Abstract
Background
Untreated celiac disease is associated with increased morbidity and mortality. Until now, no up-to-date figures have been available on the prevalence of celiac disease among children and adolescents in Germany, or on the percentage of undiagnosed cases.
Methods
To estimate the prevalence of celiac disease, serum samples obtained from 2003 to 2006 from participants in the German Health Interview and Examination Survey for Children and Adolescents (KiGGS) were studied for celiac disease–specific autoantibodies and total IgA.
Results
Of the 12 741 study participants aged 1 to 17 years (6546 boys, 6195 girls), 9 (0.07%) had a reported history of celiac disease. An elevated concentration of serum autoantibodies to tissue transglutaminase was found in 91 children with a normal IgA concentration and in 7 with IgA deficiency. The prevalence of undiagnosed celiac disease, based on positive autoantibody findings, was 0.8% (95% confidence interval 0.6–1.0%), and the overall prevalence of the disease was 0.9%. Seropositive children and adolescents had lower ferritin and red blood cell folate concentrations than seronegative ones; they also tended to be shorter and to weigh less as reflected by age- and sex-standardized z-scores.
Conclusion
The 0.9% prevalence of celiac disease in Germany, as determined from a combination of serological findings and clinical histories, is similar to reported prevalences elsewhere in Europe and North America. Pediatricians, primary care physicians, internists, and other specialists should be aware of the broad spectrum of clinical manifestations of this disease. Children who have symptoms suggestive of celiac disease or belong to a group at risk for it should be tested for antibodies against tissue transglutaminase, as should symptomatic adults after the exclusion of other possible causes. It is not yet clear whether asymptomatic adults from high-risk groups should be tested.
Celiac disease is an immune-mediated systemic disorder triggered by gluten-containing grains in genetically predisposed individuals (1– 3). Its defining characteristics are enteropathy and evidence of celiac disease–specific autoantibodies to tissue transglutaminase (3). Celiac disease is usually lifelong but with a gluten-free diet it is very treatable: the mucosa of the small intestine return to their normal condition and subsequent tests for celiac disease–specific autoantibodies are negative.
Celiac disease can develop at any age. Symptoms range from the classic signs of malabsorption syndrome—diarrhea, weight loss, growth failure, osteoporosis, and anemia—to nonspecific symptoms such as chronic constipation or abdominal pain (1, 2). Screening examinations in children have revealed that 50 to 70% of those affected are asymptomatic (4, 5). Celiac disease cannot develop without a genetic predisposition to HLA markers DQ2 or DQ8. Approximately one-third of the Caucasian population is DQ2-positive or DQ8-positive.
If celiac disease is suspected, the first step should be to determine serum levels of IgA autoantibodies to tissue transglutaminase IgA (tTG-IgA) or to endomysium (EmA-IgA), in addition to determining total IgA to rule out IgA deficiency (2, 3).
The highly sensitive and specific autoantibody tests identify patients with celiac disease and make it possible to document its prevalence and incidence, regardless of whether patients suffer any clinical complaints. The frequency of classic celiac disease with malabsorption is approximately 1 in 1000 children (6, 7). The prevalence of celiac disease that has been established using antibody screening in various European countries and North America is estimated at approximately 1% (8, 9). Incidence seems to have increased in recent decades (10). There are no current figures on the prevalence of celiac disease in children in Germany. A cross-sectional study conducted 20 years ago, involving 3004 children, found celiac disease confirmed by biopsy in one in 500 (0.2%) of the children (6). In the southern German KORA/MONICA population cohort, 63 of 4633 (1.4%) and 18 of 4173 (0.43%) of adults respectively were found to have tTG-IgA levels above the cut-off value in two cross-sectional studies (9, 11). When compared to seronegative individuals of the same ages, there was an approximately twofold increase in the risk of dying within nine years for seropositive men (217/2289 versus 11/44) and an approximately fourfold increase in risk for seropositive women (91/2281 versus 4/19) (11). Even when clinical symptoms are absent, celiac disease that remains untreated in the long term entails numerous health risks including lymphoproliferative diseases (12– 19). In children identified via screening there is increasing evidence that growth and bone quality can be adversely affected (15, 19). Because the complaints of celiac disease are so nonspecific, it often takes years before symptomatic patients are diagnosed (20). By then, symptoms and organic complications of celiac disease that has remained untreated for many years, such as pancreatic failure or atrophy of the spleen, are often no longer reversible (20– 22).
We used retained samples from KiGGS study participants. The KiGGS study was a study conducted by the Robert Koch Institute in Berlin as part of German nationwide health monitoring of child and adolescent health. The retained samples were used to establish the prevalence of celiac disease–specific autoantibodies in children and adolescents in Germany and to compare the anthropometric and various laboratory parameters of seropositive and seronegative individuals.
Methods
Study population
Between 2003 and 2006, 17 641 children and adolescents aged between 0 and 17 years were examined as part of the German nationwide KiGGS study. The design, conduct, and findings of this study have been published in several places (23– 27).
Questioning and anthropometric data
The KiGGS study recorded information on sociodemographic factors using questionnaires. Information on children’s diseases was reported by parents in a computer-assisted personal interview with a doctor. Weight and height were determined in standardized ways for all study participants.
Laboratory tests
Retained samples were used to determine serum levels of tTG-IgA and total IgA; serum levels of IgG antibodies to tissue transglutaminase (tTG-IgG) were also determined if IgA level was below 0.05 g/L. Levels of tTG-IgA below 7 U/mL were taken to be negative; levels of 7 U/mL or above were taken to be positive. According to the information provided by the manufacturer, with a cut-off value of 7 U/mL for symptomatic individuals sensitivity was 98.8% and specificity 95.0%. Because the risk of false negatives and false positives depends on tTG antibody level (28, 29), the percentages of children with borderline negative (4 to 7 U/mL), borderline positive (7 to 10 U/mL), mildly positive (10 to 30 U/mL), and strongly positive (70 U/mL or above) levels were recorded.
Antibodies to thyroid peroxidase (anti-TPO antibodies) and laboratory parameters that had already been measured were considered for group comparison (26).
Statistical evaluation
The KiGGS study percentiles were used to calculate z-scores for height and body-mass index (BMI). The point prevalence of celiac disease (from serum testing, from clinical history, and total) was established with 95% confidence intervals. The significance of differences between groups was tested using the Rao–Scott chi-square test for complex samples with the F distribution. Age-adjusted and sex-adjusted means for laboratory parameters were calculated in separate GLM models using the LSMEANS option. Differences with p-values less than 0.05 were taken to be statistically significant.
Results
Celiac disease–specific autoantibodies and total IgA levels were successfully determined from the retained samples of 12 741 study participants aged over 12 months (6546 boys: 51.4%; 6195 girls: 48.6%).
IgA antibodies to tissue transglutaminase
tTG-IgA levels equal to or above the cut-off value of 7 U/mL were found in 92 children and adolescents (0.7%). Table 1 shows percentages when the cut-off value was reduced to 4 U/mL or increased to 10, 30, or 70 U/mL.
Table 1. Prevalence of positive testing for antibodies to tissue transglutaminase (tTG) type IgA and IgG*1.
| Borderline value | tTG-IgAn = 12741 | Frequency, % (95% CI) | tTG-IgA or tTG-IgG*2n = 12 741 | Frequency, % (95% CI) |
|---|---|---|---|---|
| <4 u/ml | 12581 | 98.7 (98.4 to 98.9) | 12574 | 98.6 (98.3 to 98.8) |
| ≥4 U/mL | 160 | 1.3 (1.1 to 1.6) | 167 | 1.4 (1.2 to 1.7) |
| ≥7 U/mL | 92 | 0.7 (0.6 to 0.9) | 98 | 0.8 (0.6 to 1.0) |
| >10 U/mL | 80 | 0.6 (0.5 to 0.9) | 86 | 0.7 (0.6 to 0.9) |
| >30 U/mL | 56 | 0.5 (0.3 to 0.7) | 62 | 0.5 (0.4 to 0.7) |
| ≥70 U/mL | 43 | 0.4 (0.3 to 0.5) | 47 | 0.4 (0.3 to 0.6) |
*1In children and adolescents aged between 1 and 17 years in Germany, percentages with 95% confidence intervals for various cut-off values. 7 U/mL is the borderline value stipulated by the manufacturer.
*2Includes children who tested negative for tTG-IgA but positive for tTG-IgG with IgA deficiency.
95% CI: 95% confidence interval; tTG: Tissue transglutaminase; IgA: Immunoglobulin A; IgG: Immunoglobulin G
IgG antibodies to tissue transglutaminase in children and adolescents with IgA deficiency
Serum IgA levels below 0.05 g/L were recorded in 49 (0.4%) of the 12 741 children and adolescents. Seven (14.3%) of these 49 children tested positive for tTG-IgG antibodies (24 to 307 U/mL), and one tested positive for tTG-IgA and tTG-IgG.
Prevalence of celiac disease–specific autoimmunity
The prevalence of unidentified celiac disease in children and adolescents in Germany, estimated on the basis of positive tests for autoantibodies to tissue transglutaminase, is 0.8% (Table 1).
Prevalence of celiac disease based on positive serum findings and identified in clinical history
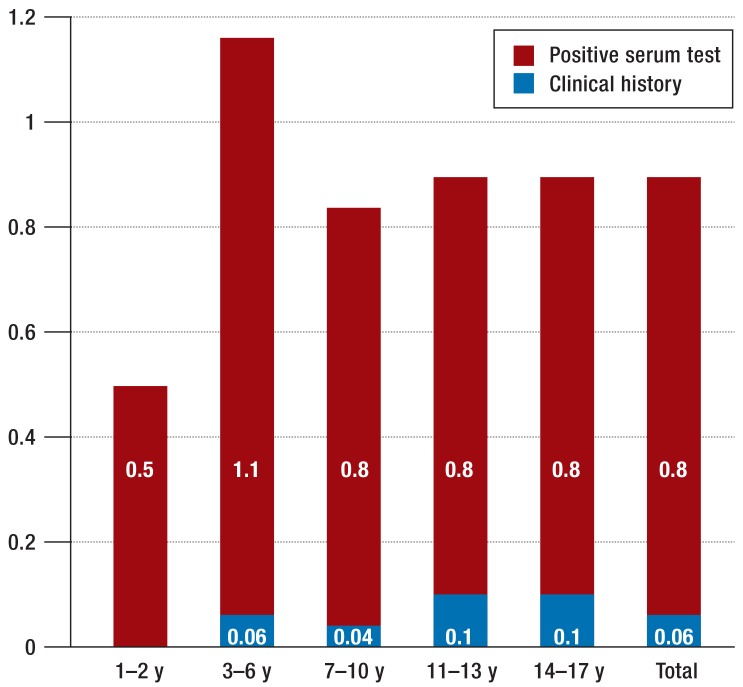
Of the total KiGGS population, parents reported known celiac disease for 13 children (0.07%). Of the 12 741 participants who underwent serum testing, celiac disease was detected in nine children between the ages of five and 16 years (mean age: 11.7 years; median age: 12 years). One child tested positive for tTG-IgA antibodies as evidence of a diet containing gluten, while the other eight had levels below the cut-off value. Adding the eight children with a positive clinical history of celiac disease to the 98 seropositive children with tTG-IgA or tTG-IgG levels above the cut-off value of 7 U/mL gives a total celiac disease prevalence of 0.9% (95% CI: 0.7 to 1.1) for children and adolescents aged between one and 17 years in Germany. Prevalence was highest among children aged between three and six years (Figure 1).
Figure 1.
Prevalence of celiac disease (%) for various age groups: celiac disease known from clinical history (blue), positive celiac disease-specific autoantibodies (red) (see also Table 3). y, years
Bodyweight, height, body mass index
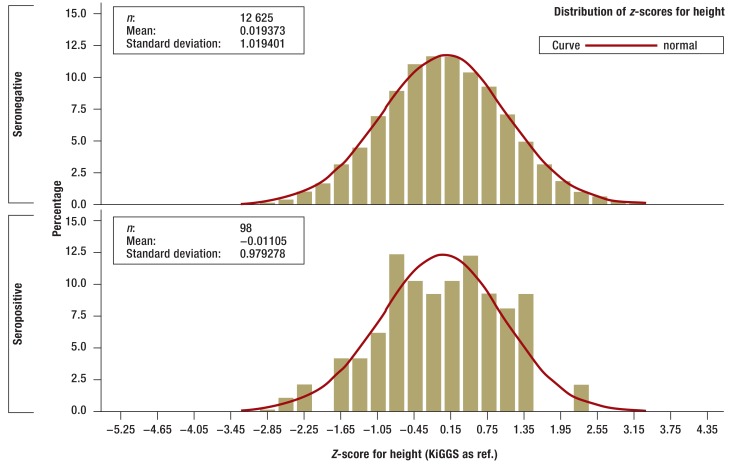
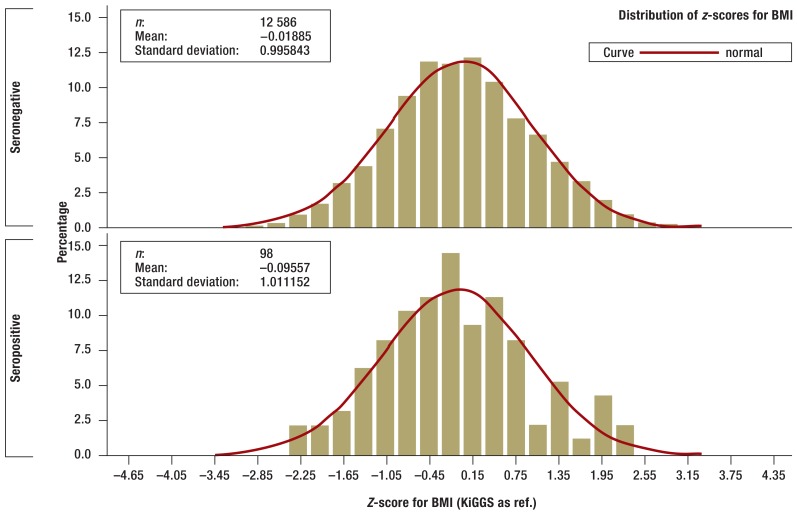
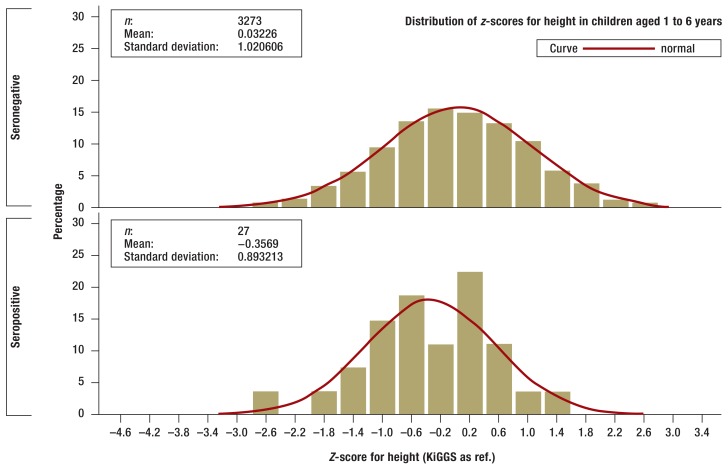
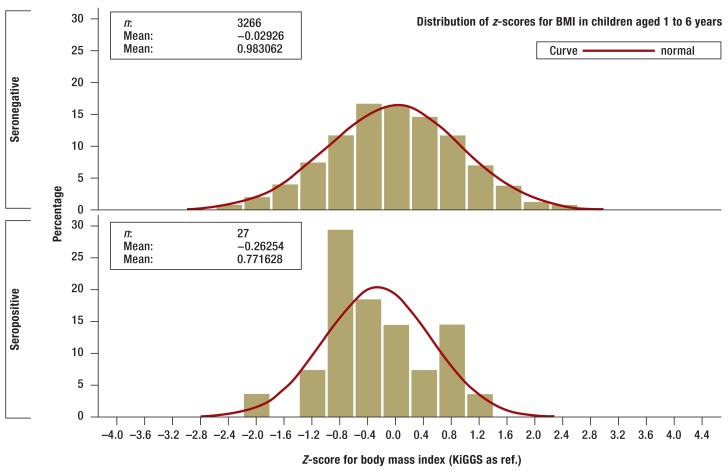
There were no statistically significant differences between children and adolescents who tested positive for tTG autoantibodies and those who were seronegative in terms of bodyweight, height, or BMI, although those who were seropositive did tend to be smaller and lighter (Figures 2a and b. In the one-year to six-year age group seropositive children (n = 27) were smaller on average than those who were seronegative (n = 3266; p= 0.0484) according to z-scores standardized for age and sex (eFigures a and b).
Figure 2a.
Z-scores for height of seronegative (above) and seropositive (below) children and adolescents in the KiGGS study (histogram). Z-scores are standardized for age and sex and adjusted to the range of values of standard normal distribution. Z-scores were calculated using the KiGGS study percentiles (30)
Figure 2b.
Z-scores for body mass index (BMI) of seronegative (above) and seropositive (below) children and adolescents in the KiGGS study (histogram). Z-scores are standardized for age and sex and adjusted to the range of values of standard normal distribution. Z-scores were calculated using the KiGGS study percentiles (30).
eFigure.a.
Z-scores for height of seronegative (above) and seropositive (below) children aged 1 to 6 years in the KiGGS study (histogram). Z-scores are standardized for age and sex and adjusted to the range of values of standard normal distribution. Z-scores were calculated using the KiGGS study percentiles.
eFigure b.
Z-scores for BMI of seronegative (above) and seropositive (below) children aged 1 to 6 years in the KiGGS study (histogram). Z-scores are standardized for age and sex and adjusted to the range of values of standard normal distribution. Z-scores were calculated using the KiGGS study percentiles.
Antibodies to thyroid peroxidase
Anti-TPO antibodies were detected in 7% of the 98 seropositive children and adolescents (n = 7, all female) but only 1.1% of the 12 643 seronegative children (n = 142, 107 female) (p<0.0001). None of the children who tested seropositive for anti-TPO antibodies had elevated TSH levels.
Comparison of laboratory parameters in children and adolescents with and without elevated tTG autoantibodies
Seropositive children and adolescents had significantly lower mean ferritin and red blood cell folate concentrations than those who were seronegative (Table 2) when adjusted for age and sex, although only 2.2% versus 0.6% had abnormally low ferritin levels (p= 0.04) and 3.9% versus 1.1% abnormally low folate (p= 0.05). For other laboratory parameters there were no significant differences, although hemoglobin, hematocrit, and mean corpuscular volume (MCV) tended to be lower in those who were seropositive (Table 2).
Table 2. Comparison of age-adjusted and sex-adjusted mean laboratory parameters*.
| Laboratory parameter | Negative serum testing (n) | Age-adjusted and sex-adjusted mean if serum tests negative | Positive serum testing (n) | Age-adjusted and sex-adjusted mean if serum tests positive | p |
|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 12508 | 13.0 | 97 | 12.9 | 0.1493 |
| Hematocrit (%) | 12509 | 38.3 | 97 | 38.0 | 0.2385 |
| MCV (fl) | 12509 | 82.9 | 97 | 82.4 | 0.1169 |
| Ferritin (μ56;g/L) | 11580 | 36.2 | 93 | 31.8 | 0.0316 |
| RBC folate (ng/mL) | 6893 | 497.8 | 51 | 448.9 | 0.0344 |
| Total protein (g/dL) | 12543 | 7.4 | 96 | 7.5 | 0.4792 |
| 25-hydroxycholecalciferol (nmol/L) | 9013 | 46.4 | 70 | 46.5 | 0.9811 |
| TSH (μ56;U/mL) | 11693 | 2.4 | 93 | 2.4 | 0.9658 |
| Gamma GT (U/L) | 12613 | 12.2 | 97 | 12.2 | 0.9990 |
| Vitamin B12 (ng/L) | 11675 | 599.8 | 93 | 591.2 | 0.7228 |
*Of 12 741 children and adolescents with negative and positive celiac disease–specific antibodies
MCV: Mean corpuscular volume; RBC: Red blood cell; TSH: Thyroid-stimulating hormone
Discussion
At 0.9%, the prevalence of celiac disease in children and adolescents in Germany based on positive tests for celiac disease–specific antibodies and celiac disease reported in clinical history is far higher than previously reported for Germany (6, 9). For every nine children with known celiac disease, there are potentially 97 newly identified children. Seroprevalence in children under the age of three years is lower, at 0.5%. Prospective screening studies have shown that the highest incidence occurs between the ages of two and five years (31, 32).
This means that the prevalence of celiac disease in children and adolescents in Germany is comparable to that reported for other European countries and North America, which is approximately 1% of the total population (10, 33). Higher prevalences of 2.2% and 2.9% for celiac disease confirmed by biopsy were reported in the ETICS screening study in Swedish schoolchildren aged 12 years born in 1997 and 1993 respectively (29). In Sweden there were only three to four newly identified patients for every child already known to have celiac disease (29); in other words, the number of unreported cases in Germany seems to be unusually high. Unlike the ETICS study, in the KiGGS study diagnosis in seropositive participants could not be confirmed histologically using biopsies of the small intestine. This makes it impossible to rule out the possibility that in some individual participants increased tTG antibody levels may be present with no enteropathy (false positive or potential celiac disease) and that prevalence may therefore have been overestimated. The sensitivity and specificity of a serum test for celiac disease are usually established unambiguously in symptomatic patients and control individuals without celiac disease, using histological methods. This means that the characteristics of the test cannot be extrapolated to asymptomatic individuals identified via screening. Comparisons with screening studies such as the ETICS study are therefore very revealing for the interpretation of our data. The ETICS study used ELISA (29), for which the manufacturer stipulates a cut-off value of 5 U/mL. Children with high normal levels of between 2 and 5 U/mL were also tested for endomysium IgA antibodies, and biopsy was offered if the test result was positive (29). Diagnosis was confirmed histopathologically in 230 of the 267 children who underwent biopsies, and of these 41 (17.8%) had levels between 2 and <5 U/mL, in other words below the cut-off level proposed by the manufacturer (41 false negatives). False positives, i.e. tTG of 5 U/mL or above, were found in eight of the 34 children with normal mucosa (Marsh 0), and in seven out of eight levels were only mildly elevated (5 to 30 U/mL) (29). In the KiGGS population investigated here, 36 children tested positive with levels between 7 and 30 U/mL (potential false positives), and 68 tested negative with levels just below the cut-off value, between 4 and <7 U/mL (potential false negatives). If, as in the ETICS study, the authors take more celiac children with high normal levels to be healthy, in the low abnormal area up to 30 U/mL, their estimate of the prevalence of celiac disease (based on serum testing and clinical history) of 0.9% actually seems conservative.
There is a well documented correlation between elevated autoantibody levels, probability of celiac disease, and severity of villous atrophy in symptomatic patients; in other words, very high autoantibody levels are highly predictive of enteropathy (4, 28). This correlation opens up the option of giving children with signs of malabsorption syndrome and tTG levels more than 10 times the cut-off value a diagnosis without histological testing, if this is done in consultation with a qualified pediatric gastroenterologist (3). Further requirements are evidence of endomysium antibodies in a second blood sample and evidence of HLA-DQ2 or HLA-DQ8. Overall, asymptomatic celiac patients with comparable damage to mucosa have lower tTG levels than symptomatic children, although there is a large overlap between the two (4).
Our findings suggest that only the tip of the iceberg of celiac disease cases has been identified in Germany. The strategy recommended in guidelines—providing individuals with symptoms suggestive of celiac disease (box) and children and adolescents in at-risk groups (Table 3) with targeted examination for celiac disease–specific autoantibodies (2, 3)—identifies only a minority of all celiac patients (5). In our study the height, weight, and BMI of seropositive individuals differed from those who were seronegative only in terms of trends. However, seropositive children aged between one and six years were significantly smaller. The longitudinal cohort study TEDDY (n = 6706) was able to show that children aged between two and four years at the time of seroconversion did not yet have delayed growth (odds ratios for mean z-scores: 0.93 to 1.15 for the various parameters) (4). In a British birth cohort, seropositive (n = 54) seven-year-old children were a median of 3.9 cm shorter and 2.2 kg lighter than seronegative children (absolute values and z-scores: p<0.0001) (35). In the Dutch Generation R Study, seropositive children who were six years old at the time of screening (n = 57) were a mean of 1.6 kg lighter (p <0.01) and 1.7 cm shorter (nonsignificant) and had a BMI 0.6 standard deviations lower (p <0.05) than seronegative children (n= 4249) (19). Bone density (total and LWS) measured using DEXA was significantly lower in seropositive children, even after adjustment (19).
Box. Symptoms for which serum testing for celiac disease should be considered after consideration of other causes*.
-
Gastrointestinal symptoms
Chronic diarrhea, suspected irritable bowel syndrome
Dyspeptic complaints with nausea, loss of appetite, vomiting
Meteorism, flatulence, protruding abdomen
Chronic constipation in children
-
Extraintestinal symptoms
Dermatitis herpetiformis
Iron deficiency anemia
Lack of weight increase, weight loss
Stunted growth or growth retardation in children
Chronic fatigue, reduced performance, concentration disorders
Relapsing mouth ulcers
Pubertas tarda, amenorrhea
Elevated transaminases
Osteoporosis, osteopenia
Infertility of unclear etiology in women
*Particularly if more than one symptom is present. In addition, serum testing to determine tissue transglutaminase IgA and total IgA levels should be always performed to rule out or confirm celiac disease before the beginning of a trial gluten-free diet for nonspecific complaints, as diagnosis is no longer possible after such a diet has been started (1–3).
Table 3. Groups at increased risk of developing celiac disease*1.
| Group/disease | Frequency of celiac disease |
|---|---|
| Children, siblings, parents of celiac patients | Sisters and daughters: 12 to 15%Brothers and sons: 6 to 7% Parents: 3 to 4% |
| Diabetes mellitus type 1 | 5 to 9%, of whom 1/3 on diagnosis |
| Trisomy 21 | 5 to 6 % |
| Ullrich–Turner syndrome | 6 to 9% |
| Selective IgA deficiency*2 | 2 to 8% |
| Autoimmune thyroiditis | 3 to 7% |
| Autoimmune hepatitis | 12 to 13% |
| IgA nephropathy | 3 to 5% |
*1Children and adolescents in these at-risk groups should undergo serum testing for celiac disease even if asymptomatic (2, 3). Whether adults in at-risk groups should be tested is still under discussion. The limited evidence on diet in terms of mortality and morbidity should be borne in mind when deciding this. *2For IgA deficiency, an IgG-based test (tTG, EmA, or DGP) is indicated
We found thyroid peroxidase antibodies in 7% of children with celiac disease–specific autoantibodies but only 1.1% of seronegative children. Our findings are comparable to Swedish data (36). There, 7.0% (17/335) of the celiac patients identified via screening were positive for anti-TPO antibodies, as were 7.5% (7/93) of the patients known to have celiac disease before screening but only 2.8% (48/1695) of the seronegative control children. The predictive value of positive testing for anti-TPO antibodies for subsequent development of autoimmune thyroiditis is unclear (37).
One limitation of this study is the lack of using a second serological control test (e.g. anti-endomysium antibodies) and lack of histological confirmation of diagnosis in children and adolescents with positive or borderline tTG autoantibody levels. In addition, there was no systematic enquiry into known celiac disease or a gluten-free diet; this may have led to prevalence being underestimated. On the other hand, this study involved a very large sample, representative of Germany, of children and adolescents who underwent serological testing. Anthropometric data and laboratory findings were combined with serum findings. The higher proportion of girls among seropositive children is compatible with the literature and supports the validity of our findings. A further strength of the study is measurement of tTG-IgG antibody levels in cases of low total IgA levels. Of the children with low IgA concentrations, 14.3% are very likely to have celiac disease. This supports the strategy recommended in the various guidelines of always determining total serum IgA in addition to celiac disease–specific IgA antibody testing (2, 3).
In view of the high number of undiagnosed cases of celiac disease in Germany, the question arises of whether asymptomatic individuals with celiac disease detected via screening would also benefit from a gluten-free diet. In comparison to children who are still growing (15, 29, 35), there is little controlled data available for adults. In a recently published Finnish study, 40 asymptomatic adults identified via screening with confirmed enteropathy were randomized to a gluten-free or normal diet (38). After one year the gluten-free group showed significantly fewer digestive problems, reflux symptoms, and anxiety and better overall scores for health values than the control group consuming a normal diet; the control group fared better in terms of social functional level. In summary, there is currently no strong evidence that a gluten-free diet reduces complications, improves quality of life, or prolongs life for asymptomatic individuals. Screening of the general population is therefore not recommended; instead, serum testing for individuals with symptoms suggestive of celiac disease (Box) is preferred (2, 39). Children who are still growing and are in an at-risk group (Table 3) should be tested even if they show no symptoms (2, 3).
Conclusion
Evaluation of the KiGGS data shows that celiac disease is probably greatly underdiagnosed in Germany. Pediatricians, primary care physicians, internal medicine specialists, and also colleagues in other specialties should be familiar with the wide range of clinical manifestations of celiac disease and should arrange for serum diagnostic tests to be performed in cases of suspected celiac disease.
Key Messages.
0.8% of children and adolescents in Germany have elevated celiac disease–specific autoantibody levels.
The total prevalence of celiac disease in children and adolescents in Germany (based on serum testing and clinical history) is higher than previously assumed, at 0.9%.
Seroprevalence suggests that the total prevalence of celiac disease in Germany is comparable to that of other Central European countries.
The number of undetected celiac disease cases seems to be particularly high in Germany.
Seropositive children under the age of six years are smaller than seronegative children of the same age and sex. Excess weight does not rule out celiac disease.
Acknowledgments
Translated from the original German by Caroline Shimakawa-Devitt, M.A.
We would like to thank the German Coeliac Society (DZG, Deutsche Zöliakiegesellschaft) for its financial support in data evaluation, and Prof. Dr. J. Henker for his help in initiating the project. We would also like to thank all the children and adolescents, and their parents, whose participation made the KiGGS study possible.
Footnotes
Conflict of interest statement
Dr. Laass has received lecture fees from Nutricia.
Prof. Uhlig holds a patent for deamidated gliadin peptide and receives license fees for it.
Dr. Laass, Prof. Uhlig, and Prof. Zimmer are involved in a study that is partly funded by Euroimmun.
Prof. Koletzko has received lecture or consultancy fees from Euroimmun, ThermoFisher. R-Biopharm, and Schär. She is leading two international studies on celiac disease that are partly funded by Euroimmun, ThermoFisher, Inova, R-Biopharm, Nestle, and Schär.
Dr. Schmitz and Mr. Thamm declare that no conflict of interest exists.
References
- 1.Schuppan D, Zimmer KP. The diagnosis and treatment of celiac disease. Dtsch Arztebl Int. 2013;110:835–846. doi: 10.3238/arztebl.2013.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felber J, Aust D, Baas S, et al. [Results of a S2k-Consensus Conference of the German Society of Gastroenterolgy, Digestive- and Metabolic Diseases (DGVS) in conjunction with the German Coeliac Society (DZG) regarding coeliac disease, wheat allergy and wheat sensitivity] Z Gastroenterol. 2014;52:711–743. doi: 10.1055/s-0034-1366687. [DOI] [PubMed] [Google Scholar]
- 3.Husby S, Koletzko S, Korponay-Szabo IR, et al. European society for pediatric gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 4.Agardh D, Lee HS, Kurppa K, et al. Clinical features of celiac disease: A prospective birth cohort. Pediatrics. 2015;135:627–634. doi: 10.1542/peds.2014-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen A, Sandstrom O, Carlsson A, et al. Usefulness of symptoms to screen for celiac disease. Pediatrics. 2014;133:211–218. doi: 10.1542/peds.2012-3765. [DOI] [PubMed] [Google Scholar]
- 6.Henker J, Lösel A, Conrad K, Hirsch T, Leupold W. [Prevalence of asymptomatic coeliac disease in children and adults in the Dresden region of Germany] Dtsch Med Wochenschr. 2002;127:1511–1515. doi: 10.1055/s-2002-32757. [DOI] [PubMed] [Google Scholar]
- 7.Kang JY, Kang AH, Green A, Gwee KA, Ho KY. Systematic review: worldwide variation in the frequency of coeliac disease and changes over time. Aliment Pharmacol Ther. 2013;38:226–245. doi: 10.1111/apt.12373. [DOI] [PubMed] [Google Scholar]
- 8.Reilly NR, Green PH. Epidemiology and clinical presentations of celiac disease. Semin Immunopathol. 2012;34:473–478. doi: 10.1007/s00281-012-0311-2. [DOI] [PubMed] [Google Scholar]
- 9.Mustalahti K, Catassi C, Reunanen A, et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med. 2010;42:587–595. doi: 10.3109/07853890.2010.505931. [DOI] [PubMed] [Google Scholar]
- 10.Ludvigsson JF, Rubio-Tapia A, VAN Dyke CT, et al. Increasing incidence of celiac disease in a North American population. Am J Gastroenterol. 2013;108:818–824. doi: 10.1038/ajg.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metzger MH, Heier M, Mäki M, et al. Mortality excess in individuals with elevated IgA anti-transglutaminase antibodies: the KORA/MONICA Augsburg cohort study 1989-98. Eur J Epidemiol. 2006;21:359–365. doi: 10.1007/s10654-006-9002-4. [DOI] [PubMed] [Google Scholar]
- 12.Ludvigsson JF, Montgomery SM, Ekbom A, Brandt L, Granath F. Small-intestinal histopathology and mortality risk in celiac disease. JAMA. 2009;302:1171–1178. doi: 10.1001/jama.2009.1320. [DOI] [PubMed] [Google Scholar]
- 13.Lebwohl B, Stephansson O, Green PH, Ludvigsson JF. Mucosal healing in patients with celiac disease and outcomes of pregnancy: a nationwide population-based study. Clin Gastroenterol Hepatol. 2014 Nov 21; doi: 10.1016/j.cgh.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner J, Pellerin G, Mager D. Prevalence of metabolic bone disease in children with celiac disease is independent of symptoms at diagnosis. J Pediatr Gastroenterol Nutr. 2009;49:589–593. doi: 10.1097/MPG.0b013e31819ca18e. [DOI] [PubMed] [Google Scholar]
- 16.Ludvigsson JF, Michaelsson K, Ekbom A, Montgomery SM. Coeliac disease and the risk of fractures—a general population-based cohort study. Aliment Pharmacol Ther. 2007;25:273–285. doi: 10.1111/j.1365-2036.2006.03203.x. [DOI] [PubMed] [Google Scholar]
- 17.Ludvigsson JF, Montgomery SM, Ekbom A. Celiac disease and risk of adverse fetal outcome: a population-based cohort study. Gastroenterology. 2005;129:454–463. doi: 10.1016/j.gastro.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 18.Ventura A, Magazzu G, Greco L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP study group for autoimmune disorders in celiac disease. Gastroenterology. 1999;117:297–303. doi: 10.1053/gast.1999.0029900297. [DOI] [PubMed] [Google Scholar]
- 19.Jansen MA, Kiefte-de Jong JC, Gaillard R, et al. Growth trajectories and bone mineral density in anti-tissue transglutaminase antibody-positive children: The Generation R Study. Clin Gastroenterol Hepatol. 2015;13:913–920. doi: 10.1016/j.cgh.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Pulido O, Zarkadas M, Dubois S, et al. Clinical features and symptom recovery on a gluten-free diet in Canadian adults with celiac disease. Can J Gastroenterol. 2013;27:449–453. doi: 10.1155/2013/741740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Sabatino, Brunetti L, Carnevale MG, Giuffrida P, Corazza GR. Is it worth investigating splenic function in patients with celiac disease? World J Gastroenterol. 2013;19:2313–2318. doi: 10.3748/wjg.v19.i15.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans KE, Leeds JS, Morley S, Sanders DS. Pancreatic insufficiency in adult celiac disease: do patients require long-term enzyme supplementation? Dig Dis Sci. 2010;55:2999–3004. doi: 10.1007/s10620-010-1261-y. [DOI] [PubMed] [Google Scholar]
- 23.Kurth BM, Kamtsiuris P, Hölling H, et al. The challenge of comprehensively mapping children’s health in a nation-wide health survey: design of the German KiGGS-Study. BMC Public Health. 2008;8 doi: 10.1186/1471-2458-8-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hölling H, Kamtsiuris P, Lange M, Thierfelder W, Thamm M, Schlack R. [The German Health Interview and Examination Survey for Children and Adolescents (KiGGS): study management and conduct of fieldwork] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2007;50:557–566. doi: 10.1007/s00103-007-0216-8. [DOI] [PubMed] [Google Scholar]
- 25.Thierfelder W, Dortschy R, Hintzpeter B, Kahl H, Scheidt-Nave C. [Biochemical measures in the German Health Interview and Examination Survey for Children and Adolescents (KiGGS)] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2007;50:757–770. doi: 10.1007/s00103-007-0238-2. [DOI] [PubMed] [Google Scholar]
- 26.Robert Koch-Institut (eds.) Beiträge zur Gesundheitsberichterstattung des Bundes. Berlin: Robert Koch-Institut; 2009. Bevölkerungsbezogene Verteilungswerte ausgewählter Laborparameter aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS) [Google Scholar]
- 27.Kohse KP, Thamm M. KiGGS-the German survey on children’s health as data base for reference intervals. Clin Biochem. 2011;44 doi: 10.1016/j.clinbiochem.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Dahlbom I, Korponay-Szabo IR, Kovacs JB, Szalai Z, Mäki M, Hansson T. Prediction of clinical and mucosal severity of coeliac disease and dermatitis herpetiformis by quantification of IgA/IgG serum antibodies to tissue transglutaminase. J Pediatr Gastroenterol Nutr. 2010;50:140–146. doi: 10.1097/MPG.0b013e3181a81384. [DOI] [PubMed] [Google Scholar]
- 29.Webb C, Norstrom F, Myleus A, et al. Celiac disease can be predicted by high levels of anti-tissue transglutaminase antibodies in population-based screening. J Pediatr Gastroenterol Nutr. 2015;60:787–791. doi: 10.1097/MPG.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 30.Robert Koch-Institut (eds.) Beiträge zur Gesundheitsberichterstattung des Bundes. 2nd revised edition. Berlin: Robert Koch-Institut; 2013. Referenzperzentile für anthropometrische Maßzahlen und Blutdruck aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS) [Google Scholar]
- 31.Liu E, Lee HS, Aronsson CA, et al. Risk of pediatric celiac disease according to HLA haplotype and country. N Engl J Med. 2014;371:42–49. doi: 10.1056/NEJMoa1313977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lionetti E, Castellaneta S, Francavilla R, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med. 2014;2(371):1295–1303. doi: 10.1056/NEJMoa1400697. [DOI] [PubMed] [Google Scholar]
- 33.Catassi C, Anderson RP, Hill ID, et al. World perspective on celiac disease. J Pediatr Gastroenterol Nutr. 2012;55:494–499. doi: 10.1097/MPG.0b013e318272adf4. [DOI] [PubMed] [Google Scholar]
- 34.Ivarsson A, Myleus A, Norstrom F, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics. 2013;131:e687–e694. doi: 10.1542/peds.2012-1015. [DOI] [PubMed] [Google Scholar]
- 35.Bingley PJ, Williams AJ, Norcross AJ, et al. Undiagnosed coeliac disease at age seven: population based prospective birth cohort study. BMJ. 2004;328:322–323. doi: 10.1136/bmj.328.7435.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Pals M, Ivarsson A, Nordstrom F, Högberg L, Svensson J, Carlsson A. Prevalence of thyroid autoimmunity in children with celiac disease compared to healthy 12-year olds. Autoimmune Dis 2014, Article ID 417356, 6 pages. doi: 10.1155/2014/417356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassio A, Ricci G, Baronio F, et al. Long-term clinical significance of thyroid autoimmunity in children with celiac disease. J Pediatr. 2010;156:292–295. doi: 10.1016/j.jpeds.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 38.Kurppa K, Paavola A, Collin P, et al. Benefits of a gluten-free diet for asymptomatic patients with serologic markers of celiac disease. Gastroenterology. 2014;147:610–617. doi: 10.1053/j.gastro.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Ludvigsson JF, Card TR, Kaukinen K, et al. Screening for celiac disease in the general population and in high-risk groups. United European Gastroenterol J. 2015;3:106–120. doi: 10.1177/2050640614561668. [DOI] [PMC free article] [PubMed] [Google Scholar]