Abstract
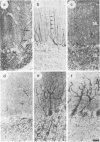
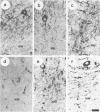
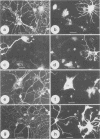
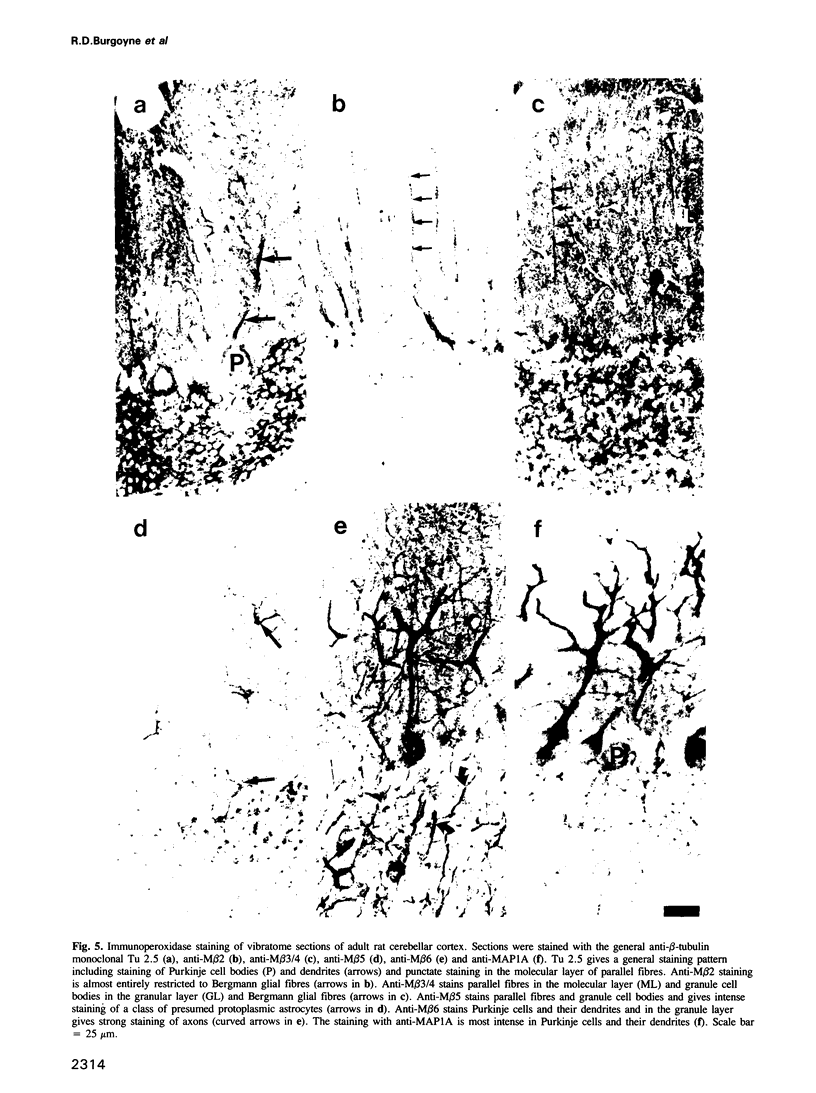
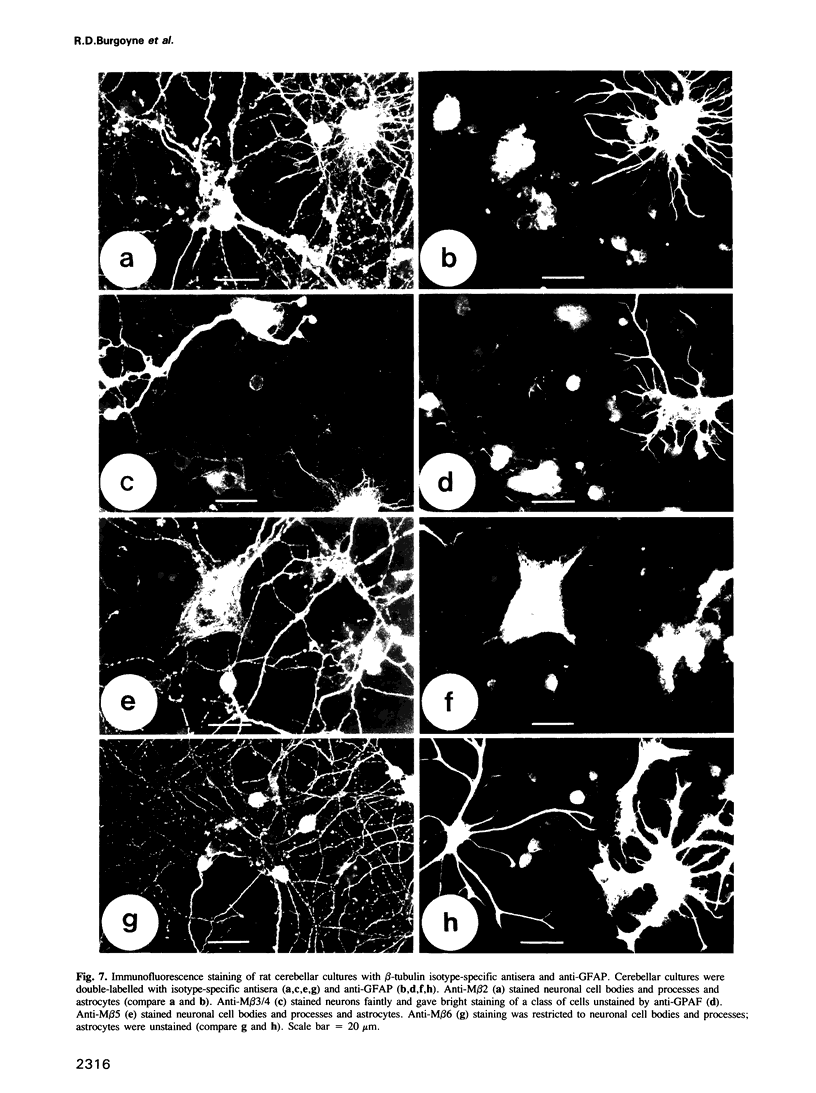
We describe the structure and expression of a mammalian beta-tubulin isotype (M beta 6) that is weakly expressed in testis but is abundant in developing brain, with transcripts declining to lower levels in the adult brain. The expression of M beta 6 was undetectable in any other mouse tissue examined. A serum specific for this isotype was prepared using a cloned fusion protein as immunogen. M beta 6 is one of five known beta-tubulin isotypes expressed in brain, and using the anti-M beta 6 serum along with sera, anti-M beta 2, anti-M beta 3/4 and anti-M beta 5, previously characterized, we have examined the pattern of expression of beta-tubulin isotypes in rat cerebellum. The isotypes each have characteristic cell-type specific patterns of localization in cerebellum. M beta 2, M beta 3/4 and M beta 5 are present in both neuronal and non-neuronal cells, but in contrast M beta 6 was only detectable in neurons in tissue sections and in dissociated cerebellar cell culture. The majority of sequence differences among the beta-tubulin isotypes lie at the carboxy terminus, the region of beta-tubulin involved in MAP binding. In the case of M beta 2 and M beta 6, the patterns of expression are similar or identical to the patterns of expression of MAP3 and MAP1A respectively. These results suggest that beta-tubulin isotypes may contribute to the determination of the specific association of MAPs with microtubules of diverse function. However, the strict subcellular segregation of other MAPs in brain may be determined by other factors.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhardt R., Huber G., Matus A. Differences in the developmental patterns of three microtubule-associated proteins in the rat cerebellum. J Neurosci. 1985 Apr;5(4):977–991. doi: 10.1523/JNEUROSCI.05-04-00977.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt R., Matus A. Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeletons. J Comp Neurol. 1984 Jun 20;226(2):203–221. doi: 10.1002/cne.902260205. [DOI] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Luca F. C., Vallee R. B. Microtubule-associated protein 1B: identification of a major component of the neuronal cytoskeleton. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5404–5408. doi: 10.1073/pnas.82.16.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. F., Fridovich-Keil J. L., Pillus L., Mulligan R. C., Solomon F. A chicken-yeast chimeric beta-tubulin protein is incorporated into mouse microtubules in vivo. Cell. 1986 Feb 14;44(3):461–468. doi: 10.1016/0092-8674(86)90467-8. [DOI] [PubMed] [Google Scholar]
- Bond J. F., Robinson G. S., Farmer S. R. Differential expression of two neural cell-specific beta-tubulin mRNAs during rat brain development. Mol Cell Biol. 1984 Jul;4(7):1313–1319. doi: 10.1128/mcb.4.7.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady S. T., Tytell M., Lasek R. J. Axonal tubulin and axonal microtubules: biochemical evidence for cold stability. J Cell Biol. 1984 Nov;99(5):1716–1724. doi: 10.1083/jcb.99.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D., Cambray-Deakin M. A. The cellular neurobiology of neuronal development: the cerebellar granule cell. Brain Res. 1988 Jan-Mar;472(1):77–101. doi: 10.1016/0165-0173(88)90006-9. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Cumming R. Ontogeny of microtubule-associated protein 2 in rat cerebellum: differential expression of the doublet polypeptides. Neuroscience. 1984 Jan;11(1):156–167. doi: 10.1016/0306-4522(84)90220-3. [DOI] [PubMed] [Google Scholar]
- Cambray-Deakin M. A., Burgoyne R. D. Acetylated and detyrosinated alpha-tubulins are co-localized in stable microtubules in rat meningeal fibroblasts. Cell Motil Cytoskeleton. 1987;8(3):284–291. doi: 10.1002/cm.970080309. [DOI] [PubMed] [Google Scholar]
- Cambray-Deakin M. A., Burgoyne R. D. Posttranslational modifications of alpha-tubulin: acetylated and detyrosinated forms in axons of rat cerebellum. J Cell Biol. 1987 Jun;104(6):1569–1574. doi: 10.1083/jcb.104.6.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambray-Deakin M. A., Norman K. M., Burgoyne R. D. Differentiation of the cerebellar granule cell: expression of a synaptic vesicle protein and the microtubule-associated protein MAP1A. Brain Res. 1987 Jul;431(1):1–7. doi: 10.1016/0165-3806(87)90190-8. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Sullivan K. F. Molecular biology and genetics of tubulin. Annu Rev Biochem. 1985;54:331–365. doi: 10.1146/annurev.bi.54.070185.001555. [DOI] [PubMed] [Google Scholar]
- Cowan N. J. Tubulin genes and the diversity of microtubule function. Oxf Surv Eukaryot Genes. 1984;1:36–60. [PubMed] [Google Scholar]
- Cumming R., Burgoyne R. D., Lytton N. A. Immunocytochemical demonstration of alpha-tubulin modification during axonal maturation in the cerebellar cortex. J Cell Biol. 1984 Jan;98(1):347–351. doi: 10.1083/jcb.98.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie D. N., Dutton G. R., Cohen J. Monolayer cultures of perikarya isolated from postnatal rat cerebellum. Experientia. 1979 Mar 15;35(3):345–347. doi: 10.1007/BF01964343. [DOI] [PubMed] [Google Scholar]
- Dutton G. R., Currie D. N., Tear K. An improved method for the bulk isolation of viable perikarya from postnatal cerebellum. J Neurosci Methods. 1981 Apr;3(4):421–427. doi: 10.1016/0165-0270(81)90029-7. [DOI] [PubMed] [Google Scholar]
- Gundersen G. G., Kalnoski M. H., Bulinski J. C. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984 Oct;38(3):779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- Huber G., Matus A. Immunocytochemical localization of microtubule-associated protein 1 in rat cerebellum using monoclonal antibodies. J Cell Biol. 1984 Feb;98(2):777–781. doi: 10.1083/jcb.98.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine J. M., Card J. P. Light and electron microscopic localization of a cell surface antigen (NG2) in the rat cerebellum: association with smooth protoplasmic astrocytes. J Neurosci. 1987 Sep;7(9):2711–2720. doi: 10.1523/JNEUROSCI.07-09-02711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Gilmartin M. E., Hall J. L., Cowan N. J. Three expressed sequences within the human beta-tubulin multigene family each define a distinct isotype. J Mol Biol. 1985 Mar 5;182(1):11–20. doi: 10.1016/0022-2836(85)90023-3. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Gu W., Cowan N. J. Free intermingling of mammalian beta-tubulin isotypes among functionally distinct microtubules. Cell. 1987 May 22;49(4):539–548. doi: 10.1016/0092-8674(87)90456-9. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Lee M. G., Cowan N. J. Five mouse tubulin isotypes and their regulated expression during development. J Cell Biol. 1985 Sep;101(3):852–861. doi: 10.1083/jcb.101.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littauer U. Z., Giveon D., Thierauf M., Ginzburg I., Ponstingl H. Common and distinct tubulin binding sites for microtubule-associated proteins. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7162–7166. doi: 10.1073/pnas.83.19.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W. In vivo microtubules are copolymers of available beta-tubulin isotypes: localization of each of six vertebrate beta-tubulin isotypes using polyclonal antibodies elicited by synthetic peptide antigens. J Cell Biol. 1987 Oct;105(4):1707–1720. doi: 10.1083/jcb.105.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parysek L. M., del Cerro M., Olmsted J. B. Microtubule-associated protein 4 antibody: a new marker for astroglia and oligodendroglia. Neuroscience. 1985 Jul;15(3):869–875. doi: 10.1016/0306-4522(85)90084-3. [DOI] [PubMed] [Google Scholar]
- Piperno G., Fuller M. T. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985 Dec;101(6):2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer B., Cohen R., Matus A. MAP5: a novel brain microtubule-associated protein under strong developmental regulation. J Neurocytol. 1986 Dec;15(6):763–775. doi: 10.1007/BF01625193. [DOI] [PubMed] [Google Scholar]
- Riederer B., Matus A. Differential expression of distinct microtubule-associated proteins during brain development. Proc Natl Acad Sci U S A. 1985 Sep;82(17):6006–6009. doi: 10.1073/pnas.82.17.6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sackett D. L., Bhattacharyya B., Wolff J. Tubulin subunit carboxyl termini determine polymerization efficiency. J Biol Chem. 1985 Jan 10;260(1):43–45. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Serrano L., Avila J., Maccioni R. B. Controlled proteolysis of tubulin by subtilisin: localization of the site for MAP2 interaction. Biochemistry. 1984 Sep 25;23(20):4675–4681. doi: 10.1021/bi00315a024. [DOI] [PubMed] [Google Scholar]
- Serrano L., de la Torre J., Maccioni R. B., Avila J. Involvement of the carboxyl-terminal domain of tubulin in the regulation of its assembly. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5989–5993. doi: 10.1073/pnas.81.19.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sullivan K. F., Cleveland D. W. Identification of conserved isotype-defining variable region sequences for four vertebrate beta tubulin polypeptide classes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4327–4331. doi: 10.1073/pnas.83.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasante A., Wang D., Dobner P., Dolph P., Lewis S. A., Cowan N. J. Six mouse alpha-tubulin mRNAs encode five distinct isotypes: testis-specific expression of two sister genes. Mol Cell Biol. 1986 Jul;6(7):2409–2419. doi: 10.1128/mcb.6.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Villasante A., Lewis S. A., Cowan N. J. The mammalian beta-tubulin repertoire: hematopoietic expression of a novel, heterologous beta-tubulin isotype. J Cell Biol. 1986 Nov;103(5):1903–1910. doi: 10.1083/jcb.103.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]