Abstract
Hundred years ago therapeutic vaccination with allergen-containing extracts has been introduced as a clinically effective, disease-modifying, allergen-specific and long-lasting form of therapy for allergy, a hypersensitivity disease affecting more than 25% of the population. Today, the structures of most of the disease-causing allergens have been elucidated and recombinant hypoallergenic allergen derivatives with reduced allergenic activity have been engineered to reduce side effects during allergen-specific immunotherapy (SIT). These recombinant hypoallergens have been characterized in vitro, in experimental animal models and in clinical trials in allergic patients. This review provides a summary of the molecular, immunological and preclinical evaluation criteria applied for this new generation of allergy vaccines. Furthermore, we summarize the mechanisms underlying SIT with recombinant hypoallergens which are thought to be responsible for their therapeutic effect.
Keywords: Allergy, Allergen, Allergy vaccination, Allergy vaccines, Hypoallergens
1. Introduction
More than 25% of the population suffer from IgE-mediated allergy, a hypersensitivity disease affecting multiple organs [1,2]. The symptoms of allergy include mild manifestations such as allergic rhinitis, conjunctivitis but also more severe forms such as dermatitis, food allergy, asthma and life-threatening anaphylaxis [2]. Hundred years ago, in 1911, the first immunotherapy trial was published by Noon [3], who had successfully treated grass pollen allergic patients by injecting grass pollen extracts subcutaneously and found a reduction of allergic symptoms in the treated patients. In 1935 Cooke and co-workers [4] identified a protective allergen-specific factor in the serum of patients who had undergone specific immunotherapy (SIT), meanwhile known as “blocking” IgG antibodies which inhibit the binding of IgE antibodies to allergens.
Since then, specific immunotherapy has been shown to be the only disease-modifying and antigen-specific treatment of allergy, and its efficacy and long lasting effect was demonstrated in numerous clinical trials [5-7]. However, the use of crude allergen extracts as active components of allergy vaccines limits the broad application of SIT. Allergen extracts are prepared from natural allergen sources and the amount of allergen molecules in the vaccine depends on many factors, like the quality of the starting material, the extraction method and the stability of the proteins. Therefore natural allergen extracts often show great variations of allergen contents, lack important allergens and may be contaminated with allergens from other sources or other unwanted substances [8-12].
Since the late nineteen-eighties efforts were made to isolate the allergen-encoding DNAs from different allergen sources. Consequently, most of the clinically relevant allergens have been produced as pure recombinant allergen molecules which were shown to mimic the epitope spectrum of natural allergen extracts[13-15]. Allergy vaccines based on recombinant allergens were successfully tested in several clinical trials and lead to promising results in the treated patients and may soon give rise to first registered allergy vaccines which fulfil the stringent criteria required for vaccines [16-18]. However, recombinant “wildtype” allergens which possess a similar allergenic activity as the natural allergens can elicit IgE-mediated side effects which are a major problem of allergen-specific immunotherapy [19].
Early attempts to reduce systemic anaphylactic reactions in the course of treatment were made already in 1940, when adjuvanted allergen preparations came into use [20]. In 1970, David Marsh [21] proposed a method for the chemical modification of allergen extracts by aldehyde treatment, which lead to a reduced allergenic activity but maintained the immunogenicity. Due to the destruction of IgE epitopes such allergoids can be applied to patients at higher concentrations but the characterization of allergoids is extremely difficult because aldehyde treatment leads to the formation of polymers and standardization with IgE-based assays is not possible.
Recombinant DNA technology has not only advanced the field of allergen characterization but also allowed the rational design and production of well-defined recombinant hypoallergenic allergen derivatives [13], which were even the first recombinant proteins to be tested for SIT in a clinical trial in allergic patients ten years ago [22].
2. Definition and construction of recombinant hypoallergens
Since the isolation of the first allergen-encoding cDNAs in the late nineteen-eighties a lot of information about the primary and three-dimensional structures, IgE and T cell epitopes of clinically relevant allergens has been collected [23]. Based on this knowledge approaches for the preparation of allergen derivatives with low or no IgE reactivity were tested with a view of targeting selectively the immune system [24]. Synthetic peptides containing allergen-specific T cell epitopes derived from the major cat allergen Fel d 1 were tested in first immunotherapy trials [25]. These peptides were thought to target allergen-specific T cells, as they were too small to induce IgE-mediated allergic reactions or a relevant allergen-specific IgG response. Interestingly, clinical trials using T cell peptides demonstrated then the induction of an IgE-independent allergic inflammation in the treated patients which indicated that these peptides despite their ability to induce tolerance in vitro can stimulate allergen-specific T cells in vivo [26,27]. The first genetically engineered recombinant allergen derivatives which were tested in clinical trials were constructed from the major birch pollen allergen Bet v 1 and represented two allergen-derived fragments and a recombinant Bet v 1 trimer [28,29]. The basic idea behind the construction of recombinant hypoallergenic allergen derivatives was to engineer a recombinant allergen derivative which exhibits reduced IgE-reactivity (i.e. the ability to bind allergen-specific IgE antibodies) and allergenic activity (i.e., the ability to induce IgE-mediated mast cells or basophil degranulation). The aim was to reduce IgE-mediated side-effects in the course of immunotherapy. Despite the fact that most of the hypoallergens lost their native conformation, it has been found that they induce upon immunization allergen-specific IgG antibody responses, which interfere with the IgE recognition of wildtype allergens [30]. Most of the recombinant hypoallergens are made in a form to preserve most allergen-specific T cell epitopes and they generally seem to induce less allergen-specific IgE antibodies than the corresponding wild type allergens upon immunization (i.e., reduced allergenicity which refers to the ability to induce an allergen-specific IgE antibody response upon allergen contact) [30,31]. Table 1 provides a summary of the types of recombinant allergen derivatives which have been made.
Table 1.
Types of recombinant hypoallergenic allergen derivatives.
The reduction of IgE reactivity can be obtained either by mutation of the amino acid residues involved in IgE-binding, or by the disruption of the three-dimensional structure of the allergen [23]. The latter approach is based on the finding that the IgE antibody response to respiratory allergens is mainly directed to conformational epitopes, probably because during allergic sensitization the immune system recognizes these allergens as intact proteins when they are taken up via the respiratory mucosa [32]. Several studies have demonstrated that the intact conformation of allergens is crucial for IgE binding and that the disruption of the three-dimensional fold leads to a reduction or loss of the IgE binding capacity [32]. The production of allergen-derived fragments lacking IgE-reactivity as described for the major birch pollen allergen Bet v 1 [28] can in principle by applied to other allergens [33-35], but may be limited by the lower immunogenicity of these fragments, resulting in a low allergen-specific IgG response. Therefore this strategy was further developed towards the construction of mosaic molecules, consisting of re-assembled allergen-derived fragments within one molecule [31,36-39]. These mosaics retained the lack of IgE-binding activity of their components due to the loss of their three-dimensional structure but were able to induce robust allergen-specific IgG antibody responses. The fusion of a hypoallergenic mosaic molecule and an allergen fragment, derived from different major grass pollen allergens, respectively, was shown to increase the immunogenicity of the included molecules [40]. The concept of producing hybrid molecules consisting of hypoallergenic allergen-derived fragments is especially attractive for the construction of hypoallergenic vaccines for complex allergen sources, like grass pollen and house dust mite, which contain several major allergens because it allows to increase the immunogenicity of the vaccine and at the same time to reduce the number of molecules which need to be included in the vaccine ([40-42], reviewed in [43]).
Interestingly it was found, that oligomerization of allergens may yield hypoallergenic oligomers for certain allergens by a mechanism of altered IgE epitope presentation [44]. The fusion of three full-length copies of an allergen-encoding gene was shown for the major birch pollen allergen Bet v 1 and resulted in a Bet v 1 trimer [29]. The trimer turned out to be a highly immunogenic protein in allergic patients but showed a strongly reduced allergenic activity [22,45,46]. When the hypoallergenic nature of the molecule was further analyzed it was found to form high molecular weight aggregates leading to altered presentation of IgE epitopes to effector cell-bound IgE which was proposed as a mechanism responsible for the hypoallergenicity [44].
Several hypoallergens were obtained by insertion of mutations in the wildtype sequence [47-63]. Hypoallergens derived from the major grass pollen allergen Phl p 5 and Phl p 6 were generated by introducing deletions in order to disrupt the protein structure[60,63]. Amino acid exchanges represent a widely used method for the generation of hypoallergens, whereby different strategies have been applied. The mutation of cysteins which are involved in the formation of disulfide bonds stabilizing the protein structure has been shown to disrupt the allergen conformation and IgE epitopes and abolish IgE recognition. Allergens belonging to the family of Ca-binding proteins nicely illustrate the relation between IgE-binding and allergen structure. As their conformation depends on the presence of protein-bound calcium, it was shown that depletion of calcium from these allergens lead to the loss of IgE reactivity [64]. The same effect was observed when mutating the calcium-binding domains, as shown for the cross-reactive grass pollen allergen Phl p 7 and the major fish allergen Cyp c 1 [55,58].
The strategies described above allow the production of well-defined molecules (regarding their biochemical and immunological characteristics as well as the production process) in a stable and reproducible way, which cannot be achieved by chemical modifications of the corresponding recombinant wildtype allergen [65,66].
Beside the loss of conformation and therefore IgE reactivity, amino acids, which are directly involved in the allergen–IgE interaction, were targeted by site-directed mutagenesis [67,68]. However, IgE epitopes are difficult to identify, as IgE directed to a conformational epitope normally does not bind to linear peptides [69]. Therefore more elaborated methods for IgE epitope mapping studies have to be applied, like the co-crystallization of allergen-IgE complexes or competition experiments using antibody probes with defined specificity to block IgE binding [70,71]. Mutational analysis introducing single amino acid exchanges might not be sufficient to distinguish amino acids involved in IgE binding from those involved in stabilizing the three dimensional fold. Furthermore, it is a moot point whether this approach ensures the disruption of all IgE epitopes recognized by polyclonal IgE antibodies from allergic patients.
3. Preclinical characterization of recombinant hypoallergens
Hypoallergens derived from many of the clinically relevant allergens have been produced, and a number of in vitro and in vivo methods for the preclinical evaluation of these molecules were established, which are summarized in Table 2.
Table 2.
Methods for the preclinical evaluation of hypoallergens.
| in vitro | in vivo | |
|---|---|---|
| IgE reactivity | Immunoblot, ELISA, quantitative IgE assays, solid-phase competition assays | |
| T cell reactivity | Proliferation of PBMC, T cell lines, clones, cytokine secretion | Atopy patch test |
| Allergenic activity | Histamine release, basophil activation | Skin testing, nasal provocation |
| Immunogenicity | Allergen-specific IgG measurement by ELISA and quantitative measurement | Immunization of animals |
| Allergenicity | Allergen-specific IgE measurement by ELISA, RBL-assay | Immunization of animals |
While recombinant allergens should mimic their natural counterparts, and therefore have to be produced in suitable expression systems, recombinant hypoallergens are normally well expressed in Escherichia coli, with the aim to yield high amounts of the recombinant proteins, which often accumulate unfolded in inclusion bodies [28]. The development of reproducible expression and purification protocols resulting in pure, stable proteins soluble under physiological conditions is a prerequisite for the further evaluation of the candidate molecules, and it is likely that certain candidate molecules are already excluded at that stage, e.g. because of low yield. SDS-PAGE, immunoblotting with specific antibody probes, mass spectrometry and size exclusion chromatography have been established for the estimation of the purity, size and integrity of the recombinant hypoallergens, whereby the latter also provides information about the aggregation behaviour[28,29,31,44]. As the binding of IgE antibodies depends on the intact fold of the allergen, the comparison of the secondary structure of hypoallergens and the corresponding natural allergens is of interest. Circular dicroism (CD) measurement allows the determination of the secondary structure content of the proteins. CD analysis of hypoallergens has confirmed that their secondary structure often differs from that of the wildtype allergen, often containing an increased amount of random coil conformation typical for unfolded proteins [28,72]. As these structural features remain constant the method represents an important tool to ensure consistency among different batches in the production process. The above mentioned physicochemical methods should be considered as tools for the characterization of the recombinant hypoallergen and the reproducibility of the production method. However, whether a particular hypoallergen qualifies as vaccine should not be determined by the physicochemical properties but by the immunological features of the protein.
We therefore would like to discuss immunological features of hypoallergens which make them desirable vaccine candidates and modes for their evaluation (Table 3). Several in vitro methods for the measurement of IgE reactivity of hypoallergens compared to wildtype allergens have been established, and in principle two test conditions have been proposed, either solid phase immunoassays, where the hypoallergens are bound to a membrane, ELISA plate or on other solid matrix and exposed to serum IgE from allergic patients, or liquid-phase assays, measuring the reduction of IgE binding to membrane bound wildtype allergens after pre-incubation of sera with the hypoallergens [28,29,33,63]. However, care must be taken when the reduction of IgE reactivity is taken as a parameter because we know that IgE reactivity does not always reflect allergenic activity [29,44,73].
Table 3.
Characteristics of hypoallergenic derivatives.
| Reduced IgE-reactivity |
| Reduced allergenic activity |
| Preservation of allergen-specific T cell epitopes |
| Reduced allergenicity |
| Induction of allergen-specific blocking IgG antibodies |
The allergenic activity of hypoallergens refers to their ability to cross-link receptor-bound IgE on the surface of basophils and mast cells leading to the release of inflammatory mediators. The classical in vitro surrogate test for measuring this reaction has been the histamine release assay, a method which has been established even before the characterization of IgE antibodies [74]. Recently an assay measuring by means of flow cytometry the surface expression of the activation marker CD203c on human blood basophils, which is up-regulated upon allergen-induced IgE cross-linking has been developed, and is meanwhile well accepted as a fast and sensitive method for the estimation of the allergenic activity of allergens and allergen derivatives [75]. The reduction of allergenic activity of a given hypoallergen should be evaluated in a representative population of allergic patients and it should be aimed at a consistently at least ten-fold reduction of allergenic activity compared to the wildtype molecules. Care should be taken because the sensitivity of patients may vary. For example, pollen allergic patients show a strongly increased sensitivity after the pollen season. Those hypoallergens which are considered suitable candidates for vaccine formulation often exhibit an even 100-fold or higher reduction of allergenic activity [76].
After in vitro evaluation of the allergenic activity and having performed the assessment of T cell reactivity and immunogenicity as well as testing for allergenicity in experimental animal models, safety studies should be performed in allergic patients which test the reduced allergenic activity in vivo. In fact, both skin testing and nasal provocations reflect the clinical symptoms of allergic patients in response to allergens well and therefore represent important tools to estimate the allergenic activity of hyperallergens [45,77]. Skin tests are easy to perform and allow the titration of sensitivity. Indeed extensive skin test studies preceded the first clinical immunotherapy trial with hypoallergenic Bet v 1 derived fragments and trimer. Both skin prick and intradermal tests in birch pollen allergic patients demonstrated the reduced capacity of these molecules to induce immediate type skin reactions leading to similar results. In addition the end-point titration represents an important method for estimating the allergenic activity of hypoallergens [46,76].
The tolerization of allergen-specific T cells in allergic patients has been proposed as one of the mechanisms underlying specific immunotherapy [78]. Initially, investigators were therefore keen to detect putative tolerogenic allergen-specific T cell epitopes and to assess their maintenance in the hypoallergens [30,79,80]. These were therefore initially evaluated regarding their ability to induce proliferation of allergen-specific T lymphocytes in vitro [29,30]. PBMC from allergic blood donors, but also allergen-specific human T cell lines and clones have been shown to proliferate in response to hypoallergens comparable to the corresponding wildtype allergens, although different cytokine patterns could be observed in some cases [29,30,63,65]. PBMC seem to be more appropriate for these kinds of tests, as only a few allergens contain only one predominant T cell epitope and the use of T cell clones may therefore not fully reflect the diversity of T cell recognition of allergens and in particular not the precursor frequency of T cells with a given specificity in different patients [81].
Whether the presence of allergen-specific T cell epitopes in hypoallergens is indeed a desirable feature has come recently into question. In fact, when immunotherapy was carried out with hypoallergens and T cell epitope-containing peptides without IgE reactivity, it could be shown that IgE-mediated side effects were almost completely abolished but still late phase, most likely T cell-mediated side effects were observed [27,82]. A further investigation of this phenomenon with hypoallergenic Bet v 1-derived fragments containing the relevant Bet v 1-specific T cell epitopes showed that these molecules could also induce a non-IgE-mediated delayed-type hypersensitivity reaction in vivo by means of atopy patch testing [83]. The results of this study together with results from earlier studies with T cell epitope-containing allergen peptides without IgE reactivity demonstrated that late phase side effects can be mediated by T cell-mediated mechanisms. As a consequence, researchers are now evaluating novel generations of hypoallergenic molecules with reduced allergen-specific T cell reactivity (reviewed in [84]).
As hypoallergens differ in sequence and structure from the naturally occurring allergens, in vivo immunization protocols in animal models had to be developed in order to test the ability of these molecules to induce an allergen-specific IgG antibody response[85,86,88]. These IgG antibodies should also cross-react with homologous allergens, block the binding of allergic patients’ IgE to the allergen and inhibit allergen-induced basophil degranulation in allergic patients. Previous studies using purified recombinant wildtype allergens for the immunization of mice showed that the development of allergen-specific IgG1 antibody responses in mice correlated quite well with the immunogenicity of these molecules in allergic patients [85]. These antibodies inhibited patients’ IgE-binding to the allergen as well as effector cell activation in vitro [85]. Therefore the mouse model was introduced for the evaluation of antibody responses to hypoallergens [88]. Furthermore, the induction of allergen-specific IgE responses upon immunization of mice with wildtype allergens was comparable to their allergenicity in humans [85,87]. Some mouse studies could provide evidence for the low allergenicity of hypoallergenic molecules, resulting in lower allergen-specific IgE antibody responses compared to immunization with the wildtype allergen, an important finding regarding prophylactic vaccination strategies [13,31]. Besides the mouse, also rabbits as out-bred species were used to study the characteristics of the IgG antibody responses which are obtained by immunization with hypoallergens [30].
Animal models for allergic sensitization are considered not too informative for the evaluation of hypoallergens because sensitization is induced under artificial conditions with high amounts of allergen, the resulting pathology is often a mixed IgE and delayed type reaction and does not fully mimick allergy in patients and most importantly, the epitopes recognized by IgE and T cells often differ substantially from those recognized by allergic patients [89].
4. Mechanisms of SIT with hypoallergens
Immunotherapy trials have been performed with hypoallergens derived from the major birch pollen allergen Bet v 1 [22,65,90,91]. The first clinical trial using purified recombinant molecules for allergy vaccination was conducted as a randomized, double-blind placebo-controlled injection trial with a mixture of two recombinant Bet v 1-derived fragments and a recombinant Bet v 1 trimer [22]. Based on the results of this trial, a recombinant hypoallergenic folding variant of Bet v 1 with similar properties as the recombinant Bet v 1 fragments was developed and successfully evaluated in clinical trials up to phase III [92,93].
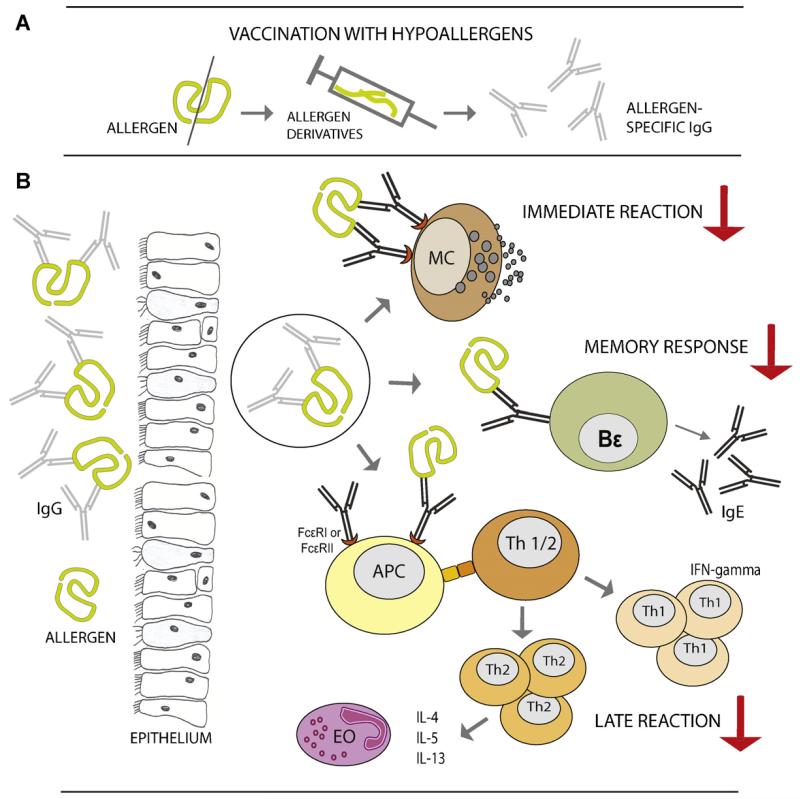
The rBet v 1 fragments and trimer as well as the folding variant exhibited a more than 100-fold reduced allergenic activity compared to the wildtype allergen [65,76]. When patients received subcutaneous injections of aluminium hydroxide-adsorbed rBet v 1 derivatives they tolerated doses of up to 80 μg per injections and cumulative doses reaching milligram amounts confirming the reduced allergenic activity of the antigens [22,82]. Patients receiving the active preparations developed high levels of allergen-specific IgG antibodies which also cross-reacted with Bet v 1-related pollen and food allergens [22,94]. The allergen-specific antibody response consisted mainly of IgG1, IgG4 and IgG2 antibodies, and was detected not only in the blood but also in mucosal secretions [22,95,96]. Only low levels of Bet v 1-specific IgA could be measured. The allergen-specific IgG response induced by vaccination was directed against new and also sequential epitopes because the immunogens (i.e., rBet v 1 fragments) represented unfolded molecules [97]. However, the therapy-induced IgG recognized areas on the Bet v 1 allergen which overlapped with the conformational epitopes recognized by IgE and thus strongly inhibited patients IgE binding to the allergens, allergen-induced basophil activation and immediate symptoms (i.e., skin and nasal sensitivity)[22,82,95] (Fig. 1). IgG antibodies induced by the Bet v 1 derivatives were shown to inhibit immediate allergic reactions in an in vitro model, measuring reduced allergen-specific sensitivity of blood basophils after addition of the IgG containing sera from patients of the actively treated groups [22]. According to the in vitro basophil histamine-release experiments, the reduction of skin sensitivity and nasal sensitivity to Bet v 1 was associated with the presence of IgG specific for the allergen [95]. SIT with the recombinant Bet v 1 hypoallergens thus induced classical “blocking antibodies” as described by Stull in 1935 [4] and Loveless in 1940 [98] and are the result of a new counter IgG and probably T cell response which antagonizes IgE-mediated allergic inflammation. Subcutaneous SIT with the hypoallergens thus has vaccination character.
Fig. 1.
Therapeutic effects of vaccination with hypoallergens mediated by blocking antibodies. (A) Vaccination with hypoallergens induces allergen-specific IgG antibodies, which inhibit IgE binding to the allergen. (B) Blocking IgG antibodies bind to the allergen and inhibit IgE-mediated effector cell degranulation, and hence the immediate allergic symptoms. They prevent the allergen-uptake by IgE via Fcε-receptors on antigen-presenting cells, the presentation of allergen-derived peptides to Th1 and Th2 cells, and thus the activation of allergen-specific T cells, which may reduce late phase allergic reactions. They also inhibit the activation of allergen-specific memory Bε cells and therefore the boost of allergen-specific IgE antibody responses. (MC, mast cell; APC, antigen presenting cell; Th, T helper cell, Bε, memory Bε cell; EO, eosinophil granulocyte.)
Previous SIT studies performed with natural pollen extracts demonstrated the reduction of CD23 expression on B cells from treated patients [99]. Facilitated antigen presentation to T cells has been described as an important effector mechanism of the low affinity receptor for IgE [100]. Allergen-uptake of antigen-presenting cells via CD23 has been shown to result in T cell activation and the release of pro-inflammatory cytokines. Allergen-specific IgG antibodies induced by SIT with pollen extracts could inhibit IgE-facilitated allergen uptake via CD23 and the activation of allergen-specific T cells [101-104]. A similar inhibiting effect was also found for IgG antibodies induced by vaccination with hypoallergens [105] using an IgE-facilitated allergen binding (FAB) assay [106]. Depletion experiments showed that the inhibition of IgE/allergen binding to CD23 was only mediated by IgG and not by other antibody classes like IgA or IgM [101]. Furthermore, the blocking activity of allergen-specific IgG was associated with the number of newly recognized Bet v 1-specific peptide epitopes, emphasizing the important role of epitope specificity of the vaccination-induced antibody response besides simply measuring allergen-specific antibody levels [105].
It is well known that IgE responses to respiratory allergens increase after allergen contact, e.g. after nasal exposure to allergens during the pollen season, due to the activation of allergen-specific epsilon memory B cells [107,108]. It could be observed, that in patients treated with the Bet v 1 derivatives the seasonally induced rise of IgE responses after the birch pollen season was blunted [22], similar as was observed for SIT with natural pollen allergen extracts[109] and for SIT with CpG-conjugated Amb a 1 [110]. Although there is not yet formal experimental proof it may be assumed that a blunting of IgE rises in the course of continuous SIT will lead to a reduction of allergen-specific IgE levels and thus play a role regarding the long-term effects of SIT even after discontinuation of treatment [6].
The possible mechanisms of subcutaneous immunotherapy with hypoallergens are summarized in Table 4. In summary, it seems that the majority of protective mechanisms induced by SIT with hypoallergens operates via the induction of allergen-specific IgG antibodies which inhibit immediate, late phase allergic inflammation as well as the boosting of the underlying allergen-specific IgE response (Fig. 1). In light of the observation, that the residual side effects observed in the course of treatment with recombinant hypoallergens is of the late phase type and most likely mediated by allergen-specific T cells it may be considered to further improve these molecules by the elimination of allergen-specific T cell epitopes. The T cell help for the induction of allergen-specific antibodies without activating allergen-specific T cells may be achieved by using carrier molecules which contain allergen-derived peptides for focusing the blocking IgG response towards the IgE epitopes of allergens [84,111-115].
Table 4.
Possible mechanisms of subcutaneous SCIT with hypoallergens.
|
Acknowledgements
Supported by grants F1815 and P23350-B11 of the Austrian Science Fund, by the Christian Doppler Research Association, the FAST project of the Framework Program 7 (FP7) of the European Commission (FAST Project), and Biomay, Vienna, Austria.
Footnotes
Disclosure statement
B.L. is funded by the Austrian Science Fund (FWF) and the FAST project of the Seventh Framework Program (FP7) of the European Commission.
References
- [1].Floistrup H, Swartz J, Bergstrom A, Alm JS, Scheynius A, van Hage M, et al. The Parsifal Study. Allergic disease and sensitization in Steiner school children. J Allergy Clin Immunol. 2006;117:59–66. doi: 10.1016/j.jaci.2005.09.039. [DOI] [PubMed] [Google Scholar]
- [2].Kay AB. Allergy and allergic diseases. Blackwell Science; Oxford: 2008. [Google Scholar]
- [3].Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;1:1572–3. [Google Scholar]
- [4].Cooke RA, Barnard JH, Hebald S, Stull A. Serological evidence of immunity with coexisting sensitization in a type of human allergy. J Exp Med. 1935;62:733–50. doi: 10.1084/jem.62.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–62. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- [6].Durham SR, Walker SM, Varga EM, Jacobson MR, O‘Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- [7].Möller C, Dreborg S, Ferdousi HA, Halken S, Høst A, Jacobsen L, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–6. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- [8].Focke M, Marth K, Flicker S, Valenta R. Heterogeneity of commercial timothy grass pollen extracts. Clin Exp Allergy. 2008;38:1400–8. doi: 10.1111/j.1365-2222.2008.03031.x. [DOI] [PubMed] [Google Scholar]
- [9].Focke M, Marth K, Valenta R. Molecular composition and biological activity of commercial birch pollen allergen extracts. Eur J Clin Invest. 2009;39:429–36. doi: 10.1111/j.1365-2362.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- [10].Curin M, Reininger R, Swoboda I, Focke M, Valenta R, Spitzauer S. Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int Arch Allergy Immunol. 2011;154:258–63. doi: 10.1159/000321113. [DOI] [PubMed] [Google Scholar]
- [11].Trivedi B, Valerio C, Slater JE. Endotoxin content of standardized allergen vaccines. J Allergy Clin Immunol. 2003;111:777–83. doi: 10.1067/mai.2003.1338. [DOI] [PubMed] [Google Scholar]
- [12].van der Veen MJ, Mulder M, Witteman AM, van Ree R, Aalberse RC, Jansen HM, et al. False-positive skin prick test responses to commercially available dog dander extracts caused by contamination with house dust mite (Dermatophagoides pteronyssinus) allergens. J Allergy Clin Immunol. 1996;98:1028–34. doi: 10.1016/s0091-6749(96)80187-4. [DOI] [PubMed] [Google Scholar]
- [13].Valenta R. The future of antigen-specific immunotherapy of allergy. Nat Rev Immunol. 2002;2:446–53. doi: 10.1038/nri824. [DOI] [PubMed] [Google Scholar]
- [14].Niederberger V, Pauli G, Grönlund H, Fröschl R, Rumpold H, Kraft D, et al. Recombinant birch pollen allergens (rBet v 1 and rBet v 2) contain most of the IgE epitopes present in birch, alder, hornbeam, hazel, and oak pollen: a quantitative IgE inhibition study with sera from different populations. J Allergy Clin Immunol. 1998;102:579–91. doi: 10.1016/s0091-6749(98)70273-8. [DOI] [PubMed] [Google Scholar]
- [15].Niederberger V, Laffer S, Fröschl R, Kraft D, Rumpold H, Kapiotis S, et al. IgE antibodies to recombinant pollen allergens (Phl p 1, Phl p 2, Phl p 5, and Bet v 2) account for a high percentage of grass pollen-specific IgE. J Allergy Clin Immunol. 1998;101:258–64. doi: 10.1016/s0091-6749(98)70391-4. [DOI] [PubMed] [Google Scholar]
- [16].Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–13. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- [17].Pauli G, Larsen TH, Rak S, Horak F, Pastorello E, Valenta R, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–60. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- [18].Larenas-Linnemann D. Oralair Birch, a recombinant major birch pollen allergen tablet for sublingual immunotherapy of allergic rhinitis caused by birch pollen. Curr Opin Investig Drugs. 2010;11:586–96. [PubMed] [Google Scholar]
- [19].Mellerup MT, Hahn GW, Poulsen LK, Malling H. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000;30:1423–9. doi: 10.1046/j.1365-2222.2000.00910.x. [DOI] [PubMed] [Google Scholar]
- [20].Stull A, Cooke RA, Sherman WB. Experimental and clinical studies of fresh and modified pollen extracts. J Allergy. 1940;11:439. [Google Scholar]
- [21].Marsh DG, Lichtenstein LM, Campbell DH. Studies on allergoids prepared from naturally occurring allergens. I. Assay of allergenicity and antigenicity of formalinized rye group I component. Immunology. 1970;18:705–22. [PMC free article] [PubMed] [Google Scholar]
- [22].Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci USA. 2004;101(Suppl. 2):14677–82. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Valenta R, Ferreira F, Focke-Tejkl M, Linhart B, Niederberger V, Swoboda I, et al. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010;28:211–41. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- [24].Linhart B, Valenta R. Molecular design of allergy vaccines. Curr Opin Immunol. 2005;17:646–55. doi: 10.1016/j.coi.2005.09.010. [DOI] [PubMed] [Google Scholar]
- [25].Norman PS, Ohman JL, Jr, Long AA, Creticos PS, Gefter MA, Shaked Z, et al. Treatment of cat allergy with T-cell reactive peptides. Am J Respir Crit Care Med. 1996;154:1623–8. doi: 10.1164/ajrccm.154.6.8970345. [DOI] [PubMed] [Google Scholar]
- [26].O‘Hehir RE, Busch R, Rothbard JB, Lamb JR. An in vitro model of peptide-mediated immunomodulation of the human T cell response to Dermatophagoides spp (house dust mite) J Allergy Clin Immunol. 1991;87:1120–7. doi: 10.1016/0091-6749(91)92158-w. [DOI] [PubMed] [Google Scholar]
- [27].Haselden BM, Kay AB, Larché M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J Exp Med. 1999;189:1885–94. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vrtala S, Hirtenlehner K, Vangelista L, Pastore A, Eichler HG, Sperr WR, et al. Conversion of the major birch pollen allergen, Bet v 1, into two nonanaphylactic T cell epitope-containing fragments: candidates for a novel form of specific immunotherapy. J Clin Invest. 1997;99:1673–81. doi: 10.1172/JCI119330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vrtala S, Hirtenlehner K, Susani M, Akdis M, Kussebi F, Akdis CA, et al. Genetic engineering of a hypoallergenic trimer of the major birch pollen allergen Bet v 1. FASEB J. 2001;15:2045–7. doi: 10.1096/fj.00-0767fje. [DOI] [PubMed] [Google Scholar]
- [30].Vrtala S, Akdis CA, Budak F, Akdis M, Blaser K, Kraft D, et al. T cell epitope-containing hypoallergenic recombinant fragments of the major birch pollen allergen, Bet v 1, induce blocking antibodies. J Immunol. 2000;165:6653–9. doi: 10.4049/jimmunol.165.11.6653. [DOI] [PubMed] [Google Scholar]
- [31].Ball T, Linhart B, Sonneck K, Blatt K, Herrmann H, Valent P, et al. Reducing allergenicity by altering allergen fold: a mosaic protein of Phl p 1 for allergy vaccination. Allergy. 2009;64:569–80. doi: 10.1111/j.1398-9995.2008.01910.x. [DOI] [PubMed] [Google Scholar]
- [32].Valenta R, Kraft D. Recombinant allergen molecules: tools to study effector cell activation. Immunol Rev. 2001;179:119–27. doi: 10.1034/j.1600-065x.2001.790112.x. [DOI] [PubMed] [Google Scholar]
- [33].Ball T, Vrtala S, Sperr WR, Valent P, Susani M, Kraft D, et al. Isolation of an immunodominant IgE hapten from an epitope expression cDNA library. Dissection of the allergic effector reaction. J Biol Chem. 1994;269:28323–8. [PubMed] [Google Scholar]
- [34].Zeiler T, Taivainen A, Rytkönen M, Rautiainen J, Karjalainen H, Mäntyjärvi R, et al. Recombinant allergen fragments as candidate preparations for allergen immunotherapy. J Allergy Clin Immunol. 1997;100:721–7. doi: 10.1016/s0091-6749(97)70264-1. [DOI] [PubMed] [Google Scholar]
- [35].Elfman LH, Whitley P, Schmidt M, van Hage-Hamsten M. IgE binding capacity of synthetic and recombinant peptides of the major storage mite (Lepidoglyphus destructor) allergen, Lep d 2. Int Arch Allergy Immunol. 1998;117:167–73. doi: 10.1159/000024006. [DOI] [PubMed] [Google Scholar]
- [36].Mothes-Luksch N, Stumvoll S, Linhart B, Focke M, Krauth MT, Hauswirth A, et al. Disruption of allergenic activity of the major grass pollen allergen Phl p 2 by reassembly as a mosaic protein. J Immunol. 2008;181:4864–73. doi: 10.4049/jimmunol.181.7.4864. [DOI] [PubMed] [Google Scholar]
- [37].Westritschnig K, Linhart B, Focke-Tejkl M, Pavkov T, Keller W, Ball T, et al. A hypoallergenic vaccine obtained by tail-to-head restructuring of timothy grass pollen profilin, Phl p 12, for the treatment of cross-sensitization to profilin. J Immunol. 2007;179:7624–34. doi: 10.4049/jimmunol.179.11.7624. [DOI] [PubMed] [Google Scholar]
- [38].Chen KW, Fuchs G, Sonneck K, Gieras A, Swoboda I, Douladiris N, et al. Reduction of the in vivo allergenicity of Der p 2, the major house-dust mite allergen, by genetic engineering. Mol Immunol. 2008;45:2486–98. doi: 10.1016/j.molimm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- [39].Campana R, Vrtala S, Maderegger B, Jertschin P, Stegfellner G, Swoboda I, et al. Hypoallergenic derivatives of the major birch pollen allergen Bet v 1 obtained by rational sequence reassembly. J Allergy Clin Immunol. 2010;126:1024–31. doi: 10.1016/j.jaci.2010.05.023. [DOI] [PubMed] [Google Scholar]
- [40].Linhart B, Mothes-Luksch N, Vrtala S, Kneidinger M, Valent P, Valenta R. A hypoallergenic hybrid molecule with increased immunogenicity consisting of derivatives of the major grass pollen allergens, Phl p 2 and Phl p 6. Biol Chem. 2008;389:925–33. doi: 10.1515/BC.2008.105. [DOI] [PubMed] [Google Scholar]
- [41].King TP, Jim SY, Monsalve RI, Kagey-Sobotka A, Lichtenstein LM, Spangfort MD. Recombinant allergens with reduced allergenicity but retaining immunogenicity of the natural allergens: hybrids of yellow jacket and paper wasp venom allergen antigen 5s. J Immunol. 2001;166:6057–65. doi: 10.4049/jimmunol.166.10.6057. [DOI] [PubMed] [Google Scholar]
- [42].Karamloo F, Schmid-Grendelmeier P, Kussebi F, Akdis M, Salagianni M, von Beust BR, et al. Prevention of allergy by a recombinant multi-allergen vaccine with reduced IgE binding and preserved T cell epitopes. Eur J Immunol. 2005;35:3268–76. doi: 10.1002/eji.200425522. [DOI] [PubMed] [Google Scholar]
- [43].Linhart B, Valenta R. Vaccine engineering improved by hybrid technology. Int Arch Allergy Immunol. 2004;134:324–31. doi: 10.1159/000079535. [DOI] [PubMed] [Google Scholar]
- [44].Campana R, Vrtala S, Maderegger B, Dall‘Antonia Y, Zafred D, Blatt K, et al. Altered IgE epitope presentation: a model for hypoallergenic activity revealed for Bet v 1 trimer. Mol Immunol. 2011;48:431–41. doi: 10.1016/j.molimm.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].van Hage-Hamsten M, Johansson E, Roquet A, Peterson C, Andersson M, Greiff L, et al. Nasal challenges with recombinant derivatives of the major birch pollen allergen Bet v 1 induce fewer symptoms and lower mediator release than rBet v 1 wild-type in patients with allergic rhinitis. Clin Exp Allergy. 2002;32:1448–53. doi: 10.1046/j.1365-2745.2002.01495.x. [DOI] [PubMed] [Google Scholar]
- [46].van Hage-Hamsten M, Kronqvist M, Zetterström O, Johansson E, Niederberger V, Vrtala S, et al. Skin test evaluation of genetically engineered hypoallergenic derivatives of the major birch pollen allergen, Bet v 1: results obtained with a mix of two recombinant Bet v 1 fragments and recombinant Bet v 1 trimer in a Swedish population before the birch pollen season. J Allergy Clin Immunol. 1999;104:969–77. doi: 10.1016/s0091-6749(99)70077-1. [DOI] [PubMed] [Google Scholar]
- [47].Olsson S, van Hage-Hamsten M, Whitley P. Contribution of disulphide bonds to antigenicity of Lep d 2, the major allergen of the dust mite Lepidoglyphus destructor. Mol Immunol. 1998;35:1017–23. doi: 10.1016/s0161-5890(98)00101-1. [DOI] [PubMed] [Google Scholar]
- [48].Ferreira F, Ebner C, Kramer B, Casari G, Briza P, Kungl AJ, et al. Modulation of IgE reactivity of allergens by site-directed mutagenesis: potential use of hypoallergenic variants for immunotherapy. FASEB J. 1998;12:231–42. doi: 10.1096/fasebj.12.2.231. [DOI] [PubMed] [Google Scholar]
- [49].Okada T, Swoboda I, Bhalla PL, Toriyama K, Singh MB. Engineering of hypoallergenic mutants of the Brassica pollen allergen, Bra r 1, for immunotherapy. FEBS Lett. 1998;434:255–60. doi: 10.1016/s0014-5793(98)00992-2. [DOI] [PubMed] [Google Scholar]
- [50].Bannon GA, Cockrell G, Connaughton C, West CM, Helm R, Stanley JS, et al. Engineering, characterization and in vitro efficacy of the major peanut allergens for use in immunotherapy. Int Arch Allergy Immunol. 2001;124:70–2. doi: 10.1159/000053672. [DOI] [PubMed] [Google Scholar]
- [51].Bonura A, Amoroso S, Locorotondo G, Di Felice G, Tinghino R, Geraci D, et al. Hypoallergenic variants of the Parietaria judaica major allergen Par j 1: a member of the non-specific lipid transfer protein plant family. Int Arch Allergy Immunol. 2001;126:32–40. doi: 10.1159/000049492. [DOI] [PubMed] [Google Scholar]
- [52].Swoboda I, De Weerd N, Bhalla PL, Niederberger V, Sperr WR, Valent P, et al. Mutants of the major ryegrass pollen allergen, Lol p 5, with reduced IgE-binding capacity: candidates for grass pollen-specific immunotherapy. Eur J Immunol. 2002;32:270–80. doi: 10.1002/1521-4141(200201)32:1<270::AID-IMMU270>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- [53].Rabjohn P, West CM, Connaughton C, Sampson HA, Helm RM, Burks AW, et al. Modification of peanut allergen Ara h 3: effects on IgE binding and T cell stimulation. Int Arch Allergy Immunol. 2002;128:15–23. doi: 10.1159/000057999. [DOI] [PubMed] [Google Scholar]
- [54].Banerjee B, Kurup VP, Greenberger PA, Kelly KJ, Fink JN. C-terminal cysteine residues determine the IgE binding of Aspergillus fumigatus allergen Asp f 2. J Immunol. 2002;169:5137–44. doi: 10.4049/jimmunol.169.9.5137. [DOI] [PubMed] [Google Scholar]
- [55].Westritschnig K, Focke M, Verdino P, Goessler W, Keller W, Twardosz A, et al. Generation of an allergy vaccine by disruption of the three-dimensional structure of the cross-reactive calcium-binding allergen, Phl p 7. J Immunol. 2004;172:5684–92. doi: 10.4049/jimmunol.172.9.5684. [DOI] [PubMed] [Google Scholar]
- [56].Buhot C, Chenal A, Sanson A, Pouvelle-Moratille S, Gelb MH, Ménez A, et al. Alteration of the tertiary structure of the major bee venom allergen Api m 1 by multiple mutations is concomitant with low IgE reactivity. Protein Sci. 2004;13:2970–8. doi: 10.1110/ps.04885404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Drew AC, Eusebius NP, Kenins L, de Silva HD, Suphioglu C, Rolland JM, et al. Hypoallergenic variants of the major latex allergen Hev b 6.01 retaining human T lymphocyte reactivity. J Immunol. 2004;173:5872–9. doi: 10.4049/jimmunol.173.9.5872. [DOI] [PubMed] [Google Scholar]
- [58].Swoboda I, Bugajska-Schretter A, Linhart B, Verdino P, Keller W, Schulmeister U, et al. A recombinant hypoallergenic parvalbumin mutant for immunotherapy of IgE-mediated fish allergy. J Immunol. 2007;178:6290–6. doi: 10.4049/jimmunol.178.10.6290. [DOI] [PubMed] [Google Scholar]
- [59].González-Rioja R, Ibarrola I, Arilla MC, Ferrer A, Mir A, Andreu C, et al. Genetically engineered hybrid proteins from Parietaria judaica pollen for allergen-specific immunotherapy. J Allergy Clin Immunol. 2007;120:602–9. doi: 10.1016/j.jaci.2007.04.039. [DOI] [PubMed] [Google Scholar]
- [60].Vrtala S, Focke M, Kopec J, Verdino P, Hartl A, Sperr WR, et al. Genetic engineering of the major timothy grass pollen allergen, Phl p 6, to reduce allergenic activity and preserve immunogenicity. J Immunol. 2007;179:1730–9. doi: 10.4049/jimmunol.179.3.1730. [DOI] [PubMed] [Google Scholar]
- [61].Marazuela EG, Rodríguez R, Barber D, Villalba M, Batanero E. Hypoallergenic mutants of Ole e 1, the major olive pollen allergen, as candidates for allergy vaccines. Clin Exp Allergy. 2007;37:251–60. doi: 10.1111/j.1365-2222.2006.02632.x. [DOI] [PubMed] [Google Scholar]
- [62].Asturias JA, Ibarrola I, Arilla MC, Vidal C, Ferrer A, Gamboa PM, et al. Engineering of major house dust mite allergens Der p 1 and Der p 2 for allergen-specific immunotherapy. Clin Exp Allergy. 2009;39:1088–98. doi: 10.1111/j.1365-2222.2009.03264.x. [DOI] [PubMed] [Google Scholar]
- [63].Wald M, Kahlert H, Weber B, Jankovic M, Keller W, Cromwell O, et al. Generation of a low immunoglobulin E-binding mutant of the timothy grass pollen major allergen Phl p 5a. Clin Exp Allergy. 2007;37:441–50. doi: 10.1111/j.1365-2222.2007.02669.x. [DOI] [PubMed] [Google Scholar]
- [64].Valenta R, Hayek B, Seiberler S, Bugajska-Schretter A, Niederberger V, Twardosz A, et al. Calcium-binding allergens: from plants to man. Int Arch Allergy Immunol. 1998;117:160–6. doi: 10.1159/000024005. [DOI] [PubMed] [Google Scholar]
- [65].Kahlert H, Suck R, Weber B, Nandy A, Wald M, Keller W, et al. Characterization of a hypoallergenic recombinant Bet v 1 variant as a candidate for allergen-specific immunotherapy. Int Arch Allergy Immunol. 2008;145:193–206. doi: 10.1159/000109288. [DOI] [PubMed] [Google Scholar]
- [66].Toda M, Reese G, Gadermaier G, Schulten V, Lauer I, Egger M, et al. Protein unfolding strongly modulates the allergenicity and immunogenicity of Pru p 3, the major peach allergen. J Allergy Clin Immunol. 2011;128:1022–30. doi: 10.1016/j.jaci.2011.04.020. [DOI] [PubMed] [Google Scholar]
- [67].Beezhold DH, Hickey VL, Sussman GL. Mutational analysis of the IgE epitopes in the latex allergen Hev b 5. J Allergy Clin Immunol. 2001;107:1069–76. doi: 10.1067/mai.2001.115482. [DOI] [PubMed] [Google Scholar]
- [68].Holm J, Gajhede M, Ferreras M, Henriksen A, Ipsen H, Larsen JN, et al. Allergy vaccine engineering: epitope modulation of recombinant Bet v 1 reduces IgE binding but retains protein folding pattern for induction of protective blocking-antibody responses. J Immunol. 2004;173:5258–67. doi: 10.4049/jimmunol.173.8.5258. [DOI] [PubMed] [Google Scholar]
- [69].Greene WK, Cyster JG, Chua KY, O‘Brien RM, Thomas WR. IgE and IgG binding of peptides expressed from fragments of cDNA encoding the major house dust mite allergen Der p I. J Immunol. 1991;147:3768–73. [PubMed] [Google Scholar]
- [70].Padavattan S, Flicker S, Schirmer T, Madritsch C, Randow S, Reese G, et al. High-affinity IgE recognition of a conformational epitope of the major respiratory allergen Phl p 2 as revealed by X-ray crystallography. J Immunol. 2009;182:2141–51. doi: 10.4049/jimmunol.0803018. [DOI] [PubMed] [Google Scholar]
- [71].Gieras A, Cejka P, Blatt K, Focke-Tejkl M, Linhart B, Flicker S, et al. Mapping of conformational IgE epitopes with peptide-specific monoclonal antibodies reveals simultaneous binding of different IgE antibodies to a surface patch on the major birch pollen allergen, Bet v 1. J Immunol. 2011;186:5333–44. doi: 10.4049/jimmunol.1000804. [DOI] [PubMed] [Google Scholar]
- [72].Verdino P, Keller W. Circular dichroism analysis of allergens. Methods. 2004;32:241–8. doi: 10.1016/j.ymeth.2003.08.017. [DOI] [PubMed] [Google Scholar]
- [73].Westritschnig K, Horak F, Swoboda I, Balic N, Spitzauer S, Kundi M, et al. Different allergenic activity of grass pollen allergens revealed by skin testing. Eur J Clin Invest. 2008;38:260–7. doi: 10.1111/j.1365-2362.2008.01938.x. [DOI] [PubMed] [Google Scholar]
- [74].Lichtenstein LM, Osler AG. Studies in the mechanisms of hypersensitivity phenomena. IX. Histamine release from human leukocytes by ragweed pollen antigen. J Exp Med. 1964;120:507–30. doi: 10.1084/jem.120.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hauswirth AW, Natter S, Ghannadan M, Majlesi Y, Schernthaner GH, Sperr WR, et al. Recombinant allergens promote expression of CD203c on basophils in sensitized individuals. J Allergy Clin Immunol. 2002;110:102–9. doi: 10.1067/mai.2002.125257. [DOI] [PubMed] [Google Scholar]
- [76].Pauli G, Purohit A, Oster JP, De Blay F, Vrtala S, Niederberger V, et al. Comparison of genetically engineered hypoallergenic rBet v 1 derivatives with rBet v 1 wild-type by skin prick and intradermal testing: results obtained in a French population. Clin Exp Allergy. 2000;30:1076–84. doi: 10.1046/j.1365-2222.2000.00869.x. [DOI] [PubMed] [Google Scholar]
- [77].Niederberger V, Stübner P, Spitzauer S, Kraft D, Valenta R, Ehrenberger K, et al. Skin test results but not serology reflects immediate type respiratory sensitivity: a study performed with recombinant allergen molecules. J Invest Dermatol. 2001;117:848–51. doi: 10.1046/j.0022-202x.2001.01470.x. [DOI] [PubMed] [Google Scholar]
- [78].Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- [79].O‘Hehir RE, Verhoef A, Panagiotopoulou E, Keswani S, Hayball JD, Thomas WR, et al. Analysis of human T cell responses to the group II allergen of Dermatophagoides species: localization of major antigenic sites. J Allergy Clin Immunol. 1993;92:105–13. doi: 10.1016/0091-6749(93)90044-g. [DOI] [PubMed] [Google Scholar]
- [80].Ebner C, Szépfalusi Z, Ferreira F, Jilek A, Valenta R, Parronchi P, et al. Identification of multiple T cell epitopes on Bet v I, the major birch pollen allergen, using specific T cell clones and overlapping peptides. J Immunol. 1993;150:1047–54. [PubMed] [Google Scholar]
- [81].Jahn-Schmid B, Kelemen P, Himly M, Bohle B, Fischer G, Ferreira F, et al. The T cell response to Art v 1, the major mugwort pollen allergen, is dominated by one epitope. J Immunol. 2002;169:6005–11. doi: 10.4049/jimmunol.169.10.6005. [DOI] [PubMed] [Google Scholar]
- [82].Purohit A, Niederberger V, Kronqvist M, Horak F, Grönneberg R, Suck R, et al. Clinical effects of immunotherapy with genetically modified recombinant birch pollen Bet v 1 derivatives. Clin Exp Allergy. 2008;38:1514–25. doi: 10.1111/j.1365-2222.2008.03042.x. [DOI] [PubMed] [Google Scholar]
- [83].Campana R, Mothes N, Rauter I, Vrtala S, Reininger R, Focke-Tejkl M, et al. Non-IgE-mediated chronic allergic skin inflammation revealed with rBet v 1 fragments. J Allergy Clin Immunol. 2008;121:528–30. doi: 10.1016/j.jaci.2007.09.014. [DOI] [PubMed] [Google Scholar]
- [84].Focke M, Swoboda I, Marth K, Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010;40:385–97. doi: 10.1111/j.1365-2222.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- [85].Vrtala S, Ball T, Spitzauer S, Pandjaitan B, Suphioglu C, Knox B, et al. Immunization with purified natural and recombinant allergens induces mouse IgG1 antibodies that recognize similar epitopes as human IgE and inhibit the human IgE-allergen interaction and allergen-induced basophil degranulation. J Immunol. 1998;160:6137–44. [PubMed] [Google Scholar]
- [86].Epstein MM. Do mouse models of allergic asthma mimic clinical disease? Int Arch Allergy Immunol. 2004;133:84–100. doi: 10.1159/000076131. [DOI] [PubMed] [Google Scholar]
- [87].Seitzer U, Bussler H, Kullmann B, Petersen A, Becker WM, Ahmed J. Characterization of immunoglobulin E responses in Balb/c mice against the major allergens of timothy grass (Phleum pratense) pollen. Clin Exp Allergy. 2003;33:669–75. doi: 10.1046/j.1365-2222.2003.01630.x. [DOI] [PubMed] [Google Scholar]
- [88].Mahler V, Vrtala S, Kuss O, Diepgen TL, Suck R, Cromwell O, et al. Vaccines for birch pollen allergy based on genetically engineered hypoallergenic derivatives of the major birch pollen allergen, Bet v 1. Clin Exp Allergy. 2004;34:115–22. doi: 10.1111/j.1365-2222.2004.01857.x. [DOI] [PubMed] [Google Scholar]
- [89].Finkelman FD, Wills-Karp M. Usefulness and optimization of mouse models of allergic airway disease. J Allergy Clin Immunol. 2008;121:603–6. doi: 10.1016/j.jaci.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Klimek L, Bachert C, Doemer C, Meyer H, Narkus A. Specific immunotherapy with recombinant birch pollen allergen rBet v 1-FV is clinically efficacious. Allergy Clin Immunol Int. 2005;2005(Suppl. 1):15. [Google Scholar]
- [91].Rak S. Clinical results with a hypoallergenic recombinant birch pollen allergen derivative; Presented at 27th Congress, EAACI 2009; 2009. [Google Scholar]
- [92].Valenta R, Linhart B, Swoboda I, Niederberger V. Recombinant allergens for allergen-specific immunotherapy: 10 years anniversary of immunotherapy with recombinant allergens. Allergy. 2011;66:775–83. doi: 10.1111/j.1398-9995.2011.02565.x. [DOI] [PubMed] [Google Scholar]
- [93].Valenta R, Niespodziana K, Focke-Tejkl M, Marth K, Huber H, Neubauer A, et al. Recombinant allergens: what does the future hold? J Allergy Clin Immunol. 2011;127:860–4. doi: 10.1016/j.jaci.2011.02.016. [DOI] [PubMed] [Google Scholar]
- [94].Niederberger V, Reisinger J, Valent P, Krauth MT, Pauli G, van Hage M, et al. Vaccination with genetically modified birch pollen allergens: immune and clinical effects on oral allergy syndrome. J Allergy Clin Immunol. 2007;119:1013–6. doi: 10.1016/j.jaci.2006.12.661. [DOI] [PubMed] [Google Scholar]
- [95].Reisinger J, Horak F, Pauli G, van Hage M, Cromwell O, König F, et al. Allergen-specific nasal IgG antibodies induced by vaccination with genetically modified allergens are associated with reduced nasal allergen sensitivity. J Allergy Clin Immunol. 2005;116:347–54. doi: 10.1016/j.jaci.2005.04.003. [DOI] [PubMed] [Google Scholar]
- [96].Gafvelin G, Thunberg S, Kronqvist M, Grönlund H, Grönneberg R, Troye-Blomberg M, et al. Cytokine and antibody responses in birch-pollen-allergic patients treated with genetically modified derivatives of the major birch pollen allergen Bet v 1. Int Arch Allergy Immunol. 2005;138:59–66. doi: 10.1159/000087358. [DOI] [PubMed] [Google Scholar]
- [97].Pree I, Reisinger J, Focke M, Vrtala S, Pauli G, van Hage M, et al. Analysis of epitope-specific immune responses induced by vaccination with structurally folded and unfolded recombinant Bet v 1 allergen derivatives in man. J Immunol. 2007;179:5309–16. doi: 10.4049/jimmunol.179.8.5309. [DOI] [PubMed] [Google Scholar]
- [98].Loveless MH. Immunological studies on pollinosis. I. The presence of two antibodies related to the same pollen antigen in the serum of treated hay-fever patients. J Immunol. 1940;38:25–50. [Google Scholar]
- [99].Jung CM, Prinz JC, Rieber EP, Ring J. A reduction in allergen-induced Fc epsilon R2/CD23 expression on peripheral B cells correlates with successful hyposensitization in grass pollinosis. J Allergy Clin Immunol. 1995;95:77–87. doi: 10.1016/s0091-6749(95)70155-9. [DOI] [PubMed] [Google Scholar]
- [100].van der Heijden FL, Joost van Neerven RJ, van Katwijk M, Bos JD, Kapsenberg ML. Serum-IgE-facilitated allergen presentation in atopic disease. J Immunol. 1993;150:3643–50. [PubMed] [Google Scholar]
- [101].van Neerven RJ, Wikborg T, Lund G, Jacobsen B, Brinch-Nielsen A, Arnved J, et al. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J Immunol. 1999;163:2944–52. [PubMed] [Google Scholar]
- [102].van Neerven RJ, Knol EF, Ejrnaes A, Würtzen PA. IgE-mediated allergen presentation and blocking antibodies: regulation of T-cell activation in allergy. Int Arch Allergy Immunol. 2006;141:119–29. doi: 10.1159/000094714. [DOI] [PubMed] [Google Scholar]
- [103].van Neerven RJ, Arvidsson M, Ipsen H, Sparholt SH, Rak S, Würtzen PA. A double-blind, placebo-controlled birch allergy vaccination study: inhibition of CD23-mediated serum-immunoglobulin E-facilitated allergen presentation. Clin Exp Allergy. 2004;34:420–8. doi: 10.1111/j.1365-2222.2004.01899.x. [DOI] [PubMed] [Google Scholar]
- [104].Würtzen PA, Lund G, Lund K, Arvidsson M, Rak S, Ipsen H. A double-blind placebo-controlled birch allergy vaccination study II: correlation between inhibition of IgE binding, histamine release and facilitated allergen presentation. Clin Exp Allergy. 2008;38:1290–301. doi: 10.1111/j.1365-2222.2008.03020.x. [DOI] [PubMed] [Google Scholar]
- [105].Pree I, Shamji MH, Kimber I, Valenta R, Durham SR, Niederberger V. Inhibition of CD23-dependent facilitated allergen binding to B cells following vaccination with genetically modified hypoallergenic Bet v 1 molecules. Clin Exp Allergy. 2010;40:1346–52. doi: 10.1111/j.1365-2222.2010.03548.x. [DOI] [PubMed] [Google Scholar]
- [106].Shamji MH, Wilcock LK, Wachholz PA, Dearman RJ, Kimber I, Wurtzen PA, et al. The IgE-facilitated allergen binding (FAB) assay: validation of a novel flow-cytometric based method for the detection of inhibitory antibody responses. J Immunol Methods. 2006;317:71–9. doi: 10.1016/j.jim.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Naclerio RM, Adkinson NF, Jr, Moylan B, Baroody FM, Proud D, Kagey-Sobotka A, et al. Nasal provocation with allergen induces a secondary serum IgE antibody response. J Allergy Clin Immunol. 1997;100:505–10. doi: 10.1016/s0091-6749(97)70143-x. [DOI] [PubMed] [Google Scholar]
- [108].Niederberger V, Ring J, Rakoski J, Jager S, Spitzauer S, Valent P, et al. Antigens drive memory IgE responses in human allergy via the nasal mucosa. Int Arch Allergy Immunol. 2007;142:133–44. doi: 10.1159/000096439. [DOI] [PubMed] [Google Scholar]
- [109].Mothes N, Heinzkill M, Drachenberg KJ, Sperr WR, Krauth MT, Majlesi Y, et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33:1198–208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
- [110].Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–55. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- [111].Focke M, Mahler V, Ball T, Sperr WR, Majlesi Y, Valent P, et al. Nonanaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. FASEB J. 2001;15:2042–4. doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- [112].Focke M, Linhart B, Hartl A, Wiedermann U, Sperr WR, Valent P, et al. Non-anaphylactic surface-exposed peptides of the major birch pollen allergen, Bet v 1, for preventive vaccination. Clin Exp Allergy. 2004;34:1525–33. doi: 10.1111/j.1365-2222.2004.02081.x. [DOI] [PubMed] [Google Scholar]
- [113].Edlmayr J, Niespodziana K, Linhart B, Focke-Tejkl M, Westritschnig K, Scheiblhofer S, et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J Immunol. 2009;182:6298–306. doi: 10.4049/jimmunol.0713622. [DOI] [PubMed] [Google Scholar]
- [114].Niespodziana K, Focke-Tejkl M, Linhart B, Civaj V, Blatt K, Valent P, et al. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J Allergy Clin Immunol. 2011;127:1562–70. doi: 10.1016/j.jaci.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Twaroch TE, Focke M, Civaj V, Weber M, Balic N, Mari A, et al. Carrier-bound, nonallergenic Ole e 1 peptides for vaccination against olive pollen allergy. J Allergy Clin Immunol. 2011;128:178–84. doi: 10.1016/j.jaci.2011.03.011. [DOI] [PubMed] [Google Scholar]