Abstract
Background
25% of all women report involuntary loss of urine, and 7% may require treatment.
Method
This review is based on a selection of pertinent literature, including guidelines and Cochrane reviews.
Results
The assessment of pelvic floor dysfunction in women begins with a basic evaluation that is followed by special diagnostic tests if indicated. The physician taking the clinical history should inquire about the patient’s behavior, personality, social and other stressors, and eating and drinking habits, as well as any mental disorders that may be present, including anxiety disorders, depression, somatization disorders, and disorders of adaptation. Conservative treatment consists mainly of lifestyle changes, physiotherapy, and medication. Stress incontinence is most commonly treated with pelvic floor exercises, with a documented success rate of 56.1% vs. 6% without such treatment (relative risk 8.38, 95% confidence interval 3.67–19.07). If incontinence persists, surgery may be indicated (implantation of suburethral tension-free slings, or colposuspension). Feedback and biofeedback training can be used to treat an overactive bladder. If these techniques and drug therapy are unsuccessful, botulinum toxin injections can be considered.
Conclusion
Well-validated treatments for pelvic floor dysfunction are available. Psychosomatic factors must be taken into account and can have a major effect on treatment outcomes.
The female pelvic floor serves multiple functions: pleasure and sexuality, parturition, urination and urinary continence, defecation and fecal continence, and keeping the pelvic organs in position. To do all these things, the pelvic floor needs an intact anatomical structure, consisting of muscle, connective tissue, an nerves. Moreover, its function is subject to control by the central nervous system. Pelvic floor function and continence can thus be impaired not only by direct anatomical injury (as in vaginal delivery), but also by dysfunctional neural control, e.g., in neurologic disease, diabetic neuropathy, and cognitive disorders.
The main types of female pelvic floor dysfunction can manifest themselves not only as urinary and/or fecal incontinence, but also as prolapse of the female reproductive organs. The percentage of women suffering from pelvic floor dysfunction ranges from 30% to 50%, depending on the definition (1). According to the Robert Koch Institute’s health report on urinary incontinence (issued in 2007), the population at large harbors many misconceptions and prejudices about pelvic floor dysfunction—mainly about urinary incontinence—that stand in the way of appropriate care and effective prevention (1). Many people believe that incontinence is a normal part of aging, and that its treatment is therefore both unnecessary and doomed to failure (e1).
Pelvic floor dysfunction.
The main types of female pelvic floor dysfunction can manifest themselves not only as urinary and/or fecal incontinence, but also as prolapse of the female reproductive organs.
As the population ages, pelvic floor dysfunction, and particularly fecal and urinary incontinence, will become more prevalent. The SHELTER study determined that 73.5% of 4156 residents of 57 old-age homes in 6 different European countries (including Germany) suffered from urinary incontinence (2). Saga et al. found that, in Norway, only 25% of old-age home residents are fully continent, 72% are incontinent of urine, 42.8% are incontinent of stool, and 40.2% are incontinent of both (e2). These figures imply that the diagnosis and treatment of incontinence is an important matter not just for the specialist, but for the general practitioner as well.
Learning objectives
Readers of this article will gain practical knowledge of the following topics:
the causes and effects of pelvic floor dysfunction
the available treatments, both conservative and surgical, along with their indications and expected outcomes
the psychosomatic aspects that should be considered in history-taking and treatment planning.
The epidemiology and pathophysiology of urinary incontinence and prolapse
In 2002, the International Continence Society (ICS) defined urinary incontinence as “any kind of involuntary loss of urine” (3), without any consideration of the severity of the problem or the degree of suffering that it causes for the patient. In the Norwegian EPINCONT study, one of the largest epidemiologic studies ever performed, 28 000 women were asked about urinary incontinence with questions that addressed both the severity of the problem and the difficulties that it caused in everyday life (4). Incontinence was considered significant when it was severe or of intermediate severity and led to moderate or severe impairment. The severity of impairment due to incontinence was rated on the validated Severity Index of Sandvik et al. (5), which incorporates both the frequency of incontinence and its extent (Box 1).
Box 1. Severity Index for urinary incontinence*.
-
Question 1: How frequent are the episodes of urine loss?
-
Question 2: How much urine is lost each time?
-
The Severity Index is the product of the answers to Questions 1 and 2.
1–2: mild urinary incontinence
3–4: moderate urinary incontinence
6–8: severe urinary incontinence
*modified from Sandvik, 1993 (5)
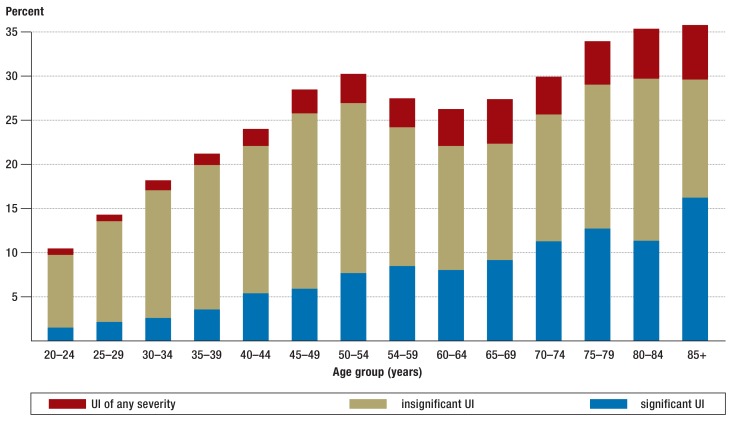
25% of the women surveyed in the EPINCONT study said they had experienced involuntary loss of urine. About 7% had significant incontinence and were thus potential candidates for treatment. The frequency of urinary incontinence of any severity, and of significant urinary incontinence, increased with age (4) (Figure 1).
Figure 1.
Figure 1: The prevalence and significance of urinary incontinence (1). Figure reproduced with the kind permission of the Robert Koch Institute. UI, urinary incontinence.
Stress incontinence.
In stress incontinence, an elevation of abdominal pressure of any cause, e.g., coughing, sneezing, jumping, or walking, can lead to loss of urine.
The commonest type of female urinary incontinence is stress incontinence (ICD-10: N 39.3), in which an elevation of abdominal pressure of any cause, e.g., coughing, sneezing, jumping, or walking, can lead to loss of urine. The overactive bladder (OAB) syndrome, formerly known as urge incontinence, comprises urinary frequency, urinary urgency (a sudden urge to urinate that is hard to suppress), and nocturia, with or without involuntary loss of urine (ICD-10: N 39.42) (3). It can be caused by an increase in the neural impulses that induce urination, a lack of central nervous inhibition, or pathological changes of the bladder wall. Mixed incontinence is a combination of stress incontinence and the overactive bladder syndrome. The different types of urinary incontinence are variably frequent with age: mixed incontinence and the overactive bladder syndrome are more common in older women (e3), stress incontinence in younger women (e4).
From the psychosomatic point of view, micturition is a hierarchically structured neurophysiological regulatory circuit with a conscious motor component that can be influenced by cognition, along with unconscious autonomic components. It is a socially regulated, emotionally charged type of learned behavior that occurs in the individual’s intimate sphere and is associated with multiple affects, including embarrassment, pleasure, tension, and relief (6).
Prevalence.
25% of the women surveyed in the EPINCONT study said they had experienced involuntary loss of urine.
Mental disorders (depression, anxiety, hypochondriasis) are more common in women with bladder dysfunction than in other women. Sexual dysfunction is also more common in women with bladder dysfunction (7– 10).
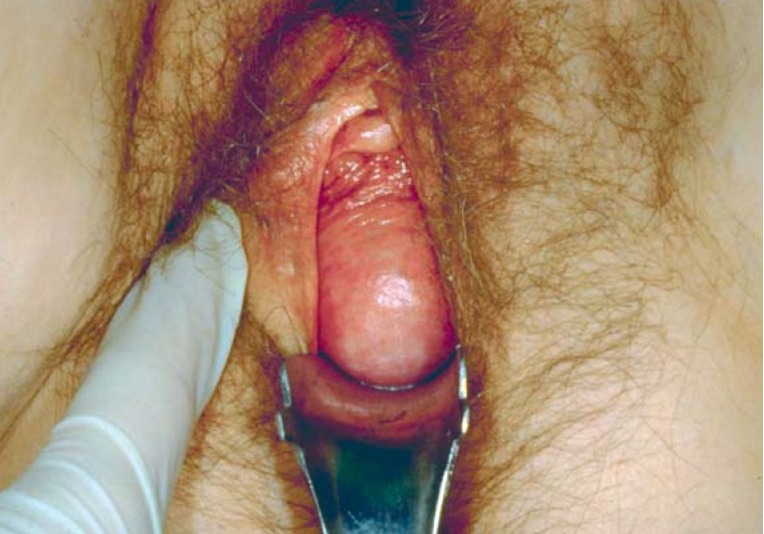
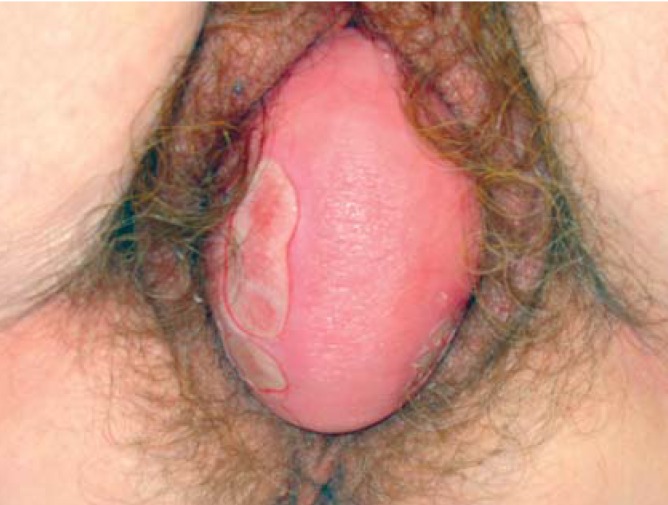
Prolapse of the female reproductive organs (ICD-10: N 81.-) is a lower than normal position of the uterus or vagina. (Figures 2, 3).
Figure 2.
Prolapse of the anterior vaginal wall down to the hymenal edge (grade II) (photograph reproduced with the kind permission of Prof. John DeLancey, Ann Arbor, USA).
Figure 3.
Total prolapse of the vagina after hysterectomy, with ulcerations of the vaginal mucosa (Grade IV).
Female reproductive-organ prolapse is often graded as follows:
grade I: descent within the vagina
grade II: descent to the level of the introitus
grade III: descent beyond the introitus
grade IV: total prolapse
Associated illnesses.
Mental disorders (depression, anxiety, hypochondriasis) are more common in women with bladder dysfunction than in other women. Sexual dysfunction is also more common in women with bladder dysfunction.
In a Swedish study, prolapse of the reproductive organs was found in 31% of women aged 20 to 59 (e5). Hendrix et al. (e6) estimated its prevalence among women aged 50 to 79 at 41%. The lifetime risk of undergoing surgery for prolapse or incontinence is 11% to 19%, and that of repeated surgery after a first procedure is 29%, according to Olsen et al. (e7– e9).
Prolapse of the reproductive organs.
In a Swedish study, prolapse of the reproductive organs was found in 31% of women aged 20 to 59.
Pregnancy and delivery are an important risk factor for urinary incontinence and prolapse of the reproductive organs. In the Norwegian EPICONT study, data on parity and urinary incontinence were obtained from 15 307 women under age 65. The prevalence of urinary incontinence of any type was 10.1% for nulliparous women, 15.9% after Cesarean section, and 21.0% after vaginal delivery. Compared to nulliparous women, women who had undergone Cesarean section had an adjusted odds ratio of 1.5 for any type of urinary incontinence (95% confidence interval [CI], 1.2–1.9) and an adjusted odds ratio of 1.4 for moderate or severe incontinence (95% CI 1.0–2.1) (11, e10).
In the Swedish SWEPOP study, 5236 women who had given birth to only one child, either vaginally or by Cesarean section, were surveyed 20 years later with a validated questionnaire about symptoms of prolapse and urinary incontinence. The prevalence of a symptomatic prolapse was twice as high after vaginal delivery as after Cesarean section (14.6% vs. 6.3%, odds ratio 2.55, 95% CI 1.98–3.28) (12).
History
The history should focus on the nature and extent of symptoms and on the degree to which they impair sexuality and cause suffering. The patient’s gynecological and obstetrical history, relevant accompanying illnesses, medications, earlier treatments, and current treatment goals should also be documented (13, e11). Medical and neurological conditions—e.g., overweight, diabetes mellitus, multiple sclerosis, and other neurological conditions such as trauma, Parkinson’s disease, and lumbar spinal disorders—can also affect micturition, as can medications including anticholinergic agents, calcium antagonists, antidepressants, and diuretics (e11).
From a psychosomatic point of view, attention must be paid to the following: the patient’s biographical history, social stressors, eating and drinking behavior, anxiety disorders, depression, somatization disorders, and disorders of adaptation (6). A history of having been a victim of violence should also be asked about. A study by the authors of this review revealed that women with an overactive bladder were more often victims of sexual and physical violence than control patients or women with stress incontinence (30.6% [26/85] vs. 17.5% [10/57] and 17.8% [18/101], respectively) (14).
Diagnostic evaluation
The process of diagnostic evaluation is comprehensively summarized in Box 2.
Box 2. The evaluation of pelvic floor dysfunction.
-
Basic evaluation
carried out by a general practitioner, gynecologist, or urologist; suffices to establish the indication for initial conservative treatment, with antibiotic treatment of urinary tract infections (if necessary), pelvic floor exercises, behavior modification, or anticholinergic medication
history, including basic psychosomatic questions
urinalysis to rule out infection
determination of post-void residual volume
micturition diary
-
Extended basic evaluation
carried out by a gynecologist or urologist (with the aid of a psychiatrist and/or specialist in psychosomatic medicine, if indicated); suffices to establish the indication for a further trial of conservative treatment
vaginal inspection (prolapse, local hormone deficiency, contractility of pelvic floor)
perineal/introital ultrasonography (bladder neck mobility, bladder neck funneling, prolapse)
clinical stress test (urine loss upon coughing while standing)
Urinary pad test
-
Special tests
carried out by a specialist (perhaps at a specialized continence and pelvic floor center); necessary to establish the indication for a surgical procedure when conservative treatment has failed
urodynamic testing
cystoscopy
spezialized perineal/introital ultrasonography
endoanal intrasonography, if indicated
dynamic magnetic resonance defecography, if indicated
Symptomatic prolapse.
In the Swedish SWEPOP study, the prevalence of a symptomatic prolapse was found to be twice as high after vaginal delivery as after Cesarean section (14.6% vs. 6.3%, odds ratio 2.55, 95% CI 1.98–3.28).
Basic diagnostic evaluation
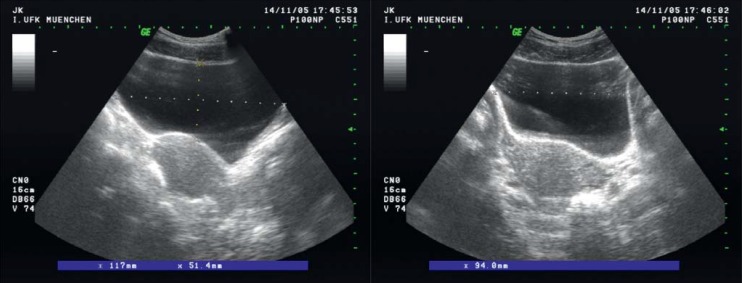
First, a urinary tract infection should be excluded by urinalysis (13). A gynecologic examination is performed for the assessment of prolapse of the reproductive organs, genital atrophy, and the strength of levator contraction (e12). Stress incontinence can be demonstrated by a cough test with a full bladder. Post-void residual urine measurement with ultrasound or a single-use catheter provides information about bladder emptying (Figure 4). The frequency and volume of micturition can be recorded in a micturition diary (15): the patient measures the amount of urine voided each time and records it in the diary along with the time of voiding.
Figure 4.
Ultrasonic imaging to determine urinary residual volume: abdominal image in three planes.
Perineal or introital ultrasound can be used to assess the mobility of the bladder neck when the patient presses or coughs (hypermobility of the urethra, funnel formation), to detect vesical and urethral diverticula, to visualize elevation of the bladder neck on contraction of the levator ani muscle (pelvic floor exercise), and to clarify the extent of prolapse of the anterior vaginal wall. It can also be used to visualize avulsions of the levator ani muscle and to monitor the condition of suburethral artificial slings and meshes (16, e7, e10, e13– e17).
Special diagnostic tests
The basic diagnostic evaluation suffices to establish an indication for conservative treatment. If conservative treatment fails or surgery is planned, further diagnostic tests must be performed.
History.
The history should focus on the nature and extent of symptoms and on the degree to which they impair sexuality and cause suffering.
In a urodynamic study, the filling behavior of the bladder is evaluated (cystometry), the closing function of the urethra is measured (profilometry), and micturition is evaluated (uroflow, mictiometry). Urodynamic studies can reveal a relevant component of urinary urgency, if present, and can be used to study complex functional disorders (17).
Video urodynamic studies are mainly useful for the demonstration of neurogenic bladder dysfunction. In such studies, the bladder and urethra are depicted on video imaging while urodynamic testing is carried out simultaneously. If an overactive bladder disorder has not responded to conservative treatment, cystoscopy should be performed (18).
Questionnaires
Validated questionnaires are preferable to (merely) standardized questionnaires for the documentation of the severity of the disturbance, its effect on the quality of life, and improvement or worsening after treatment. Self-administered questionnaires are now the international gold standard (e18). Questionnaires are mainly used in clinical studies; for more information, see the eBox (e19– e21).
Psychosomatic considerations.
Women with urinary incontinence suffer from depression more commonly than continent women. Anxiety disorders and a general fear of illness are also associated with incontinence.
Treatment
Numerous studies have been published on the conservative and surgical treatment of stress incontinence, overactive bladder syndrome, and prolapse of the female reproductive organs. These studies have also been discussed in multiple meta-analyses and Cochrane reviews (19– 28).
Psychosomatic considerations for treatment
A Canadian survey showed that 18- to 44-year-old women with urinary incontinence suffered from depression more commonly than continent controls (30% vs. 9.2%) (29). Anxiety disorders and a general fear of illness were also associated with incontinence (30). These findings underscore the importance of the psychosomatic approach. Psychosomatic treatment generally incorporates elements of cognitive behavioral therapy, even though their efficacy against incontinence is not yet supported by any high-level evidence. In behavior therapy, the patient is helped to develop a basic understanding of the problem from which she suffers. Elementary knowledge of the anatomy and physiology of micturition is imparted, with illustrations and explanations of the process of voiding and the disturbances that can affect it. Therapeutic steps can include keeping a micturition diary and analyzing the situations most commonly associated with incontinence in the patient’s particular case. In a further step, the patient can be motivated to undergo micturition training. Physical exercises, such as pelvic-floor exercises and biofeedback, can be helpful in this regard.
The treatment of stress incontinence
Conservative treatment – Conservative treatment consists of lifestyle changes, such as the following:
a change in drinking behavior (mainly abstention from bladder-irritating drinks such as coffee, tea, alcohol, and carbonated beverages, and an even distribution of fluid intake over the course of the day)
smoking cessation
topical estrogen treatment of the vagina in case of tissue atrophy.
Intravaginal support devices (e.g., the Arabin urethral pessary or incontinence tampons) can reinforce the urethrovesical junction and help prevent incontinence (e23, e24).
The treatment of stress incontinence.
Weight loss, physiotherapy, and the use of special urethral pessaries are examples of conservative treatment.
Duloxetine is the sole drug used in the treatment of stress incontinence. Two systematic reviews concluded that duloxetine, in a daily dose of 80 mg, does not eliminate incontinence but does lessen the frequency of episodes of both stress and urge incontinence. Because severe nausea is a frequent side effect at the beginning of treatment, this drug should be introduced with a slow escalation to the final dose (e25, e26).
Pelvic floor exercises – Pelvic floor exercises are intended to increase the strength of contraction and to improve coordination. Increased muscle tone significantly lessens the mobility of the bladder neck when the patient coughs. Because of the wide variation in training methods and outcome criteria, it is hard to make any reliable estimate of the success rate of pelvic floor exercises to treat incontinence, yet their efficacy in general has been confirmed in a Cochrane analysis by Dumoulin et al. (20). In this meta-analysis of four trials involving a total of 165 patients (20), there was a cure rate of 56.1% in those who performed pelvic floor exercises, and only 6% in those who did not (relative risk [RR] 8.38, 95% CI 3.68–19.07).
Pelvic floor exercises: evidence for efficacy.
The wide variation in training methods and outcome criteria makes the success rate of pelvic floor exercises for incontinence hard to estimate, yet their general efficacy has been confirmed in a Cochrane analysis by Dumoulin et al.
In multiple meta-analyses, women with stress incontinence or other types of urinary incontinence who underwent treatment with pelvic floor exercises had significantly better outcomes with respect to their quality of life, satisfaction with treatment, and need for further treatment (20) compared with control groups. Bø (24) also reported grade 1, level A evidence for the efficacy of physiotherapy.
Surgery – According to the AWMF guideline for stress incontinence, surgery should be considered only after pelvic floor exercises have been tried without success (13). The principal type of surgical intervention is the implantation of a suburethral, tension-free sling (TVT, “tension-free vaginal tape”). Single-incision slings and postoperatively adjustable systems are now available (e27, e28).
The potential complications include (e29):
bladder perforation (2–5%)
persistent bladder emptying dysfunction (5–10%)
de novo urge incontinence (5–20%)
bleeding/hematoma (1–2%)
erosion of the tape into the urethra, bladder, or vagina (2–10%); nerve injury (< 1%).
Surgery.
According to the AWMF guideline for stress incontinence, surgery should be considered only after pelvic floor exercises have been tried without success.
The objective cure rate after suburethral band implantation is 85–90% at one year or later, with a somewhat lower subjective cure rate (77%). 17-year follow-up data for the TVT operation are now available, with very good results: in 71 patients, the subjective rate of cure or improvement was 87% (32, e30). Colposuspension is an alternative surgical procedure in which the paraurethral vaginal facial tissue is anchored to Cooper’s ligaments via an open or laparascopic transabdominal approach. Potential complications include bladder emptying dysfunction (5–25%) and recto-/enterocele (5–20%) (33).
Conservative treatment of overactive bladder.
Behavior-therapeutic measures include micturition and toilet training (i.e., optimal timing of voiding), perhaps supplemented by pelvic floor exercises in group or individual sessions, or intensified by electrostimulation and biofeedback.
Studies with long-term follow-up for up to five years have not revealed any difference in the efficacy of colposuspension versus sling procedures (e31, e32). Open colposuspension and autologous fascial slings were found to be equally effective at five years (level of evidence [LOE] 1b) (13). Moreover, laparoscopic and open colposuspension were found to be equally effective at 2 years (LOE 1a), but the laparoscopic procedure was associated with less postoperative pain and faster recovery (LOE 1a) (13).
A further surgical method involves the injection of so-called bulking agents into the submucosa of the proximal or middle portion of the urethra or into the external urethral sphincter. The materials used include autologous fat, collagen, silicone, dextranomer + hyaluronic acid, polyacrylamide, and others. This intervention can bring about short- or intermediate-term symptomatic improvement; its long-term results have not been reported (22). Inadequate evidence for it was found in a Cochrane review (34). Serious complications occasionally arise when autologous fat is used.
Treatment of the overactive bladder syndrome
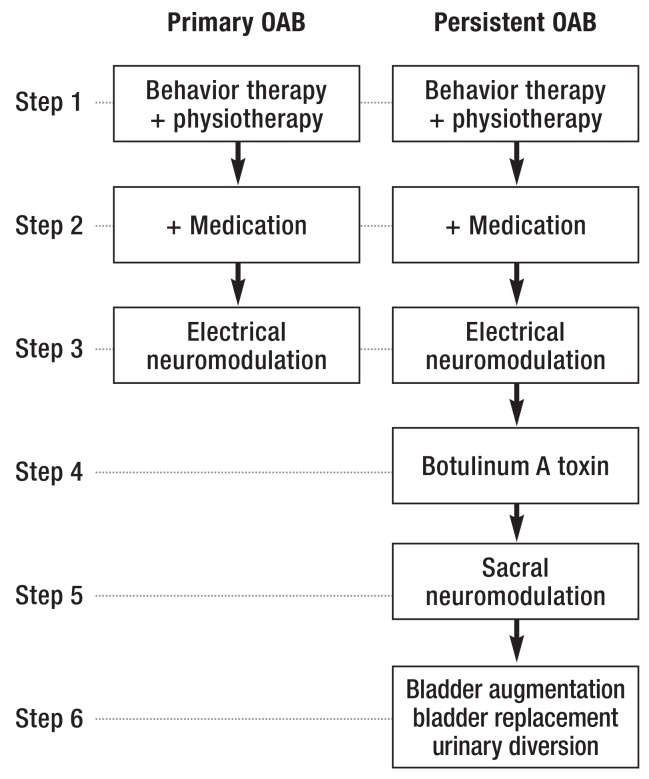
The goals of treating the overactive bladder syndrome are to increase the stable storage volume of the bladder, prolong the interval between one voiding and the next, and bring about secure continence and social reintegration. An incremental treatment algorithm is recommended in the AWMF guideline (18) (Figure 5).
Figure 5.
Stepwise treatment algorithm for urge incontinence. OAB, overactive bladder syndrome.
(With the kind permission of the Overactive Bladder Guideline Comittee of the German Society of Gynecology and Obstetrics [Deutsche Gesellschaft für Gynäkologie und Geburtshilfe]) (18]).
Conservative treatment—Behavior-therapeutic measures include micturition and toilet training (i.e., optimal timing of voiding), perhaps supplemented by pelvic floor exercises in group or individual sessions, or intensified by electrostimulation and biofeedback. The efficacy of feedback and biofeedback was confirmed in a meta-analysis of 17 studies, in which pelvic floor exercises alone were used as a control (RR 0.75, 95% CI 0.66–0.86) (35). The utility of physiotherapy was documented in a Cochrane analysis (20).
The electrostimulation (neurostimulation) of afferent nerves, e.g., the pudendal nerve or the posterior tibial nerve, can improve treatment outcomes (e33).
Electrostimulation.
The electrostimulation (neurostimulation) of afferent nerves, e.g., the pudendal nerve or the posterior tibial nerve, can improve treatment outcomes.
Drugs for the treatment of the overactive bladder syndrome include topical estrogen and, above all, anticholinergic and antimuscarinic agents. The drugs approved for use in Germany include darifenacin, fesoterodine, oxybutynine, propiverine, solifenacin, tolterodine, and trospium chloride; their utility has been documented in multiple randomized trials (36, e34). Anticholinergic use significantly lowers the frequency of urination and of incontinence episodes. The main side effects—dry mouth, constipation, and visual disturbances—adversely affect long-term compliance: 24 months after the initial prescription, only 6–12% patients were still taking the drug (e35).
Surgery—In 2013, on the basis of multiple randomized, controlled trials, botulinum toxin injection was approved for the treatment of idiopathic overactive bladder syndrome in case of non-response or intolerance to treatment with anticholinergic agents (37). A dilute solution containing 100 I/U or more of botulinum toxin (depending on the manufacturer) is injected into the detrusor muscle at 20 different sites, under cystoscopic guidance. In a randomized trial on 557 subjects, botulinum toxin injections lowered the number of urge incontinence episodes by 51% (e36), and 27% of the treated subjects achieved continence. The effect wears off in approximately six months, at which time the injection must be repeated. Temporary bladder emptying dysfunction may arise (e37).
Sacral neuromodulation is a technique in which electrical stimulation is delivered via electrodes implanted in the S2–S4 neuroforamina on one or both sides in order to suppress detrusor hyperactivity. After test stimulation, a permanent impulse generator (pacemaker) is implanted (38). Surgical bladder augmentation, bladder replacement, and urinary diversion should be considered treatments of last resort.
Treatment of prolapse of the female reproductive organs
Conservative treatment—Conservative treatments include clinical observation, reduction of known risk factors such as obesity, smoking, and chronic constipation, and targeted pelvic-floor exercises. Four randomized trials of pelvic floor exercises for prolapse were evaluated in a Cochrane analysis in 2011: the probability of improvement of prolapse by at least one stage was 17% higher after pelvic floor exercises than after management in a control group (25). Topical estrogen is an established treatment of irritative symptoms, as well as an essential accompaniment to treatment with a pessary to prevent vaginal ulceration (e38). There have only been a few trials of treatment with a pessary (26); Observational studies show that one can be successfully fitted in 50–73% of patients (39, e39). A large-scale, long-term randomized trial comparing pessary treatment to pelvic floor exercises was begun in the Netherlands in 2009 and has not yet been completed (e40).
Botulinum toxin.
Botulinum toxin injection Is approved for the treatment of idiopathic overactive bladder syndrome in case of non-response or intolerance to treatment with anticholinergic agents.
Surgery—An appropriate operation should be planned in consideration of the patient’s age, risk factors such as obesity and hard physical labor, and the patient’s wishes with respect to keeping the uterus versus undergoing a hysterectomy.
The pelvic floor should be considered as a functional unit, rather than an assemblage of multiple compartments, because it is rare for a defect to involve only a single compartment (anterior, middle, or posterior). All defects must be diagnosed preoperatively; incomplete repair elevates the risk of an unsatisfactory outcome, possibly necessitating further surgery. We will briefly describe the surgical interventions that can be performed on the middle, anterior, and posterior compartments.
The uterus, if present, must be either fixed (e.g., to the sacrospinal ligament or the sacrum) or removed (hysterectomy) with fixation of the vaginal vault. Open abdominal or laparoscopic sacrocolpopexy for fixation of the vaginal vault has success rates of 78–100%, with a reoperation rate of 4.4% for prolapse (e41). Transvaginal sacrospinal fixation has similarly good results, with success rates of 79–97% (e42). It was concluded in a Cochrane meta-analysis, however, that sacrocolpopexy is more effective than sacrospinal fixation in direct comparisons (28).
Correction of pelvic organ prolapse at the anterior vaginal wall with autologous tissue was found to have success rates of 30–100% (cumulative success rate: 63%) in randomized trials with at least 12 months of follow-up. If surgery is simultaneously performed to support the apical (middle) compartment, the risk of recurrence is significantly lower (RR 0.7, 95% CI 0.6–0.81) (28, 40).
The reported success rates of mesh implantation in the anterior vaginal wall are highly inconsistent. After the U. S. Food and Drug Administration (FDA), in 2011, issued a warning against the use of meshes in pelvic floor surgery (except in clinical trials), a number of systems that had been studied in randomized trials were removed from the market. A meta-analysis of the randomized trials revealed that, without the additional implantation of a mesh, the risk of recurrence rises by a factor of three (RR 3.5, 95% CI 2.7–4.4). The success rate of anterior colporrhaphy was 52% without mesh augmentation, and 86% with it (p < 0.001) (28).
Conservative treatment of prolapse.
Conservative treatments include reduction of known risk factors such as obesity, smoking, and chronic constipation, targeted pelvic-floor exercises, and the use of a pessary.
In a prospective observational study with at least 12 months of follow-up per patient, posterior colporrhaphy with the use of autologous tissue for midline fascial tightening (without mesh implantation) had success rates of 82–93% (cumulative success rate, 86%) (e43). This is thus a good option for initial surgery. The use of meshes in the posterior compartment is not yet supported by any data from randomized trials (28, 40).
Surgical correction of prolapse.
Correction of pelvic organ prolapse at the anterior vaginal wall with autologous tissue was found to have success rates of 30–100% in randomized trials with at least 12 months of follow-up.
Further Information on CME.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education. Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire. See the following website: cme.aerzteblatt.de.
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
This CME unit can be accessed until 8 November 2015, and earlier CME units until the dates indicated:
–“Mental Disorders in Early Childhood” (Issue 21/2015) until 16 August 2015,
–“Animal and Human Bite Wounds” (Issue 25/2015) until 13 September 2015,
–“Transfusion of Packed Red Cells: Indications, Triggers and Adverse Events” (Issue 29-30/2015) until 11 October 2015.
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What are the main symptoms of female pelvic floor dysfunction?
urinary and fecal incontinence and prolapse of the reproductive organs
nocturia, a pulling sensation in the pelvis, and anorgasmia
flatulence, micturition disturbance, and cystitis
weak urinary stream, recurrent urinary tract infections, and rectal prolapse
dyspareunia, colpitis, and vulvar atrophy
Question 2
What is a typical symptom of overactive bladder syndrome?
incontinence on jumping
urethral stricture
alguria
nocturia
dysuria
Question 3
A 38-year-old woman has been suffering from an overactive bladder for the past 4 years. Behavior therapy, physiotherapy combined with medication, and electrical neuromodulation have all been tried without any lasting benefit.
According to the treatment algorithm, what is the appropriate next step?
Jacobsen relaxation training
keeping a micturition diary
hypnosis
botulinum A toxin treatment
bladder replacement
Question 4
According to the SHELTER study, what percentage of residents of old age homes suffer from urinary incontinence?
15%
30%
75%
90%
100%
Question 5
How many of the 28 000 women surveyed in the EPINCONT study reported the involuntary loss of urine?
15%
20%
25%
30%
35%
Question 6
A woman’s lifetime risk of undergoing surgery for incontinence or prolapse of the pelvic organs is 11–19%. According to Olsen et al., what is the risk of reoperation after initial surgery?
29%
35%
41%
47%
53%
Question 7
Which of the following is part of the basic diagnostic evaluation of pelvic floor dysfunction?
colposcopy
urodynamic testing
cystoscopy
endoanal ultrasonography
measurement of post-void residual volume
Question 8
A 49-year-old mother of three has complained ever since her second delivery of involuntary urine loss upon coughing or sneezing. What should be the next step in the management of her problem?
urogynecological assessment followed by a trial of conservative treatment
surgery, because early surgery offers the best chance of cure
no treatment, because this is a normal, early symptom of menopause
explanation to the patient that she needs surgery for prolapse of the pelvic organs
prescription of an anticholinergic drug to treat stress incontinence
Question 9
What disease is often associated with urinary incontinence?
pneumonia
angina pectoris
depression
herpes zoster
rubella
Question 10
Which of the following is a major risk factor for urinary incontinence and prolapse of the female reproductive organs?
high-performance sports
vaginal delivery
menopause
chronic cystitis
pyelonephritis
eBox. Validated questionnaires for assessing the severity of urinary incontinence, its effect on the quality of life, and its improvement or worsening after treatment.
The questionnaires of the International Consultation on Incontinence (ICI) assess vaginal symptoms, symptoms of the lower urinary tract, urinary incontinence, sexuality, nocturia, and overactive bladder syndrome (www.iciq.net) (e18).
The German version of the King’s Health Questionnaire assesses urinary incontinence symptoms and the quality of life (e19).
The Australian Pelvic Floor Questionnaire (also available in a German version) assesses symptoms relating to the bladder, bowel, prolapse, and pelvic organ prolapse, as well as the quality of life. It was especially designed and validated for patients with urogenital problems. It also has a validated follow-up module that can be used after treatment (e20, e21).
Case illustration.
A 56-year-old woman comes to the urogynecology outpatient clinic complaining of urinary incontinence. She says: “As soon I feel that my bladder is full, I have to go immediately, and I often don’t even get to the bathroom on time. I often lose urine on the way there. I can’t even leave the house anymore. The need to urinate in a hurry has taken over my life.”
The patient is under medical treatment for depression. She is the mother of two children (both born by vaginal delivery) and has not had any surgery other than an appendectomy. Her only medication at present is citalopram, which, she reports, has negative effects on her sexual sensations and on her ability to sleep.
The urinalysis is normal, and examination reveals mild genital atrophy, no prolapse of the reproductive organs, and only moderate strength of contraction of the levator ani. Perineal ultrasonography reveals no evidence of urethral funnel formation. Micturition is brisk and without any residual volume.
A micturition diary, kept for three days, reveals urinary volumes ranging from 30 to 100 mL (normal range: 300–500 mL) in 12 to 14 voiding episodes per day. She is incontinent only once per day.
The patient is treated at first with topical estrogen (estriol ointment 1%, 0.5 g every other night) and is then advised to carry out “bladder training,” with urination by the clock and a gradual increase of the interval from one voiding to the next. She says, however, that there is no way she can do this. Only in this second interview does the patient relate that she is caring for her paralyzed husband at home. She had wanted a divorce from him, but then he had a stroke and she felt morally obliged to look after him. He is very demanding and insists that she be there for him at all times.
The possible psychosomatic background of her symptoms is discussed with her at length, and psychological support is recommended to help her cope with the conflict situation at home. She is also given a prescription for an anticholinergic drug.
On her next visit, she reports that she feels much less stress owing to the psychological help she has received, as she has been able to get rid of her feelings of guilt and aggression. She also has assistance from her children and a nursing service so that she does not have to care for her disabled husband by herself. Her micturition frequency has come down to 7–8 times a day.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Peschers has served as a paid consultant for Astellas and Allergan and has received reimbursement of medical meeting participation fees from Pfizer. She has received lecture honoraria and reimbursement of travel and accommodation expenses from Coloplast, Allergan, AMS, and Astellas and has been paid for carrying out clinical trials on behalf of Coloplast and Allergan.
Prof. Kentenich and PD Jundt declare that they have no conflict of interest.
References
- 1.Harninkontinenz. Gesundheitsberichterstattung des Bundes. Robert-Koch-Institut. 2007;Band 39 [Google Scholar]
- 2.Onder G, Carpenter I, Finne-Soveri H, et al. SHELTER project: Assessment of nursing home residents in Europe: the Services and Health for Elderly in Long TERm care (SHELTER) study. BMC Health Serv Res. 2012;12 doi: 10.1186/1472-6963-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Subcommittee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 4.Hannestad YS, Rortveit G, Sandvik H, et al. A community-based epidemiological survey of female urinary incontinence: The Norwegian EPINCONT Study. J Clin Epidemiol. 2000;53:1150–1157. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 5.Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47:497–499. doi: 10.1136/jech.47.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitzer J. Psychosomatik der Miktionsstörungen der Frau. In: Stauber M, Kentenich H, Richter D, editors. Psychosomatische Geburtshilfe und Gynäkologie. Berlin Heidelberg: Springer-Verlag; 1999. pp. 522–531. [Google Scholar]
- 7.Walters MD, Taylor S, Schoenfeld LS. Psychosexual study of women with detrusor instability. Obstet Gynecol. 1990;75:22–26. [PubMed] [Google Scholar]
- 8.Schoenfeld M, Fuermetz A, Muenster M, et al. Sexuality in German urogynecological patients and healthy controls: is there a difference with respect to the diagnosis? Eur J Obstet Gynecol Reprod Biol. 2013;170:567–570. doi: 10.1016/j.ejogrb.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Coyne KS, Sexton CC, Kopp ZS, et al. The impact of overactive bladder on mental health, work productivity and health-related quality of life in the UK and Sweden : results from EpiLUTS. BJU Int. 2011;108:1459–1471. doi: 10.1111/j.1464-410X.2010.10013.x. [DOI] [PubMed] [Google Scholar]
- 10.Heidler S, Mert C, Wehrberger C, et al. Impact of overactive bladder symptoms on sexuality in both sexes. Urol Int. 2010;85:443–446. doi: 10.1159/000321003. [DOI] [PubMed] [Google Scholar]
- 11.Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S. Urinary incontinence after vaginal delivery or cesarean sectionN. Engl J Med. 2003;348:900–907. doi: 10.1056/NEJMoa021788. [DOI] [PubMed] [Google Scholar]
- 12.Gyhagen M, Bullarbo M, Nielsen TF, Milsom I. Prevalence and risk factors for pelvic organ prolapse 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. BJOG. 2013;120:152–160. doi: 10.1111/1471-0528.12020. [DOI] [PubMed] [Google Scholar]
- 13.Reisenauer C, Muche-Borowski C, Anthuber C, et al. Interdisziplinäre S2e-Leitlinie für die Diagnostik und Therapie der Belastungsinkontinenz der Frau. Geburtsh Frauenheilk. 2013;73:1–5. [Google Scholar]
- 14.Jundt K, Scheer I, Schiessl B, Pohl K, Haertl K, Peschers UM. Physical and sexual abuse in patients with overactive bladder: is there an association? Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:449–453. doi: 10.1007/s00192-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 15.Lucas MG, Bosch RJ, Burkhard FC, et al. EAU guidelines on surgical treatment of urinary incontinence. Actas Urol Esp. 2013;37:459–472. doi: 10.1016/j.acuro.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Viereck V, Rautenberg O, Kociszewski J, Grothey S, Welter J, Eberhard J. Midurethral sling incision: indications and outcomes. Int Urogynecol J. 2013;24:645–653. doi: 10.1007/s00192-012-1895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schäfer W, Abrams P, Liao L, et al. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21:261–274. doi: 10.1002/nau.10066. [DOI] [PubMed] [Google Scholar]
- 18.AMWF. Die überaktive Blase. 2010. AWMF 015/007 (S2k) www.awmf.org/uploads/tx_szleitlinien/015-007l_S2k_Ueberaktive_Blase_2010-abgelaufen.pdf. (last accessed on 10 July 2015)
- 19.Cody JD, Jacobs ML, Richardson K, Moehrer B, Hextall A. Oestrogen therapy for urinary incontinence in postmenopausal women. Cochrane Database Syst Rev. 2012;10 doi: 10.1002/14651858.CD001405.pub3. CD001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumoulin C, Hay-Smith EJ, Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014; 5. doi: 10.1002/14651858.CD005654.pub3. CD005654. [DOI] [PubMed] [Google Scholar]
- 21.Hay-Smith EJC, Dumoulin C. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database of Syst Rev. 2006;1 doi: 10.1002/14651858.CD005654. CD005654. [DOI] [PubMed] [Google Scholar]
- 22.Kirchin V, Page T, Keegan PE, Atiemo K, Cody JD, McClinton S. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2012;2 doi: 10.1002/14651858.CD003881.pub3. CD003881. [DOI] [PubMed] [Google Scholar]
- 23.Novara G, Artibani W, Barber MD, et al. Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol. 2010;58:218–238. doi: 10.1016/j.eururo.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Bø K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J Urol. 2012;30:437–443. doi: 10.1007/s00345-011-0779-8. [DOI] [PubMed] [Google Scholar]
- 25.Hagen S, Stark D. Conservative prevention and management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2011;12 doi: 10.1002/14651858.CD003882.pub4. CD003882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bugge C, Adams EJ, Gopinath D, Reid F. Pessaries (mechanical devices) for pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;2 doi: 10.1002/14651858.CD004010.pub3. CD004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang FW, Wei F, Wang HL, et al. Does pelvic floor muscle training augment the effect of surgery in women with pelvic organ prolapse? A systematic review of randomized controlled trials. Neurourol Urodyn. 2015 doi: 10.1002/nau.22784. doi: 10.1002/nau.22784. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Maher CM, Feiner B, Baessler K, Glazener CM. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011;22:1445–1457. doi: 10.1007/s00192-011-1542-9. [DOI] [PubMed] [Google Scholar]
- 29.Vigod SN, Steward DE. Major depression in female urinary incontinence. Psychosomatics. 2006;47:147–151. doi: 10.1176/appi.psy.47.2.147. [DOI] [PubMed] [Google Scholar]
- 30.Debus G, Kästner R. Psychosomatic aspects of urinary incontinence in women. Geburtshilfe Frauenheilkd. 2015;75:165–169. doi: 10.1055/s-0034-1396257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunskaar S. A systematic review of overweight and obesity as risk factors and targets for clinical intervention for urinary incontinence in women. Neurourol Urodyn. 2008;27:749–757. doi: 10.1002/nau.20635. [DOI] [PubMed] [Google Scholar]
- 32.Fong ED, Nitti VW. Review article: Mid-urethral synthetic slings for female stress urinary incontinence. BJU Int. 2010;106:596–608. doi: 10.1111/j.1464-410X.2010.09544.x. [DOI] [PubMed] [Google Scholar]
- 33.Lapitan MC, Cody JD. Open retropubic colposuspension for urinary incontinence in women. Cochrane Database Syst Rev. 2012;6 doi: 10.1002/14651858.CD002912.pub5. CD002912. [DOI] [PubMed] [Google Scholar]
- 34.Kirchin V, Page T, Keegan PE, Atiemo K, Cody JD, McClinton S. Urethral injection therapy for urinary incontinence in women. Cochrane Database of Syst Rev. 2012;2 doi: 10.1002/14651858.CD003881.pub3. CD003881. [DOI] [PubMed] [Google Scholar]
- 35.Herderschee R, Hay-Smith EJC., Herbison GP, Roovers JP, Heineman MJ. Feedback or biofeedback to augment pelvic floor muscle training for urinary incontinence in women. Cochrane Database of Syst Rev. 2011;7 doi: 10.1002/14651858.CD009252. CD009252. [DOI] [PubMed] [Google Scholar]
- 36.Rai BP, Cody JD, Alhasso A, Stewart L. Anticholinergic drugs versus non-drug active therapies for non-neurogenic overactive bladder syndrome in adults. Cochrane Database Syst Rev. 2012;12 doi: 10.1002/14651858.CD003193.pub4. CD003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duthie JB, Vincent M, Herbison GP, Wilson DIain, Wilson D. Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Database Syst Rev. 2011;12 doi: 10.1002/14651858.CD005493.pub3. CD005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noblett K, Siegel S, Mangel J, et al. Results of a prospective, multicenter study evaluating quality of life, safety, and efficacy of sacral neuromodulation at twelve months in subjects with symptoms of overactive bladder. Neurourol Urodyn. 2014 doi: 10.1002/nau.22707. doi: 10.1002/nau.22707. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Bugge C, Hagen S, Thakar R. Vaginal pessaries for pelvic organe prolapse and urinary incontinence: a multiprofessional survey of practice. Int Urogynecol J. 2013;24:1017–1024. doi: 10.1007/s00192-012-1985-7. [DOI] [PubMed] [Google Scholar]
- 40.AWMF. Aktuelle Version 2015 im Druck (S2e) Deszensus genitalis der Frau. AWMF 015/006 (S1) [Google Scholar]
- e1.Fantl JA, Newman DK, Colling J., et al. Urinary incontinence in adults: acute and chronic management. Clinical Practice Guideline No. 2. 1996 Update. Rockville, MD. [Google Scholar]
- e2.Saga S, Vinsnes AG, Mørkved S, Norton C, Seim A. What characteristics predispose to continence in nursing home residents?: A population-based cross-sectional study. Neurourol Urodyn. 2015;34:362–367. doi: 10.1002/nau.22563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e3.Nuotio M, Jylha M, Luukkaala T, Tammela TL. Urinary incontinence in a Finish population aged 70 and over Prevalence of types, associated factors and self-reported treatments. Scan J Prim Health Care. 2003;21:182–187. [PubMed] [Google Scholar]
- e4.Samuelsson E, Victor A, Tibblin G. A population study of urinary incontinence and nocturia among women aged 20-59 years. Prevalence, well-being and wish for treatment. Acta Obstet Gynecol Scand. 1997;76:74–80. doi: 10.3109/00016349709047789. [DOI] [PubMed] [Google Scholar]
- e5.Samuelsson EC, Victor FT, Tibblin G, Svärdsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180:299–305. doi: 10.1016/s0002-9378(99)70203-6. [DOI] [PubMed] [Google Scholar]
- e6.Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160–1166. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- e7.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- e8.Fialkow MF, Newton KM, Lentz GM, Weiss NS. Lifetime risk of surgical management for pelvic organ prolapse or urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:437–433. doi: 10.1007/s00192-007-0459-9. [DOI] [PubMed] [Google Scholar]
- e9.Smith FJ, Holman CD, Moorin RE, Tsokos N. Lifetime risk undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010;116:1096–1100. doi: 10.1097/AOG.0b013e3181f73729. [DOI] [PubMed] [Google Scholar]
- e10.Yang JM, Yang SH, Yang SY, Yang E, Huang WC, Tzeng CR. Clinical and pathophysiological correlates of the symptom severity of stress urinary incontinence. Int Urogynecol J. 2010;21:637–643. doi: 10.1007/s00192-009-1094-4. [DOI] [PubMed] [Google Scholar]
- e11.Ghoniem G, Stanford E, Kenton K, et al. Evaluation and outcome measures in the treatment of female urinary stress incontinence: International Urogynecological Association (IUGA) guidelines for research and clinical practice. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:5–33. doi: 10.1007/s00192-007-0495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Bø K, Finckenhagen HB. Vaginal palpation of pelvic floor muscle strength: inter-test reproducibility and comparison between palpation and vaginal squeeze pressure. Acta Obstet Gynecol Scand. 2001;80:883–887. doi: 10.1034/j.1600-0412.2001.801003.x. [DOI] [PubMed] [Google Scholar]
- e13.Jundt K, Scheer I, von Bodungen V, Krumbachner F, Friese K, Peschers UM. What harm does a second delivery to the pelvic floor? Eur J Med Res. 2010;15:362–366. doi: 10.1186/2047-783X-15-8-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e14.Shek KL, Chantarasorn V, Dietz HP. The urethral motion profile before and after suburethral sling placement. J Urol. 2010;183:1450–1454. doi: 10.1016/j.juro.2009.12.028. [DOI] [PubMed] [Google Scholar]
- e15.Dickie KJ, Shek KL, Dietz HP. The relationship between urethral mobility and parity. BJOG. 2010;117:1220–1224. doi: 10.1111/j.1471-0528.2010.02649.x. [DOI] [PubMed] [Google Scholar]
- e16.Huang WC, Yang SH, Yang SY, Yang E, Yang JM. The correlations of incontinence-related quality of life measures with symptom severity and pathophysiology in women with primary stress urinary incontinence. World J Urol. 2010;28:619–623. doi: 10.1007/s00345-009-0485-y. [DOI] [PubMed] [Google Scholar]
- e17.Steensma AB, Konstantinovic ML, Burger CW, de Ridder D, Timmerman D, Deprest J. Prevalence of major levator abnormalities in symptomatic patients with an underactive pelvic floor contraction. Int Urogynecol J. 2010;21:861–867. doi: 10.1007/s00192-010-1111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e18.Avery KN, Bosch JL, Gotoh M, et al. Questionnaires to assess urinary and anal incontinence: review and recommendations. J Urol. 2007;177:39–49. doi: 10.1016/j.juro.2006.08.075. [DOI] [PubMed] [Google Scholar]
- e19.Bjelic-Radisic V, Dorfer M, Tamussino K, Greimel E. Psychometric properties and validation of the German-language King’s Health Questionnaire in women with stress urinary incontinence. Neurourol Urodyn. 2005;24:63–68. doi: 10.1002/nau.20092. [DOI] [PubMed] [Google Scholar]
- e20.Baessler K, Kempkensteffen C. [Validation of a comprehensive pelvic floor questionnaire for the hospital, private practice and research] Validierung eines umfassenden Beckenboden-Fragebogens fur Klinik, Praxis und Forschung. Gynakol Geburtshilfliche Rundsch. 2009;49:299–307. doi: 10.1159/000301098. [DOI] [PubMed] [Google Scholar]
- e21.Baessler K, Junginger B. [Validation of a pelvic floor questionnaire with improvement and satisfaction scales to assess symptom severity, bothersomeness and quality of life before and after pelvic floor therapy]. Beckenboden-Fragebogen fur Frauen. Validierung eines Instrumentes mit posttherapeutischem Modul zur Evaluation von Symptomen, Leidensdruck, Lebensqualitat, Verbesserung und Zufriedenheit. Aktuelle Urol. 2011;42:316–322. doi: 10.1055/s-0031-1271544. [DOI] [PubMed] [Google Scholar]
- e22.Imamura M, Abrams P, Bain C, et al. Systematic review and economic modelling of the effectiveness and cost-effectiveness of non-surgical treatments for women with stress urinary incontinence. Health Technol Assess. 2010;14:1–188. doi: 10.3310/hta14400. [DOI] [PubMed] [Google Scholar]
- e23.Farrell SA, Baydock S, Amir B, Fanning C. Effectiveness of a new self-positioning pessary for the management of urinary incontinence in women. Am J Obstet Gynecol. 2007;196(474):e1–e8. doi: 10.1016/j.ajog.2006.11.038. [DOI] [PubMed] [Google Scholar]
- e24.Noblett KL, McKinney A, Lane FL. Effects of the incontinence dish pessary on urethral support and urodynamic parameters. Am J Obstet Gynecol. 2008;198(592):e1–e5. doi: 10.1016/j.ajog.2008.02.004. [DOI] [PubMed] [Google Scholar]
- e25.Mariappan P, Alhasso A, Ballantyne Z, Grant A, N’Dow J. Duloxetine, a serotonin and noradrenaline reuptake inhibitor (SNRI) for the treatment of stress urinary incontinence: a systematic review. Eur Urol. 2007;51:67–74. doi: 10.1016/j.eururo.2006.08.041. [DOI] [PubMed] [Google Scholar]
- e26.Shamliyan TA, Kane RL, Wyman J, Wilt TJ. Systematic review: randomized, controlled trials of nonsurgical treatments for urinary incontinence in women. Ann Intern Med. 2008;148:459–473. doi: 10.7326/0003-4819-148-6-200803180-00211. [DOI] [PubMed] [Google Scholar]
- e27.Mostafa A, Lim CP, Hopper L, Madhuvrata P, Abdel-Fattah M. Single-incision mini-slings versus standard midurethral slings in surgical management of female stress urinary incontinence: an updated systematic review and meta-analysis of effectiveness and complications. Eur Urol. 2014;65:402–427. doi: 10.1016/j.eururo.2013.08.032. [DOI] [PubMed] [Google Scholar]
- e28.Agur W, Riad M, Secco S, et al. Surgical treatment of recurrent stress urinary incontinence in women: a systematic review and meta-analysis of randomised controlled trials. Eur Urol. 2013;64:323–336. doi: 10.1016/j.eururo.2013.04.034. [DOI] [PubMed] [Google Scholar]
- e29.Magee G, Roy S, Hinoul P, et al. A real-world comparative assessment of complications following various mid-urethral sling procedures for the treatment of stress urinary incontinence. J Long Term Eff Med Implants. 2012;22:329–340. doi: 10.1615/jlongtermeffmedimplants.2013007383. [DOI] [PubMed] [Google Scholar]
- e30.Nilsson CG, Palva K, Aarnio R, Morcos E, Falconer C. Seventeen years’ follow-up of the tension-free vaginal tape procedure for female stress urinary incontinence. Int Urogynecol J. 2013;24:1265–1269. doi: 10.1007/s00192-013-2090-2. [DOI] [PubMed] [Google Scholar]
- e31.Ward K, Hilton P. Prospective multicentre randomised trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;325 doi: 10.1136/bmj.325.7355.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e32.Jelovsek JE, Barber MD, Karram MM, Walters MD, Paraiso MF. Randomised trial of laparoscopic Burch colposuspension versus tension-free vaginal tape: long-term follow up. BJOG. 2008;115:219–225. doi: 10.1111/j.1471-0528.2007.01592.x. discussion 225. [DOI] [PubMed] [Google Scholar]
- e33.Wang AC, Wang YY, Chen MC. Single-blind, randomized trial of pelvic floor muscle training, biofeedback-assisted pelvic floor muscle training, and electrical stimulation in the management of overactive bladder. Urology. 2004;63:61–66. doi: 10.1016/j.urology.2003.08.047. [DOI] [PubMed] [Google Scholar]
- e34.Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: An update of a systematic review and meta-analysis. Eur Urol. 2008;54:543–562. doi: 10.1016/j.eururo.2008.06.047. [DOI] [PubMed] [Google Scholar]
- e35.Veenboer PW, Bosch JL. Long-term adherence to antimuscarinic therapy in everyday practice: a systematic review. J Urol. 2014;191:1003–1008. doi: 10.1016/j.juro.2013.10.046. [DOI] [PubMed] [Google Scholar]
- e36.Nitti VW, Dmochowski R, Herschorn S, et al. EMBARK Study Group: OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013;189:2186–2193. doi: 10.1016/j.juro.2012.12.022. [DOI] [PubMed] [Google Scholar]
- e37.Schurch B, Schmid DM, Stöhrer M. Treatment of neurogenic incontinence with botulinum toxin A. N Engl J Med. 2000;342 doi: 10.1056/NEJM200003023420918. [DOI] [PubMed] [Google Scholar]
- e38.Hanson LA, Schulz JA, Flood CG, Cooley B, Tam F. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:155–159. doi: 10.1007/s00192-005-1362-x. [DOI] [PubMed] [Google Scholar]
- e39.Clemons JL, Aguilar VC, Sokol ER, Jackson ND, Myers DL. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159–164. doi: 10.1016/j.ajog.2004.04.048. [DOI] [PubMed] [Google Scholar]
- e40.Wiegersma M, Panman CM, Kollen BJ, et al. Pelvic floor muscle training versus watchful waiting or pessary treatment for pelvic organ prolapse (POPPS): Design and participant baseline characteristics of two parallel pragmatic randomized controlled trials in primary care. Maturitas. 2014;77:168–173. doi: 10.1016/j.maturitas.2013.10.014. [DOI] [PubMed] [Google Scholar]
- e41.Hsiao KC, Latchamsetty K, Govier FE, Kozlowski P, Kobashi KC. Comparison of laparoscopic and abdominal sacrocolpopexy for the treatment of vaginal vault prolapse. J Endourol. 2007;21:926–930. doi: 10.1089/end.2006.0381. [DOI] [PubMed] [Google Scholar]
- e42.Barber MD, Maher C. Apical prolapse. Int Urogynecol J. 2013;24:1815–1833. doi: 10.1007/s00192-013-2172-1. [DOI] [PubMed] [Google Scholar]
- e43.Abramov Y, Gandhi S, Goldberg RP, Botros SM, Kwon C, Sand PK. Site-specific rectocele repair compared with standard posterior colporrhaphy. Obstet Gynecol. 2005;105:314–318. doi: 10.1097/01.AOG.0000151990.08019.30. [DOI] [PubMed] [Google Scholar]