Abstract
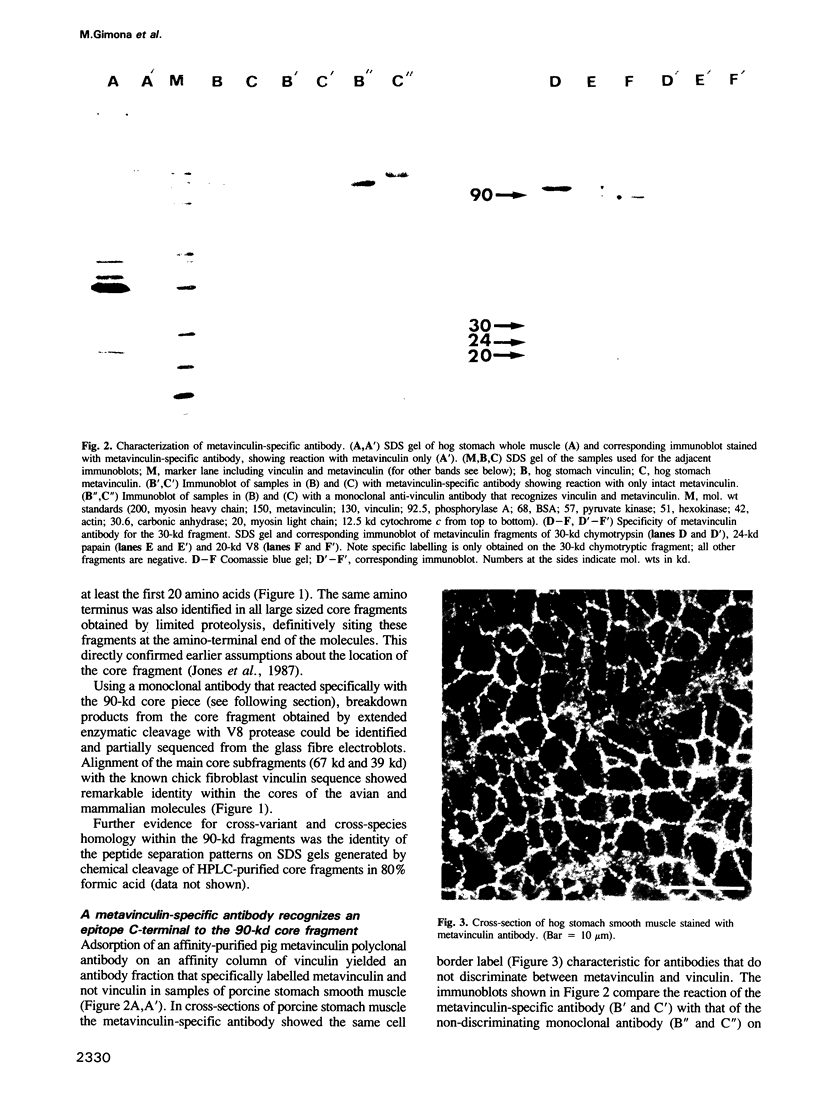
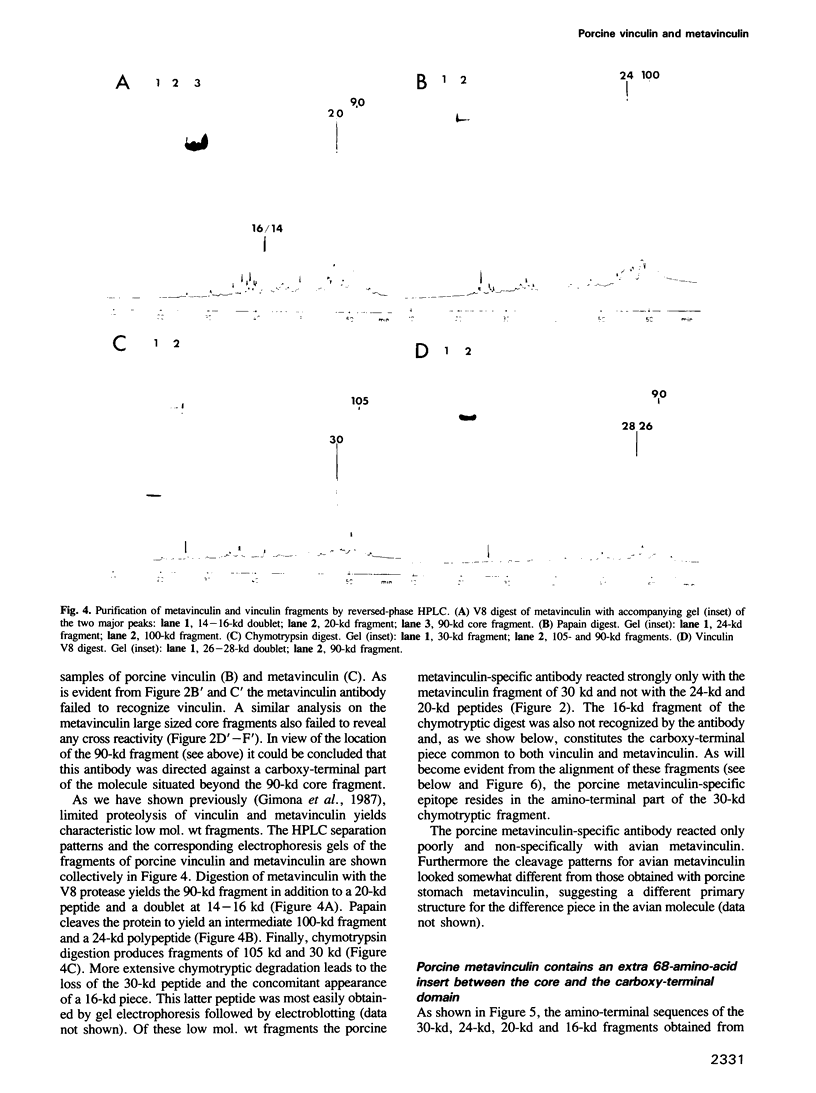
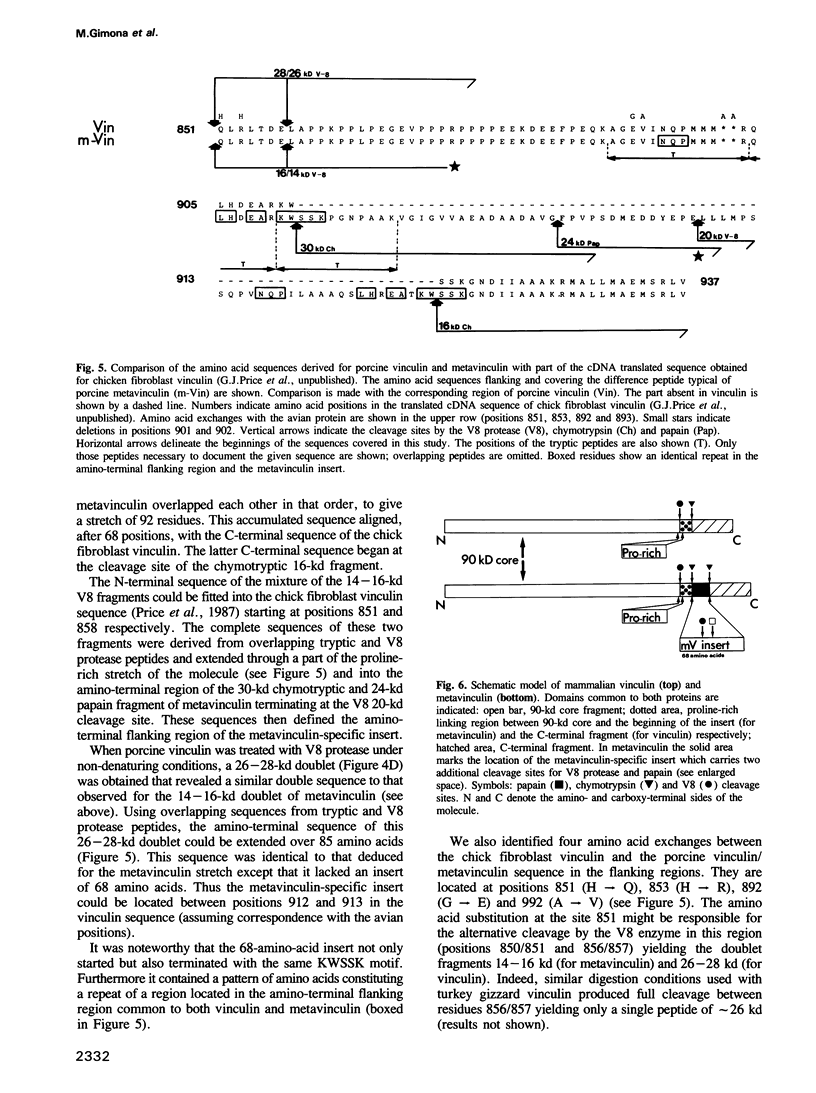
Metavinculin is a higher mol. wt variant of vinculin expressed only in muscle tissue. Using amino acid sequencing methods on the intact molecules and their proteolytic subfragments, together with a polyclonal antibody specific only for metavinculin from porcine stomach, we have been able to identify and sequence the difference peptide in the porcine metavinculin molecule. By alignment with the complete sequence of chick fibroblast vinculin (communicated by G.J. Price, P. Jones, M.D. Davison, R. Bendori, S. Griffiths, B. Patel, B. Geiger and D.R. Critchley, prior to publication) the exact location of the insert could be determined. In porcine metavinculin, this insert lies between the 90-kd protease-resistant amino-terminal core and the carboxy terminus of the molecule. It contains 68 amino acids and is flanked by KWSSK sequences, one of which is present in vinculin. The identity of the mapped vinculin and metavinculin sequences outside this difference peptide is consistent with the two proteins arising via alternative splicing at the mRNA level. The lack of reactivity of the porcine metavinculin antibody with metavinculin from chicken as well as the finding of different proteolytic cleavage sites in avian metavinculin indicate a species-specific amino acid sequence in the difference piece of the metavinculin molecule.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauw G., De Loose M., Inzé D., Van Montagu M., Vandekerckhove J. Alterations in the phenotype of plant cells studied by NH(2)-terminal amino acid-sequence analysis of proteins electroblotted from two-dimensional gel-separated total extracts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4806–4810. doi: 10.1073/pnas.84.14.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendori R., Salomon D., Geiger B. Contact-dependent regulation of vinculin expression in cultured fibroblasts: a study with vinculin-specific cDNA probes. EMBO J. 1987 Oct;6(10):2897–2905. doi: 10.1002/j.1460-2075.1987.tb02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Crick F. Split genes and RNA splicing. Science. 1979 Apr 20;204(4390):264–271. doi: 10.1126/science.373120. [DOI] [PubMed] [Google Scholar]
- De B. K., Misono K. S., Lukas T. J., Mroczkowski B., Cohen S. A calcium-dependent 35-kilodalton substrate for epidermal growth factor receptor/kinase isolated from normal tissue. J Biol Chem. 1986 Oct 15;261(29):13784–13792. [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Geiger B. Membrane-cytoskeleton interaction. Biochim Biophys Acta. 1983 Aug 11;737(3-4):305–341. doi: 10.1016/0304-4157(83)90005-9. [DOI] [PubMed] [Google Scholar]
- Gimona M., Fürst D. O., Small J. V. Metavinculin and vinculin from mammalian smooth muscle: bulk isolation and characterization. J Muscle Res Cell Motil. 1987 Aug;8(4):329–341. doi: 10.1007/BF01568889. [DOI] [PubMed] [Google Scholar]
- Glukhova M. A., Kabakov A. E., Belkin A. M., Frid M. G., Ornatsky O. I., Zhidkova N. I., Koteliansky V. E. Meta-vinculin distribution in adult human tissues and cultured cells. FEBS Lett. 1986 Oct 20;207(1):139–141. doi: 10.1016/0014-5793(86)80027-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Jones P., Price G. J., Davison M. D., Critchley D. R. Isolation and characterization of a vinculin cDNA from chick embryo fibroblasts. Biochem Soc Trans. 1987 Oct;15(5):794–796. doi: 10.1042/bst0150794. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mangeat P., Burridge K. Actin-membrane interaction in fibroblasts: what proteins are involved in this association? J Cell Biol. 1984 Jul;99(1 Pt 2):95s–103s. doi: 10.1083/jcb.99.1.95s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Moeremans M., Daneels G., Van Dijck A., Langanger G., De Mey J. Sensitive visualization of antigen-antibody reactions in dot and blot immune overlay assays with immunogold and immunogold/silver staining. J Immunol Methods. 1984 Nov 30;74(2):353–360. doi: 10.1016/0022-1759(84)90303-x. [DOI] [PubMed] [Google Scholar]
- Price G. J., Jones P., Davison M. D., Patel B., Eperon I. C., Critchley D. R. Isolation and characterization of a vinculin cDNA from chick-embryo fibroblasts. Biochem J. 1987 Jul 15;245(2):595–603. doi: 10.1042/bj2450595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga S., Hamaguchi M., Hoshino M., Kojima K. Expression of meta-vinculin associated with differentiation of chicken embryonal muscle cells. Exp Cell Res. 1985 Jan;156(1):45–56. doi: 10.1016/0014-4827(85)90260-5. [DOI] [PubMed] [Google Scholar]
- Siliciano J. D., Craig S. W. Meta-vinculin--a vinculin-related protein with solubility properties of a membrane protein. Nature. 1982 Dec 9;300(5892):533–535. doi: 10.1038/300533a0. [DOI] [PubMed] [Google Scholar]
- Siliciano J. D., Craig S. W. Properties of smooth muscle meta-vinculin. J Cell Biol. 1987 Mar;104(3):473–482. doi: 10.1083/jcb.104.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V., Fürst D. O., De Mey J. Localization of filamin in smooth muscle. J Cell Biol. 1986 Jan;102(1):210–220. doi: 10.1083/jcb.102.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunón P., Johansson K. E. Yet another improved silver staining method for the detection of proteins in polyacrylamide gels. J Biochem Biophys Methods. 1984 May;9(2):171–179. doi: 10.1016/0165-022x(84)90008-3. [DOI] [PubMed] [Google Scholar]






