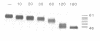Abstract
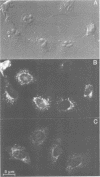
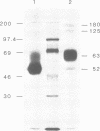
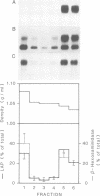
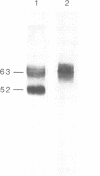
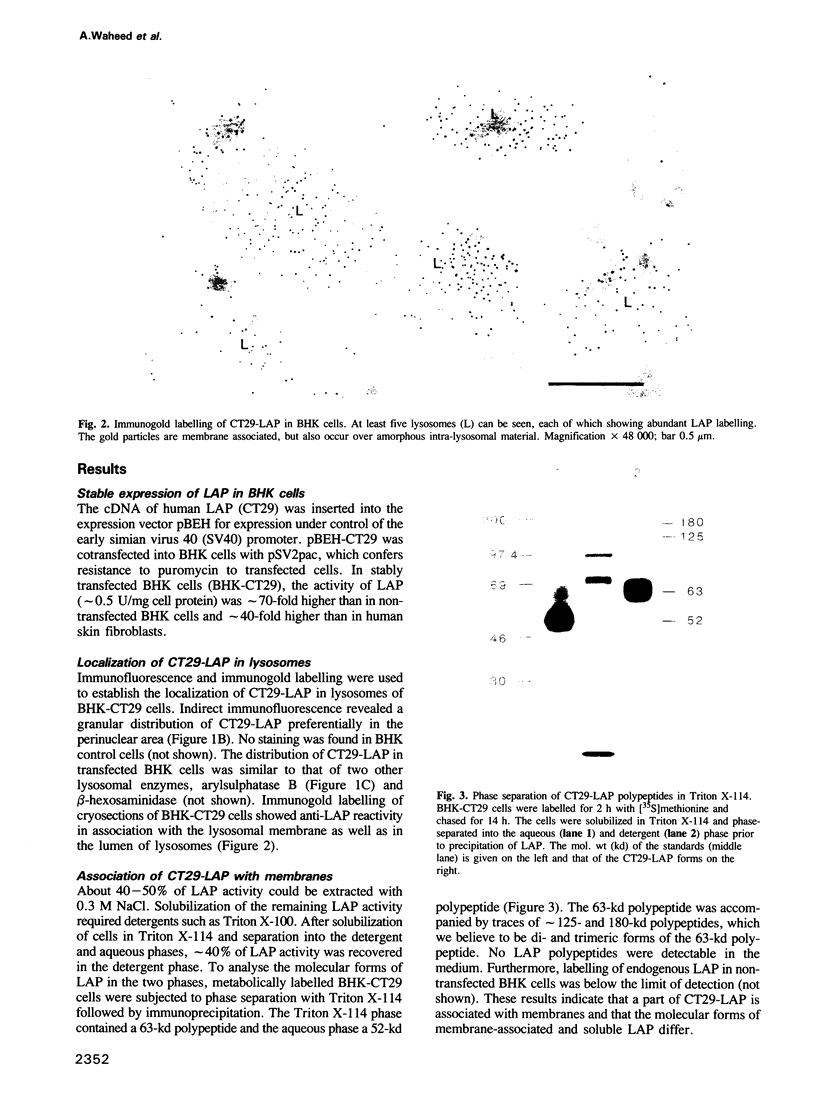
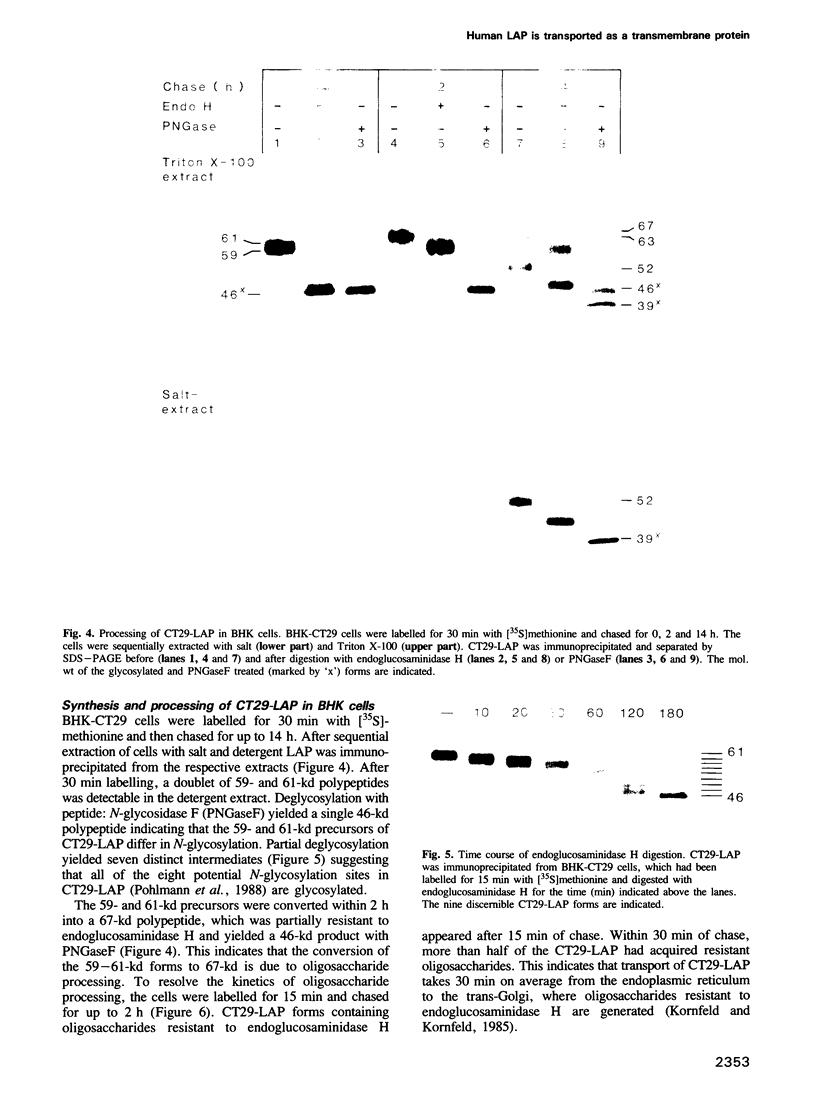
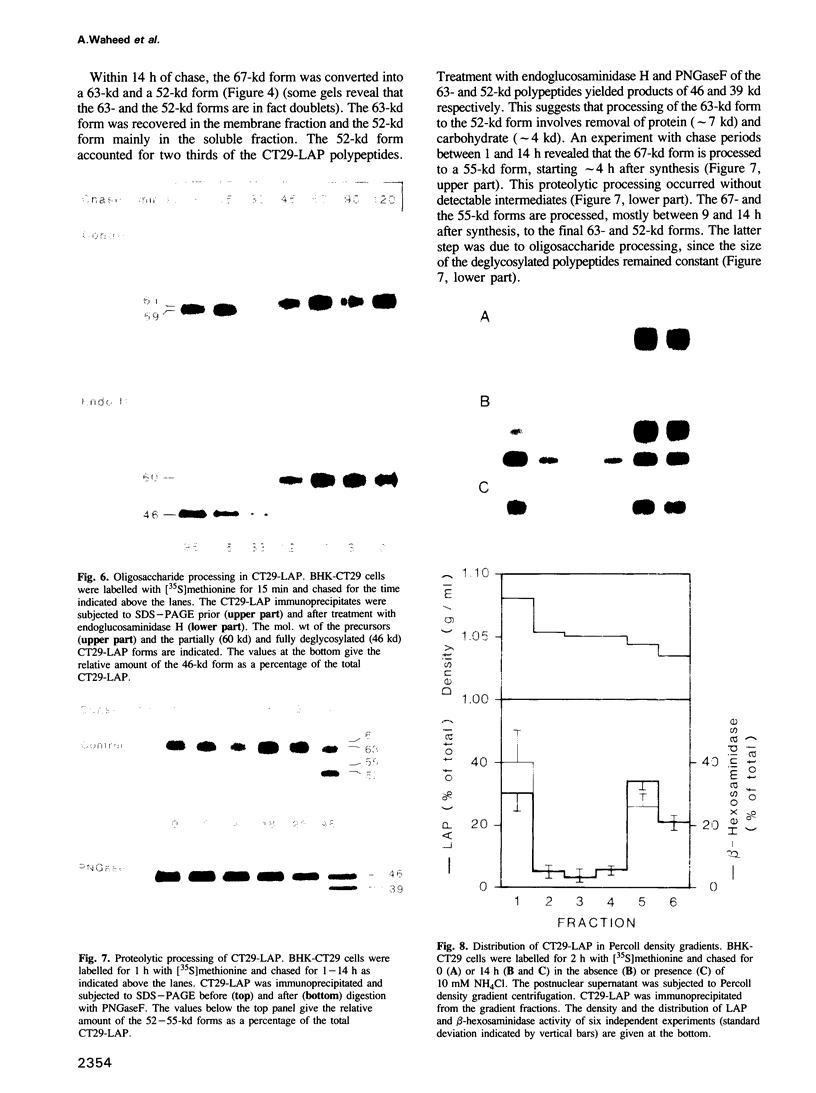
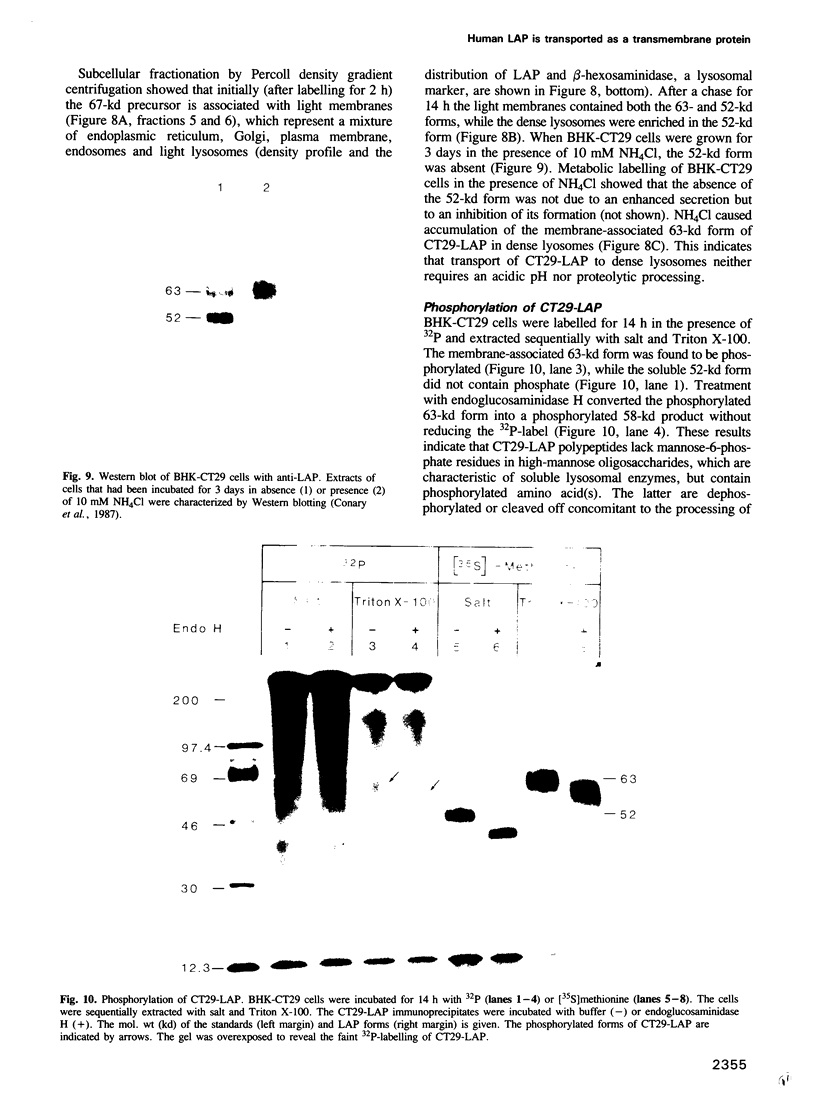
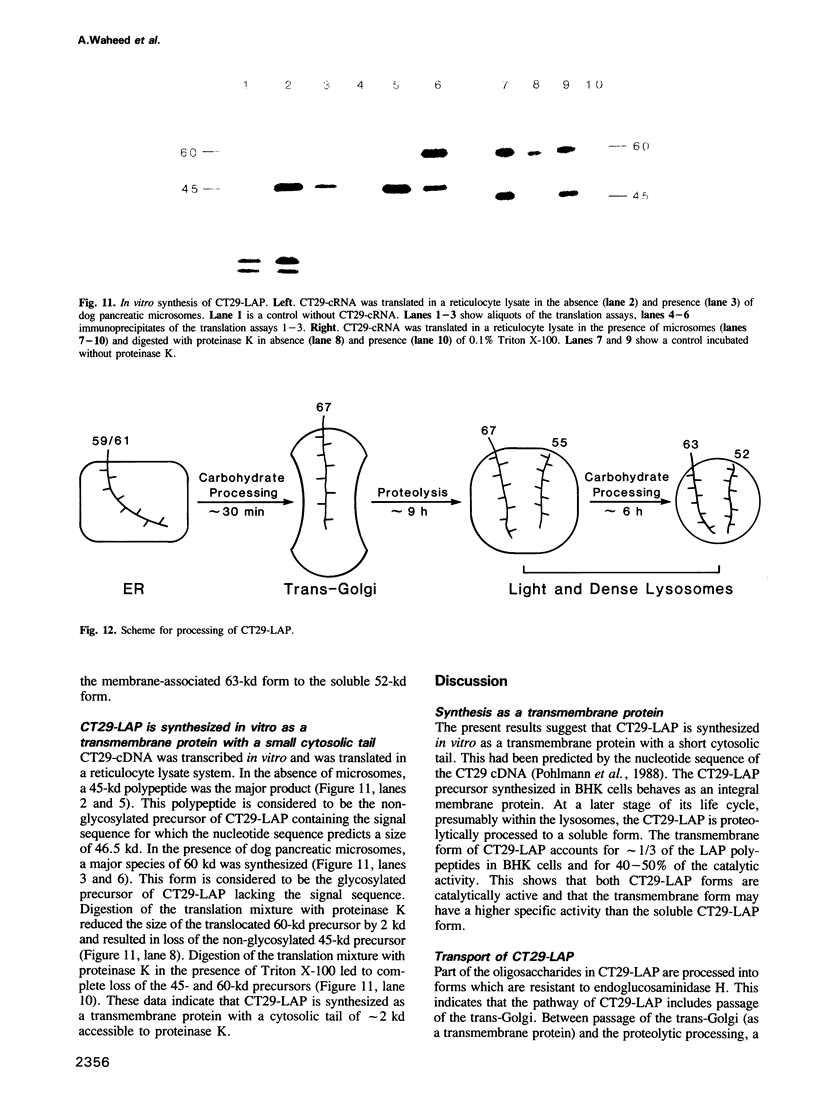
BHK cells transfected with human lysosomal acid phosphatase (LAP) cDNA (CT29) expressed 70-fold higher enzyme activities of acid phosphatase than non-transfected BHK cells. The CT29-LAP was synthesized in BHK cells as a heterogeneously glycosylated precursor that was tightly membrane associated. Transfer to the trans-Golgi was associated with a small increase in size (approximately 7 kd) and partial processing of the oligosaccharides to complex type structures. CT29-LAP was transferred into lysosomes as shown by subcellular fractionation, immunofluorescence and immunoelectron microscopy. Lack of mannose-6-phosphate residues suggested that transport does not involve mannose-6-phosphate receptors. Part of the membrane-associated CT29-LAP was processed to a soluble form. The mechanism that converts CT29-LAP into a soluble form was sensitive to NH4Cl, and reduced the size of the polypeptide by 7 kd. In vitro translation of CT29-derived cRNA in the presence of microsomal membranes yielded a CT29-LAP precursor that is protected from proteinase K except for a small peptide of approximately 2 kd. In combination with the sequence data available for LAP, these observations suggest that CT29-LAP is synthesized and transported to lysosomes as a transmembrane protein. In the lysosomes, CT29-LAP is released from the membrane by proteolytic cleavage, which removes a C-terminal peptide including the transmembrane domain and the cytosolic tail of 18 amino acids.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barriocanal J. G., Bonifacino J. S., Yuan L., Sandoval I. V. Biosynthesis, glycosylation, movement through the Golgi system, and transport to lysosomes by an N-linked carbohydrate-independent mechanism of three lysosomal integral membrane proteins. J Biol Chem. 1986 Dec 15;261(35):16755–16763. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chen J. W., Murphy T. L., Willingham M. C., Pastan I., August J. T. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985 Jul;101(1):85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conary J. T., Lorkowski G., Schmidt B., Pohlmann R., Nagel G., Meyer H. E., Krentler C., Cully J., Hasilik A., von Figura K. Genetic heterogeneity of steroid sulfatase deficiency revealed with cDNA for human steroid sulfatase. Biochem Biophys Res Commun. 1987 Apr 29;144(2):1010–1017. doi: 10.1016/s0006-291x(87)80064-5. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Takeyasu K., Lippincott-Schwarz J., Siegel N. R. Structure of LEP100, a glycoprotein that shuttles between lysosomes and the plasma membrane, deduced from the nucleotide sequence of the encoding cDNA. J Cell Biol. 1988 Jan;106(1):61–67. doi: 10.1083/jcb.106.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W. Disproportional immunostaining patterns of two secretory proteins in guinea pig and rat exocrine pancreatic cells. An immunoferritin and fluorescence study. Eur J Cell Biol. 1980 Apr;21(1):93–100. [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980 May 25;255(10):4946–4950. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes in normal and disease states. J Clin Invest. 1986 Jan;77(1):1–6. doi: 10.1172/JCI112262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemansky P., Gieselmann V., Hasilik A., von Figura K. Cathepsin D and beta-hexosaminidase synthesized in the presence of 1-deoxynojirimycin accumulate in the endoplasmic reticulum. J Biol Chem. 1984 Aug 25;259(16):10129–10135. [PubMed] [Google Scholar]
- Lemansky P., Gieselmann V., Hasilik A., von Figura K. Synthesis and transport of lysosomal acid phosphatase in normal and I-cell fibroblasts. J Biol Chem. 1985 Jul 25;260(15):9023–9030. [PubMed] [Google Scholar]
- Lewis V., Green S. A., Marsh M., Vihko P., Helenius A., Mellman I. Glycoproteins of the lysosomal membrane. J Cell Biol. 1985 Jun;100(6):1839–1847. doi: 10.1083/jcb.100.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Fambrough D. M. Lysosomal membrane dynamics: structure and interorganellar movement of a major lysosomal membrane glycoprotein. J Cell Biol. 1986 May;102(5):1593–1605. doi: 10.1083/jcb.102.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann R., Krentler C., Schmidt B., Schröder W., Lorkowski G., Culley J., Mersmann G., Geier C., Waheed A., Gottschalk S. Human lysosomal acid phosphatase: cloning, expression and chromosomal assignment. EMBO J. 1988 Aug;7(8):2343–2350. doi: 10.1002/j.1460-2075.1988.tb03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckel F., Hasilik A., von Figura K. Biosynthesis and maturation of arylsulfatase B in normal and mutant cultured human fibroblasts. J Biol Chem. 1983 Dec 10;258(23):14322–14326. [PubMed] [Google Scholar]
- Stein M., Braulke T., Krentler C., Hasilik A., von Figura K. 46-kDa mannose 6-phosphate-specific receptor: biosynthesis, processing, subcellular location and topology. Biol Chem Hoppe Seyler. 1987 Aug;368(8):937–947. doi: 10.1515/bchm3.1987.368.2.937. [DOI] [PubMed] [Google Scholar]
- Stein M., Zijderhand-Bleekemolen J. E., Geuze H., Hasilik A., von Figura K. Mr 46,000 mannose 6-phosphate specific receptor: its role in targeting of lysosomal enzymes. EMBO J. 1987 Sep;6(9):2677–2681. doi: 10.1002/j.1460-2075.1987.tb02559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T., Singer S. J. Improved procedures for immunoferritin labeling of ultrathin frozen sections. J Cell Biol. 1976 Dec;71(3):894–906. doi: 10.1083/jcb.71.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara J. A., Portela A., Ortín J., Jiménez A. Expression in mammalian cells of a gene from Streptomyces alboniger conferring puromycin resistance. Nucleic Acids Res. 1986 Jun 11;14(11):4617–4624. doi: 10.1093/nar/14.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A., Hasilik A., von Figura K. Enhanced breakdown of arylsulfatase A in multiple sulfatase deficiency. Eur J Biochem. 1982 Apr 1;123(2):317–321. doi: 10.1111/j.1432-1033.1982.tb19770.x. [DOI] [PubMed] [Google Scholar]
- Waheed A., Van Etten R. L. Biosynthesis and processing of lysosomal acid phosphatase in cultured human cells. Arch Biochem Biophys. 1985 Nov 15;243(1):274–283. doi: 10.1016/0003-9861(85)90796-9. [DOI] [PubMed] [Google Scholar]
- Waheed A., Van Etten R. L., Gieselmann V., von Figura K. Immunological characterization of human acid phosphatase gene products. Biochem Genet. 1985 Apr;23(3-4):309–319. doi: 10.1007/BF00504327. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- von Figura K., Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]
- von Figura K., Steckel F., Hasilik A. Juvenile and adult metachromatic leukodystrophy: partial restoration of arylsulfatase A (cerebroside sulfatase) activity by inhibitors of thiol proteinases. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6066–6070. doi: 10.1073/pnas.80.19.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]