMTX) followed by surgery, while 30 were randomized to surgery alone. Neoadjuvant FAMTX did not significantly improve survival compared to surgery alone (30 vs. 18 months, P=0.17), although the trial was likely underpowered (38).
The United Kingdom Medical Research Council MAGIC trial was the first large RCT to demonstrate a benefit for perioperative chemotherapy in GC and gastroesophageal (GE) cancer. Five hundred three patients with stage T2 or higher potentially resectable gastric (74%), distal esophageal (11%), or esophagogastric junction (EGJ) adenocarcinomas (15%) were randomly assigned to surgery alone or surgery plus perioperative chemotherapy with epirubicin, cisplatin, and fluorouracil (ECF). This regimen consisted of three preoperative and three postoperative cycles of intravenous epirubicin (50 mg/m2) and cisplatin (60 mg/m2) on day 1, and a continuous intravenous infusion of fluorouracil (200 mg/m2/day) for 21 days. The primary end point was OS. Patients in the perioperative chemotherapy group had similar rates of postoperative complications and death within 30 days compared to those in the surgery alone group. Although only 42% of patients were able to complete protocol treatment, more patients in the perioperative chemotherapy group were able to undergo surgery (79% vs. 70%), and resected tumors were significantly smaller (T1/T2 52% vs. 37%) with fewer regional nodal metastases (N0/N1 84% vs. 71%) compared to the surgery alone cohort. In addition, patients in the perioperative chemotherapy group had improved OS compared with patients in the surgery only group (HR for death 0.75, 95% CI: 0.60-0.93; P=0.009; 5-year survival rate, 36% vs. 23%) as well as a progression-free survival benefit (HR 0.66; 95% CI: 0.53 to 0.81; P<0.001). The most notable ECF-related adverse effect was neutropenia (23%); however, less than 12% of patients experienced serious (grade 3 or 4) toxicity. Notably, nearly half of patients in the perioperative chemotherapy group did not complete the adjuvant ECF component of their treatment plan, suggesting that this survival benefit was largely derived from neoadjuvant ECF (39).
A similar benefit was reported in the French FNLCC/FFCD trial, where a similar population was randomized to receive 2-3 cycles of preoperative and 3-4 cycles of postoperative chemotherapy (infused fluorouracil 800 mg/m2 daily for five days plus cisplatin 100 mg/m2 on day 1 or 2, every four weeks) or surgery alone (113 vs. 111 patients, respectively). Higher rates of R0 resection (84% vs. 73%, P=0.04), improved 5-year DFS (34% vs. 19%, HR 0.65; 95% CI: 0.48-0.89, P=0.003), and improved OS (38% vs. 24%, HR 0.69; 95% CI: 0.50-0.95, P=0.02) were achieved in the neoadjuvant group compared to the surgery alone group (40).
A trial from the European Organization for Research and Treatment of Cancer (EORTC 40954) did not support increased survival with neoadjuvant chemotherapy compared with surgery alone, although it was terminated early due to poor recruitment. In recruited patients, however, two 48-day cycles of neoadjuvant cisplatin, d-L-folinic acid, and fluorouracil did improve R0 resection (81.9% vs. 66.7%, P=0.036) and decreased lymph node metastasis rates (61.4% vs. 76.5%, P=0.018) compared with surgery alone (41).
Neoadjuvant CRT in GC
The efficacy of adjuvant CRT demonstrated in the Intergroup 0116 trial (25) and the advantages of a neoadjuvant approach in other aggressive malignancies have motivated application of neoadjuvant CRT in GC. Single arm studies demonstrating improvements in R0 resection rate and the achievement of pathological complete response (pCR) with preoperative CRT have been promising. In a phase I multi-institutional single-arm trial, Ajani et al. demonstrated R0 resection and pCR rates of 70% and 30%, respectively, with preoperative CRT consisting of two 28-day cycles of induction fluorouracil, leucovorin, and cisplatin followed by fluorouracil-based CRT to 45 Gy (42). Pathological complete response, pathologic partial response, R0 resection, and postoperative T/N stage were associated with improved OS (37). Another multi-institutional phase II trial of preoperative induction chemotherapy with two cycles of fluorouracil, leucovorin, and cisplatin followed by paclitaxel, fluorouracil, and concurrent 45 Gy radiotherapy demonstrated R0 resection rates of 77% and pCR rate of 26% (43). Similarly, in a recent Dutch phase I/II study, patients with locally advanced GC had an R0 resection rate of 72%, pCR rate of 16%, and near-complete response rate of 24% following neoadjuvant therapy with carboplatin and paclitaxel with concurrent radiotherapy (44). Other phase I and II trials of neoadjuvant CRT for GC in both Eastern and Western patients have been equally promising and are summarized in Table 1 (45-50).
Table 1. Summary of phase I and II trials investigating impact of neoadjuvant chemoradiotherapy for locally advanced gastric cancer.
| Trial (References) | N | Schedule | Number proceeding to surgery [%] | Post-operative mortality [%] | Pathological complete response [%] | Pathological complete or partial response [%] | R0 resection [%] |
|---|---|---|---|---|---|---|---|
| Lowy et al. [2001] (45) | 24 | 5-FU, XRT, intraoperative XRT | 19/24 [83] | 5/19 [26] | 2/24 [8] | NR | NR |
| Roth et al. [2003] (46) | 19 | Cisplatin, 5-FU, leucovorin, XRT | 19/19 [100] | 0 | 1/19 [5] | 9/19 [47] | NR |
| Ajani et al. [2004] (42) | 34 | 5-FU, leucovorin, cisplatin, XRT | 28/33 [85] | 2/28 [7] | 10/34 [29] | 18/34 [53] | 23/34 [68] |
| Ajani et al. [2006] (43) | 43 | 5-FU, leucovorin, cisplatin, XRT | 36/43 [84] | 0 | 11/43 [26] | NR | 27/43 [63] |
| Wydmański et al. [2007] (47) | 40 | 5-FU, leucovorin, XRT | 32/40 [80] | NR | 7/40 [18] | 8/40 [20] | 30/40 [75] |
| Inoue et al. [2012] (48) | 12 | S-1, XRT | 12/12 [100] | 0 | 2/12 [17] | 10/12 [83] | 11/12 [92] |
| Lee et al. [2012] (49) | 12 | S-1, oxaliplatin, XRT | 12/12 [100] | 0 | 1/12 [8] | 6/12 [50] | 11/12 [92] |
| Pera et al. [2012] (50) | 41 | Oxaliplatin, cisplatin, 5-FU | 19/25 [76] | 3/31 [10]* | 3/25 [12] | 11/25 [44] | 29/41 [71]* |
| Trip et al. [2014] (44) | 25 | Carboplatin, paclitaxel, XRT | 24/25 [96] | 1/24 [4] | 4/25 [16] | 10/25 [40] | 18/25 [72] |
*, contained patients with both esophageal and gastric cancer. Outcomes other than mortality were reported individually; 5-FU, fluorouracil; XRT, radiotherapy; NR, not recorded.
Despite these promising results, phase III RCTs demonstrating a survival benefit and/or improved R0 resection rates following neoadjuvant CRT in distal GC are lacking. However, studies including patients with EGJ cancers have recently been conducted. A 2009 German phase III RCT (POET study) evaluated the impact of adding CRT to neoadjuvant chemotherapy with cisplatin, fluorouracil, and leucovorin versus chemotherapy alone for tumors of the lower esophagus and gastric cardia (51). Although this study found a trend toward improved survival with the addition of preoperative CRT to chemotherapy alone (47.4% vs. 27.7% 3-year survival, P=0.07), it was inadequately powered and primarily included patients with esophageal cancer.
In the more recent Dutch CROSS trial, patients with potentially resectable esophageal or EGJ cancer (3/4 adenocarcinomas, 1/4 SCC, majority distal esophageal, 11% EGJ) were randomized to preoperative CRT using weekly paclitaxel 50 mg/m2 plus carboplatin (AUC of 2) plus concurrent radiotherapy (41.4 Gy over 5 weeks) or surgery alone. Preoperative CRT was well tolerated, with grade 3 or worse hematologic toxicity in 7%, and grade 3 or higher non-hematologic toxicity in <13%; there were also no differences in postoperative morbidity or mortality between the two groups. The R0 resection rate was higher with CRT (92% vs. 69%), and 29% of those treated with CRT had pCR. At a median follow-up of 32 months, median OS was significantly better with preoperative CRT (HR for death 0.657, 95% CI: 0.495-0.871, P=0.003; 3-year survival rate: 58% vs. 44%) (35).
Based on these data and encouraging phase I-III evidence, well-designed and adequately powered phase III RCTs comparing neoadjuvant CRT versus chemotherapy alone in distal gastric tumors are warranted.
Patient selection for neoadjuvant therapy
While a neoadjuvant approach can be applied broadly, its advantages may be most pronounced in specific patient subsets. Gastric resection is associated with substantial morbidity, particularly in certain high-risk patients groups. Using the ACS-NSQIP database, we recently demonstrated that older age, preoperative weight loss, and concomitant splenectomy and/or pancreatectomy are associated with increased risk of morbidity following total gastrectomy for GC (29). Such morbidity may preclude timely initiation of adjuvant therapy. Using a large cohort from the National Cancer Data Base (NCDB), we directly explored factors predicting omission of adjuvant therapy following gastric resection. Advancing age, medical comorbidities, non-privately insured/uninsured status, proximal tumor location, and clinical T1/2 and N0 classification were associated with adjuvant therapy omission (30). It is plausible; therefore, that a neoadjuvant approach may increase the likelihood of multimodality treatment completion in select patient subsets at highest risk for either morbidity from gastrectomy and/or omission of adjuvant therapy. Ultimately, such factors must be weighed against the risk of attrition due to treatment-related toxicity following neoadjuvant therapy, in order to develop a personalized treatment plan that optimizes multimodality therapy delivery.
Limitations of clinical staging may also influence selection of a neoadjuvant approach. Endoscopic ultrasound (EUS), a mainstay of preoperative staging, provides a relatively crude estimate of T- and N-classfications (52-54). While there is significant heterogeneity in the accuracy of EUS in the literature, a recent meta-analysis found a pooled EUS accuracy for N stage of only 64% and T stage of 75%, with better accuracy for T3 and T4 tumors than T1 and T2 tumors (55). Such limitations may favor primary surgery in lower risk patients who can be salvaged with adjuvant CRT if pathologic stage renders them eligible for multimodality therapy. Conversely, more liberal use of neoadjuvant therapy may be appropriate in ambiguously staged patients at higher risk for omission of adjuvant therapy.
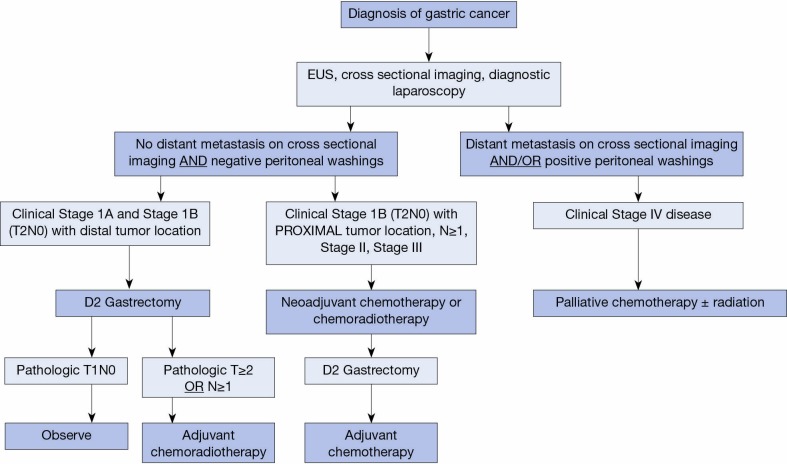
Acknowledging the limitations of preoperative staging by EUS and the factors associated with high risk of morbidity from gastrectomy or adjuvant therapy omission, we propose an algorithm for the approach to adjuvant or neoadjuvant treatment of GC based on both clinical stage and baseline patient characteristics (Figure 1). In this algorithm, all patients with a diagnosis of GC are formally staged with a combination of cross sectional imaging, EUS, and diagnostic laparoscopy. Patients with clinical T1-2, N0 and distal tumors proceed to surgery, while those with T3-4 tumors, evidence of regional LN metastases, or T2 proximal tumors receive neoadjuvant chemotherapy or CRT followed by surgery. Exceptions are made on an individual basis using nuanced clinical judgment. Given the implications of inadequate LN staging on long-term outcomes, patients should undergo sound oncologic operations with examination of at least 15 LNs (24); in our practice this is achieved by pancreas and spleen-preserving D2 gastrectomy (56). When surgery is the initial treatment modality, adjuvant treatment is selected on the basis of pathological stage at resection (i.e., IB-III based on intergroup criteria).
Figure 1.
Proposed treatment algorithm for a multimodality approach to gastric cancer. EUS, Endoscopic ultrasound.
Trials in progress
Several ongoing studies have been designed to further characterize the optimal sequencing of multimodality therapy in GC. As would be expected from current regional variations in treatment of locally advanced GC, these sequencing combinations and schedules are quite heterogeneous and merit discussion.
The ongoing Dutch CRITICS trial compares preoperative chemotherapy alone with epirubicin, cisplatin, and capecitabine (ECX) followed by surgery and postoperative ECX alone versus preoperative ECX followed by surgery and postoperative CRT (NCT00407186) (57). TOPGEAR is an Australasian, Canadian, and European study evaluating preoperative ECF chemotherapy alone versus preoperative ECF plus CRT (NCT01924819). The UK-based MAGIC-B/MRC-ST03 trial compares perioperative ECX with or without bevacizumab for localized GC (NCT00450203).
In Asia, the PRODIGY trial is evaluating preoperative docetaxel, oxaliplatin, and S-1 followed by surgery and adjuvant S-1 versus surgery followed by S-1 alone (NCT01515748). The ARTIST II trial is a follow-up to the previously discussed ARTIST trial, further dissecting survival differences between CRT and chemotherapy alone in patients with LN-positive disease. In ARTIST II, patients with positive LNs found at the time of D2 gastrectomy will receive adjuvant S-1, adjuvant S-1 plus oxaliplatin, or adjuvant S-1, oxaliplatin, and CRT (NCT01761461).
Future directions
While biomarker-targeted therapy has received enormous attention in recent years, its potential remains largely untapped in GC. Indeed, a recent novel molecular classification of GC from the Cancer Genome Atlas may revolutionize targeted treatment paradigms in this disease (58). Consequently, future efforts will likely require the addition of targeted and biologic therapy to standard surgery, chemotherapy, and radiotherapy in GC in order to optimize long-term outcomes. While a comprehensive discussion of these agents is beyond the scope of this review, a few existing therapies deserve discussion.
Trastuzumab is a humanized monoclonal antibody that inhibits the HER2/neu receptor. In pivotal RCTs in both the adjuvant and neoadjuvant settings, trastuzumab has been shown to be effective in treating HER2-positive breast cancer; in GC, the role of trastuzumab in improving survival has only been elucidated in patients with advanced, non-operable HER2-positive GC (59). As is the case in breast cancer, HER2-positivity has been characterized as a negative prognostic factor for survival in GC. However, in a prospective observational study, HER2-positive advanced GC patients treated with trastuzumab and chemotherapy demonstrated comparable survival to HER2-negative patients treated with chemotherapy alone (60).
Other targeted therapies have been examined in advanced GC patients with encouraging results. Bevacizumab, a vascular endothelial growth factor A (VEGF-A) inhibitor, has been used as a chemotherapy adjunct in other GI malignancies including colon and rectal cancer (61-63). In the aforementioned AVAGAST trial, the addition of bevacizumab to cisplatin and capecitabine or fluorouracil in advanced GC patients improved progression-free survival and overall response rates, although it did not significantly impact OS (23). A prospective evaluation of biomarkers that might predict response to bevacizumab was incorporated in the trial design; interestingly, patients with baseline high VEGF-A and low neuropilin-1 levels displayed a trend toward improved survival with bevacizumab, suggesting that the optimal effect from bevacizumab may be realized in appropriately selected patients (64). The addition of bevacizumab to perioperative ECX demonstrated acceptable toxicity in a phase II trial (65).
Ramucirumab is a VEGF receptor-2 antagonist that has been demonstrated to improve survival in advanced GC, either as a single agent or in combination with taxane-based chemotherapy, with an additive advantage in response rate outcomes (66,67).
While utilization of such approaches in a salvage setting for metastatic disease are supported by strong rationale and some evidence, benefit of targeted agents for resectable disease has been less extensively explored. Treatment related toxicity will emerge as a critical determinant of the applicability of such regimens in the non-metastatic disease population.
Conclusions
Benefits of a neoadjuvant approach have been demonstrated in the management of gastrointestinal malignancies. Neoadjuvant therapy is probably underutilized in the management of GC. The morbidity associated with gastric resection precludes timely adjuvant therapy in a subset of patients and multimodality therapy has proven efficacy. Neoadjuvant therapy allows for an assessment of response to therapy, may improve treatment compliance and can inform selection of patients for surgical resection. Future studies are warranted and may establish a role for more effective and better tolerated neoadjuvant regimens.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol 2001;2:533-43. [DOI] [PubMed] [Google Scholar]
- 4.Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. [DOI] [PubMed] [Google Scholar]
- 5.Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387-93. [DOI] [PubMed] [Google Scholar]
- 6.Nakajima T, Kinoshita T, Nashimoto A, et al. Randomized controlled trial of adjuvant uracil-tegafur versus surgery alone for serosa-negative, locally advanced gastric cancer. Br J Surg 2007;94:1468-76. [DOI] [PubMed] [Google Scholar]
- 7.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [DOI] [PubMed] [Google Scholar]
- 8.Grau JJ, Estapé J, Alcobendas F, et al. Positive results of adjuvant mitomycin-C in resected gastric cancer: a randomised trial on 134 patients. Eur J Cancer 1993;29A:340-2. [DOI] [PubMed] [Google Scholar]
- 9.Engstrom PF, Lavin PT, Douglass HO, Jr, et al. Postoperative adjuvant 5-fluorouracil plus methyl-CCNU therapy for gastric cancer patients. Eastern Cooperative Oncology Group study (EST 3275). Cancer 1985;55:1868-73. [DOI] [PubMed] [Google Scholar]
- 10.Coombes RC, Schein PS, Chilvers CE, et al. A randomized trial comparing adjuvant fluorouracil, doxorubicin, and mitomycin with no treatment in operable gastric cancer. International Collaborative Cancer Group. J Clin Oncol 1990;8:1362-9. [DOI] [PubMed] [Google Scholar]
- 11.Krook JE, O'Connell MJ, Wieand HS, et al. A prospective, randomized evaluation of intensive-course 5-fluorouracil plus doxorubicin as surgical adjuvant chemotherapy for resected gastric cancer. Cancer 1991;67:2454-8. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald JS, Fleming TR, Peterson RF, et al. Adjuvant chemotherapy with 5-FU, adriamycin, and mitomycin-C (FAM) versus surgery alone for patients with locally advanced gastric adenocarcinoma: A Southwest Oncology Group study. Ann Surg Oncol 1995;2:488-94. [DOI] [PubMed] [Google Scholar]
- 13.Lise M, Nitti D, Marchet A, et al. Final results of a phase III clinical trial of adjuvant chemotherapy with the modified fluorouracil, doxorubicin, and mitomycin regimen in resectable gastric cancer. J Clin Oncol 1995;13:2757-63. [DOI] [PubMed] [Google Scholar]
- 14.Tsavaris N, Tentas K, Kosmidis P, et al. A randomized trial comparing adjuvant fluorouracil, epirubicin, and mitomycin with no treatment in operable gastric cancer. Chemotherapy 1996;42:220-6. [DOI] [PubMed] [Google Scholar]
- 15.Bajetta E, Buzzoni R, Mariani L, et al. Adjuvant chemotherapy in gastric cancer: 5-year results of a randomised study by the Italian Trials in Medical Oncology (ITMO) Group. Ann Oncol 2002;13:299-307. [DOI] [PubMed] [Google Scholar]
- 16.Popiela T, Kulig J, Czupryna A, et al. Efficiency of adjuvant immunochemotherapy following curative resection in patients with locally advanced gastric cancer. Gastric Cancer 2004;7:240-5. [DOI] [PubMed] [Google Scholar]
- 17.Bouché O, Ychou M, Burtin P, et al. Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the FFCD randomized phase III trial (8801). Ann Oncol 2005;16:1488-97. [DOI] [PubMed] [Google Scholar]
- 18.Nitti D, Wils J, Dos Santos JG, et al. Randomized phase III trials of adjuvant FAMTX or FEMTX compared with surgery alone in resected gastric cancer. A combined analysis of the EORTC GI Group and the ICCG. Ann Oncol 2006;17:262-9. [DOI] [PubMed] [Google Scholar]
- 19.De Vita F, Giuliani F, Orditura M, et al. Adjuvant chemotherapy with epirubicin, leucovorin, 5-fluorouracil and etoposide regimen in resected gastric cancer patients: a randomized phase III trial by the Gruppo Oncologico Italia Meridionale (GOIM 9602 Study). Ann Oncol 2007;18:1354-8. [DOI] [PubMed] [Google Scholar]
- 20.Cascinu S, Labianca R, Barone C, et al. Adjuvant treatment of high-risk, radically resected gastric cancer patients with 5-fluorouracil, leucovorin, cisplatin, and epidoxorubicin in a randomized controlled trial. J Natl Cancer Inst 2007;99:601-7. [DOI] [PubMed] [Google Scholar]
- 21.GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group , Paoletti X, Oba K, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA 2010;303:1729-37. [DOI] [PubMed] [Google Scholar]
- 22.Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer 2000;88:921-32. [PubMed] [Google Scholar]
- 23.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [DOI] [PubMed] [Google Scholar]
- 24.Datta J, Lewis RS, Jr, Mamtani R, et al. Implications of inadequate lymph node staging in resectable gastric cancer: a contemporary analysis using the National Cancer Data Base. Cancer 2014;120:2855-65. [DOI] [PubMed] [Google Scholar]
- 25.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [DOI] [PubMed] [Google Scholar]
- 26.Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012;30:2327-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Lim do H, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012;30:268-73. [DOI] [PubMed] [Google Scholar]
- 28.Ajani JA, Mansfield PF, Lynch PM, et al. Enhanced staging and all chemotherapy preoperatively in patients with potentially resectable gastric carcinoma. J Clin Oncol 1999;17:2403-11. [DOI] [PubMed] [Google Scholar]
- 29.Bartlett EK, Roses RE, Kelz RR, et al. Morbidity and mortality after total gastrectomy for gastric malignancy using the American College of Surgeons National Surgical Quality Improvement Program database. Surgery 2014;156:298-304. [DOI] [PubMed] [Google Scholar]
- 30.Datta J, McMillan MT, Shang EK, et al. Omission of adjuvant therapy following gastric cancer resection: development of a validated risk model. J Natl Compr Canc Netw 2015;13:531-41. [DOI] [PubMed] [Google Scholar]
- 31.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [DOI] [PubMed] [Google Scholar]
- 32.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [DOI] [PubMed] [Google Scholar]
- 33.Howard JH, Gonzalez Q, Arnoletti JP, et al. Prognostic factors and preoperative radiation therapy associated with sphincter preservation in patients with resectable rectal cancer. Am J Surg 2008;195:239-43. [DOI] [PubMed] [Google Scholar]
- 34.Crane CH, Skibber JM, Birnbaum EH, et al. The addition of continuous infusion 5-FU to preoperative radiation therapy increases tumor response, leading to increased sphincter preservation in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2003;57:84-9. [DOI] [PubMed] [Google Scholar]
- 35.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [DOI] [PubMed] [Google Scholar]
- 36.Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999;340:908-14. [DOI] [PubMed] [Google Scholar]
- 37.Ajani JA, Mansfield PF, Crane CH, et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol 2005;23:1237-44. [DOI] [PubMed] [Google Scholar]
- 38.Hartgrink HH, van de Velde CJ, Putter H, et al. Neo-adjuvant chemotherapy for operable gastric cancer: long term results of the Dutch randomised FAMTX trial. Eur J Surg Oncol 2004;30:643-9. [DOI] [PubMed] [Google Scholar]
- 39.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [DOI] [PubMed] [Google Scholar]
- 40.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [DOI] [PubMed] [Google Scholar]
- 41.Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010;28:5210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ajani JA, Mansfield PF, Janjan N, et al. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol 2004;22:2774-80. [DOI] [PubMed] [Google Scholar]
- 43.Ajani JA, Winter K, Okawara GS, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol 2006;24:3953-8. [DOI] [PubMed] [Google Scholar]
- 44.Trip AK, Poppema BJ, van Berge Henegouwen MI, et al. Preoperative chemoradiotherapy in locally advanced gastric cancer, a phase I/II feasibility and efficacy study. Radiother Oncol 2014;112:284-8. [DOI] [PubMed] [Google Scholar]
- 45.Lowy AM, Feig BW, Janjan N, et al. A pilot study of preoperative chemoradiotherapy for resectable gastric cancer. Ann Surg Oncol 2001;8:519-24. [DOI] [PubMed] [Google Scholar]
- 46.Roth AD, Allal AS, Bründler MA, et al. Neoadjuvant radiochemotherapy for locally advanced gastric cancer: a phase I-II study. Ann Oncol 2003;14:110-5. [DOI] [PubMed] [Google Scholar]
- 47.Wydmański J, Suwinski R, Poltorak S, et al. The tolerance and efficacy of preoperative chemoradiotherapy followed by gastrectomy in operable gastric cancer, a phase II study. Radiother Oncol 2007;82:132-6. [DOI] [PubMed] [Google Scholar]
- 48.Inoue T, Yachida S, Usuki H, et al. Pilot feasibility study of neoadjuvant chemoradiotherapy with S-1 in patients with locally advanced gastric cancer featuring adjacent tissue invasion or JGCA bulky N2 lymph node metastases. Ann Surg Oncol 2012;19:2937-45. [DOI] [PubMed] [Google Scholar]
- 49.Lee DJ, Sohn TS, Lim do H, et al. Phase I study of neoadjuvant chemoradiotherapy with S-1 and oxaliplatin in patients with locally advanced gastric cancer. Cancer Chemother Pharmacol 2012;69:1333-8. [DOI] [PubMed] [Google Scholar]
- 50.Pera M, Gallego R, Montagut C, et al. Phase II trial of preoperative chemoradiotherapy with oxaliplatin, cisplatin, and 5-FU in locally advanced esophageal and gastric cancer. Ann Oncol 2012;23:664-70. [DOI] [PubMed] [Google Scholar]
- 51.Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851-6. [DOI] [PubMed] [Google Scholar]
- 52.Bentrem D, Gerdes H, Tang L, et al. Clinical correlation of endoscopic ultrasonography with pathologic stage and outcome in patients undergoing curative resection for gastric cancer. Ann Surg Oncol 2007;14:1853-9. [DOI] [PubMed] [Google Scholar]
- 53.Spolverato G, Ejaz A, Kim Y, et al. Use of endoscopic ultrasound in the preoperative staging of gastric cancer: a multi-institutional study of the US gastric cancer collaborative. J Am Coll Surg 2015;220:48-56. [DOI] [PubMed] [Google Scholar]
- 54.Willis S, Truong S, Gribnitz S, et al. Endoscopic ultrasonography in the preoperative staging of gastric cancer: accuracy and impact on surgical therapy. Surg Endosc 2000;14:951-4. [DOI] [PubMed] [Google Scholar]
- 55.Cardoso R, Coburn N, Seevaratnam R, et al. A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer. Gastric Cancer 2012;15 Suppl 1:S19-26. [DOI] [PubMed] [Google Scholar]
- 56.Strong VE, D’Amico TA, Kleinberg L, et al. Impact of the 7th Edition AJCC staging classification on the NCCN clinical practice guidelines in oncology for gastric and esophageal cancers. J Natl Compr Canc Netw 2013;11:60-6. [DOI] [PubMed] [Google Scholar]
- 57.Dikken JL, van Sandick JW, Maurits Swellengrebel HA, et al. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer 2011;11:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cancer Genome Atlas Research Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [DOI] [PubMed] [Google Scholar]
- 60.Qiu MZ, Li Q, Wang ZQ, et al. HER2-positive patients receiving trastuzumab treatment have a comparable prognosis with HER2-negative advanced gastric cancer patients: a prospective cohort observation. Int J Cancer 2014;134:2468-77. [DOI] [PubMed] [Google Scholar]
- 61.Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 2007;25:4779-86. [DOI] [PubMed] [Google Scholar]
- 62.Giantonio BJ, Levy DE, O’dwyer PJ, et al. A phase II study of high-dose bevacizumab in combination with irinotecan, 5-fluorouracil, leucovorin, as initial therapy for advanced colorectal cancer: results from the Eastern Cooperative Oncology Group study E2200. Ann Oncol 2006;17:1399-403. [DOI] [PubMed] [Google Scholar]
- 63.Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 2014;32:513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Cutsem E, de Haas S, Kang YK, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119-27. [DOI] [PubMed] [Google Scholar]
- 65.Okines AF, Langley RE, Thompson LC, et al. Bevacizumab with peri-operative epirubicin, cisplatin and capecitabine (ECX) in localised gastro-oesophageal adenocarcinoma: a safety report. Ann Oncol 2013;24:702-9. [DOI] [PubMed] [Google Scholar]
- 66.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [DOI] [PubMed] [Google Scholar]
- 67.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [DOI] [PubMed] [Google Scholar]