Abstract
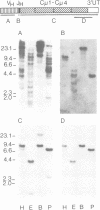
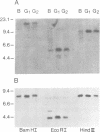
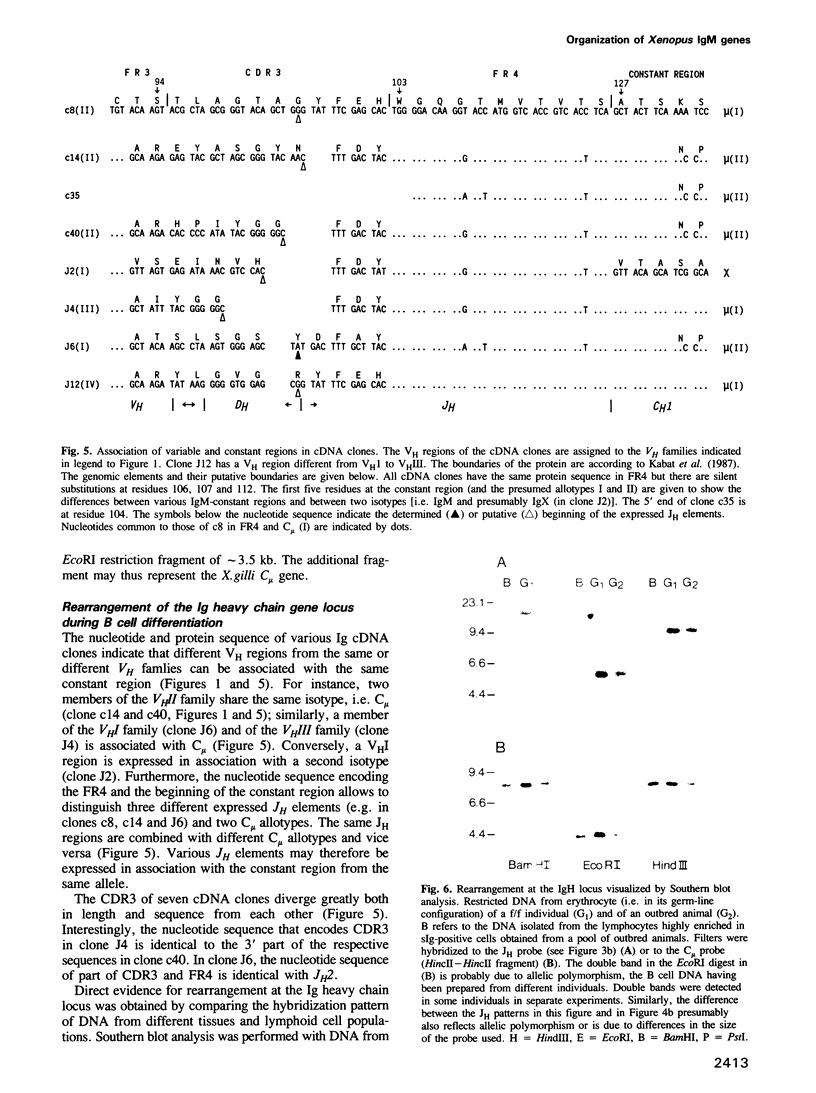
Sequences of immunoglobulin (Ig) cDNA clones of Xenopus laevis show that at least three different VH families are expressed in association with different JH elements and different isotypes of Ig constant regions. In genomic Southern blot analysis, the VH probes for each family hybridize to a distinct set of multiple DNA fragments. In contrast, the genomic JH elements and the IgM constant region gene are localized in a single DNA fragment of approximately 15 kb. Genomic VH elements contain regulatory sequences similar to those in VH genes of shark, fish and mammals and have a leader peptide sequence that contains an intron; they encode the VH region until residue 95 and have heptamer--23-bp--nonamer motifs similar to the rearrangement signal sequences (RSS) in all other vertebrate VH elements. The six genomic JH elements so far sequenced have a nonamer--23-bp--heptamer motif at their 5' end. These RSS motifs imply the existence of DH elements. The comparison of cDNA clones that contain similar constant regions but different VH regions or JH elements suggest rearrangement events. This is shown by Southern blot analysis of erythrocyte and B cell DNA with a JH probe. Thus, the overall organization of the Xenopus Ig gene locus is similar to that of mammals but strikingly different from shark.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera R. J., Akira S., Okazaki K., Sakano H. A pre-B cell nuclear protein that specifically interacts with the immunoglobulin V-J recombination sequences. Cell. 1987 Dec 24;51(6):909–917. doi: 10.1016/0092-8674(87)90578-2. [DOI] [PubMed] [Google Scholar]
- Akira S., Okazaki K., Sakano H. Two pairs of recombination signals are sufficient to cause immunoglobulin V-(D)-J joining. Science. 1987 Nov 20;238(4830):1134–1138. doi: 10.1126/science.3120312. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicher P. A., Cohen N. Monoclonal anti-IgM can separate T cell from B cell proliferative responses in the frog, Xenopus laevis. J Immunol. 1981 Oct;127(4):1549–1555. [PubMed] [Google Scholar]
- Brandt D. C., Griessen M., Du Pasquier L., Jaton J. C. Antibody diversity in amphibians: evidence for the inheritance of idiotypic specificities in isogenic Xenopus. Eur J Immunol. 1980 Oct;10(10):731–736. doi: 10.1002/eji.1830101002. [DOI] [PubMed] [Google Scholar]
- Brodeur P. H., Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984 Oct;14(10):922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Collins M. L., Hunsaker W. R. Improved hybridization assays employing tailed oligonucleotide probes: a direct comparison with 5'-end-labeled oligonucleotide probes and nick-translated plasmid probes. Anal Biochem. 1985 Dec;151(2):211–224. doi: 10.1016/0003-2697(85)90168-x. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L. Antibody diversity in lower vertebrates--why is it so restricted? Nature. 1982 Mar 25;296(5855):311–313. doi: 10.1038/296311a0. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L., Wabl M. R. Antibody diversity in amphibians: inheritance of isoelectric focusing antibody patterns in isogenic frogs. Eur J Immunol. 1978 Jun;8(6):428–433. doi: 10.1002/eji.1830080611. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flajnik M. F., Kaufman J. F., Riegert P., Du Pasquier L. Identification of class I major histocompatibility complex encoded molecules in the amphibian Xenopus. Immunogenetics. 1984;20(4):433–442. doi: 10.1007/BF00345617. [DOI] [PubMed] [Google Scholar]
- Gaudernack G., Leivestad T., Ugelstad J., Thorsby E. Isolation of pure functionally active CD8+ T cells. Positive selection with monoclonal antibodies directly conjugated to monosized magnetic microspheres. J Immunol Methods. 1986 Jun 24;90(2):179–187. doi: 10.1016/0022-1759(86)90074-8. [DOI] [PubMed] [Google Scholar]
- Hinds K. R., Litman G. W. Major reorganization of immunoglobulin VH segmental elements during vertebrate evolution. Nature. 1986 Apr 10;320(6062):546–549. doi: 10.1038/320546a0. [DOI] [PubMed] [Google Scholar]
- Kurosawa Y., Tonegawa S. Organization, structure, and assembly of immunoglobulin heavy chain diversity DNA segments. J Exp Med. 1982 Jan 1;155(1):201–218. doi: 10.1084/jem.155.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman G. W., Murphy K., Berger L., Litman R., Hinds K., Erickson B. W. Complete nucleotide sequences of three VH genes in Caiman, a phylogenetically ancient reptile: evolutionary diversification in coding segments and variation in the structure and organization of recombination elements. Proc Natl Acad Sci U S A. 1985 Feb;82(3):844–848. doi: 10.1073/pnas.82.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parslow T. G., Blair D. L., Murphy W. J., Granner D. K. Structure of the 5' ends of immunoglobulin genes: a novel conserved sequence. Proc Natl Acad Sci U S A. 1984 May;81(9):2650–2654. doi: 10.1073/pnas.81.9.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Dahan A., Weill J. C. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985 Feb;40(2):283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Grimal H., Weill J. C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987 Feb 13;48(3):379–388. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]
- Sakano H., Kurosawa Y., Weigert M., Tonegawa S. Identification and nucleotide sequence of a diversity DNA segment (D) of immunoglobulin heavy-chain genes. Nature. 1981 Apr 16;290(5807):562–565. doi: 10.1038/290562a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwager J., Mikoryak C. A., Steiner L. A. Amino acid sequence of heavy chain from Xenopus laevis IgM deduced from cDNA sequence: implications for evolution of immunoglobulin domains. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2245–2249. doi: 10.1073/pnas.85.7.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. C., Tung E., Fudenberg H. H., Hadji-Azimi I. Immunoglobulin evolution: chemical study of clawed toad (Xenopus laevis) heavy and light chains. J Immunogenet. 1978 Dec;5(6):355–364. doi: 10.1111/j.1744-313x.1978.tb00665.x. [DOI] [PubMed] [Google Scholar]
- Weiss N., Du Pasquier L. Factors affecting the reactivity of amphibian lymphocytes in a miniaturized technique of the mixed lymphocyte culture. J Immunol Methods. 1973 Nov;3(3):273–285. doi: 10.1016/0022-1759(73)90023-9. [DOI] [PubMed] [Google Scholar]
- Wilson M. R., Middleton D., Warr G. W. Immunoglobulin heavy chain variable region gene evolution: structure and family relationships of two genes and a pseudogene in a teleost fish. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1566–1570. doi: 10.1073/pnas.85.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki-Kataoka Y., Honjo T. Nucleotide sequences of variable region segments of the immunoglobulin heavy chain of Xenopus laevis. Nucleic Acids Res. 1987 Jul 24;15(14):5888–5888. doi: 10.1093/nar/15.14.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]