Abstract
Protein I is a hetero-tetramer which contains two copies each of p11 and p36. p36 (calpactin I, lipocortin II) is a major substrate of retrovirally encoded tyrosine protein kinases, while p11 modulates several Ca2+-induced properties also displayed by p36 alone. Here we have characterized the p11 binding site on p36 by fluorescence spectroscopy using porcine p36 labelled at cysteine 8 with the fluorophore Prodan (6-proprionyl-2-dimethylamino-naphthalene). We have used peptides of differing length from the amino-terminal domain of p36 to restrict the major binding site to the first 12 residues. Noticeable binding is still observed with a peptide containing only the first nine residues. Interestingly the N-terminal acetyl group of p36 forms a functional part of the p11 binding site. CD studies indicate that the binding region can form an alpha-helix, which seems to have amphiphatic properties when projected on a helical wheel. This structural element is also known for a calmodulin binding protein. Thus the question is raised whether other p11/calmodulin-related proteins interact with their target proteins via a similar mechanism. We also discuss how p11 could modulate p36 associated properties.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal D. K., Takio K., Edelman A. M., Charbonneau H., Titani K., Walsh K. A., Krebs E. G. Identification of the calmodulin-binding domain of skeletal muscle myosin light chain kinase. Proc Natl Acad Sci U S A. 1985 May;82(10):3187–3191. doi: 10.1073/pnas.82.10.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Calabretta B., Battini R., Kaczmarek L., de Riel J. K., Baserga R. Molecular cloning of the cDNA for a growth factor-inducible gene with strong homology to S-100, a calcium-binding protein. J Biol Chem. 1986 Sep 25;261(27):12628–12632. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Clark D. J., Hill C. S., Martin S. R., Thomas J. O. Alpha-helix in the carboxy-terminal domains of histones H1 and H5. EMBO J. 1988 Jan;7(1):69–75. doi: 10.1002/j.1460-2075.1988.tb02784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M. R., Owens R. J., Totty N. F., Moss S. E., Waterfield M. D., Crumpton M. J. Primary structure of the human, membrane-associated Ca2+-binding protein p68 a novel member of a protein family. EMBO J. 1988 Jan;7(1):21–27. doi: 10.1002/j.1460-2075.1988.tb02779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson F. F., Dennis E. A., Powell M., Glenney J. R., Jr Inhibition of phospholipase A2 by "lipocortins" and calpactins. An effect of binding to substrate phospholipids. J Biol Chem. 1987 Feb 5;262(4):1698–1705. [PubMed] [Google Scholar]
- De B. K., Misono K. S., Lukas T. J., Mroczkowski B., Cohen S. A calcium-dependent 35-kilodalton substrate for epidermal growth factor receptor/kinase isolated from normal tissue. J Biol Chem. 1986 Oct 15;261(29):13784–13792. [PubMed] [Google Scholar]
- Dorin J. R., Novak M., Hill R. E., Brock D. J., Secher D. S., van Heyningen V. A clue to the basic defect in cystic fibrosis from cloning the CF antigen gene. Nature. 1987 Apr 9;326(6113):614–617. doi: 10.1038/326614a0. [DOI] [PubMed] [Google Scholar]
- Drust D. S., Creutz C. E. Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature. 1988 Jan 7;331(6151):88–91. doi: 10.1038/331088a0. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Takio K., Blumenthal D. K., Hansen R. S., Walsh K. A., Titani K., Krebs E. G. Characterization of the calmodulin-binding and catalytic domains in skeletal muscle myosin light chain kinase. J Biol Chem. 1985 Sep 15;260(20):11275–11285. [PubMed] [Google Scholar]
- Erikson E., Tomasiewicz H. G., Erikson R. L. Biochemical characterization of a 34-kilodalton normal cellular substrate of pp60v-src and an associated 6-kilodalton protein. Mol Cell Biol. 1984 Jan;4(1):77–85. doi: 10.1128/mcb.4.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi B., Borin G., Marchiori F. The influence of amino acid side-chains on alpha-helix stability: S-peptide analogues and related ribonucleases S'. J Mol Biol. 1976 Sep 15;106(2):315–324. doi: 10.1016/0022-2836(76)90088-7. [DOI] [PubMed] [Google Scholar]
- Funakoshi T., Hendrickson L. E., McMullen B. A., Fujikawa K. Primary structure of human placental anticoagulant protein. Biochemistry. 1987 Dec 15;26(25):8087–8092. doi: 10.1021/bi00399a011. [DOI] [PubMed] [Google Scholar]
- Geisow M. J., Fritsche U., Hexham J. M., Dash B., Johnson T. A consensus amino-acid sequence repeat in Torpedo and mammalian Ca2+-dependent membrane-binding proteins. Nature. 1986 Apr 17;320(6063):636–638. doi: 10.1038/320636a0. [DOI] [PubMed] [Google Scholar]
- Gerke V., Weber K. Calcium-dependent conformational changes in the 36-kDa subunit of intestinal protein I related to the cellular 36-kDa target of Rous sarcoma virus tyrosine kinase. J Biol Chem. 1985 Feb 10;260(3):1688–1695. [PubMed] [Google Scholar]
- Gerke V., Weber K. Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J. 1984 Jan;3(1):227–233. doi: 10.1002/j.1460-2075.1984.tb01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V., Weber K. The regulatory chain in the p36-kd substrate complex of viral tyrosine-specific protein kinases is related in sequence to the S-100 protein of glial cells. EMBO J. 1985 Nov;4(11):2917–2920. doi: 10.1002/j.1460-2075.1985.tb04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Boudreau M., Galyean R., Hunter T., Tack B. Association of the S-100-related calpactin I light chain with the NH2-terminal tail of the 36-kDa heavy chain. J Biol Chem. 1986 Aug 15;261(23):10485–10488. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Tack B. F. Amino-terminal sequence of p36 and associated p10: identification of the site of tyrosine phosphorylation and homology with S-100. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7884–7888. doi: 10.1073/pnas.82.23.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Tack B., Powell M. A. Calpactins: two distinct Ca++-regulated phospholipid- and actin-binding proteins isolated from lung and placenta. J Cell Biol. 1987 Mar;104(3):503–511. doi: 10.1083/jcb.104.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. Phospholipid-dependent Ca2+ binding by the 36-kDa tyrosine kinase substrate (calpactin) and its 33-kDa core. J Biol Chem. 1986 Jun 5;261(16):7247–7252. [PubMed] [Google Scholar]
- Gould K. L., Woodgett J. R., Isacke C. M., Hunter T. The protein-tyrosine kinase substrate p36 is also a substrate for protein kinase C in vitro and in vivo. Mol Cell Biol. 1986 Jul;6(7):2738–2744. doi: 10.1128/mcb.6.7.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Hexham J. M., Totty N. F., Waterfield M. D., Crumpton M. J. Homology between the subunits of S100 and a 10kDa polypeptide associated with p36 of pig lymphocytes. Biochem Biophys Res Commun. 1986 Jan 14;134(1):248–254. doi: 10.1016/0006-291x(86)90554-1. [DOI] [PubMed] [Google Scholar]
- Huang K. S., Wallner B. P., Mattaliano R. J., Tizard R., Burne C., Frey A., Hession C., McGray P., Sinclair L. K., Chow E. P. Two human 35 kd inhibitors of phospholipase A2 are related to substrates of pp60v-src and of the epidermal growth factor receptor/kinase. Cell. 1986 Jul 18;46(2):191–199. doi: 10.1016/0092-8674(86)90736-1. [DOI] [PubMed] [Google Scholar]
- Jackson-Grusby L. L., Swiergiel J., Linzer D. I. A growth-related mRNA in cultured mouse cells encodes a placental calcium binding protein. Nucleic Acids Res. 1987 Aug 25;15(16):6677–6690. doi: 10.1093/nar/15.16.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
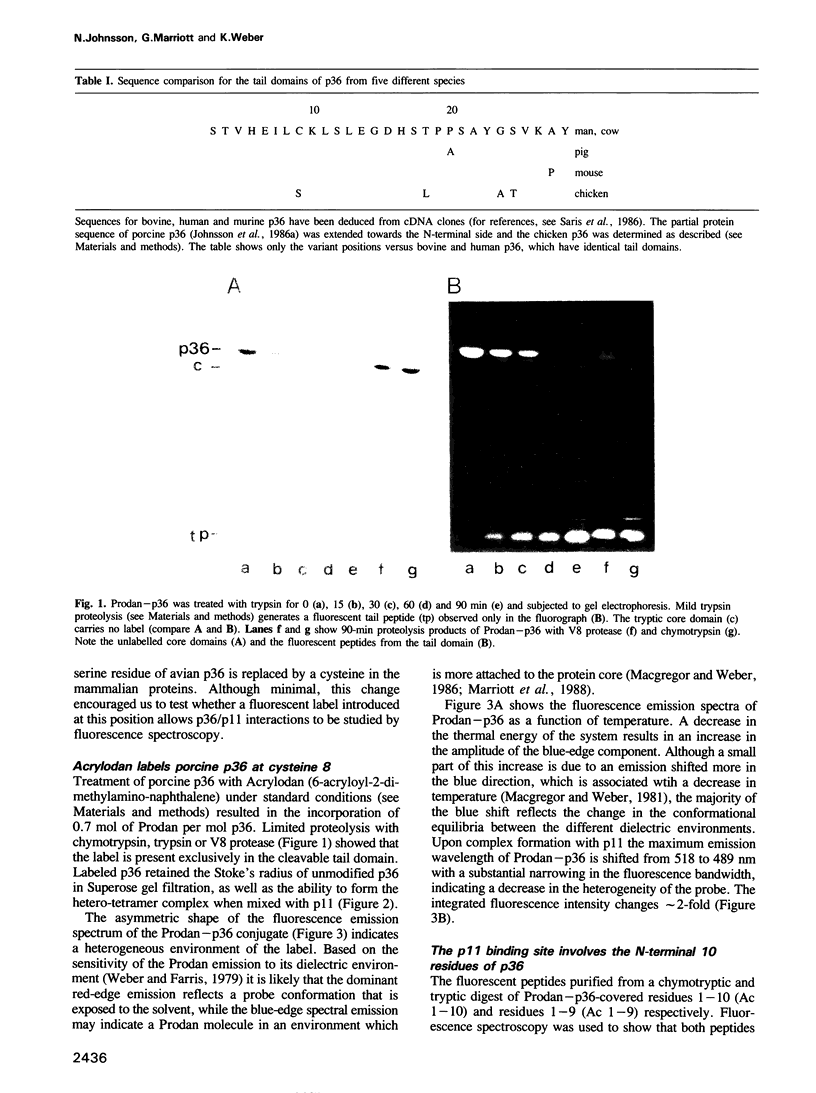
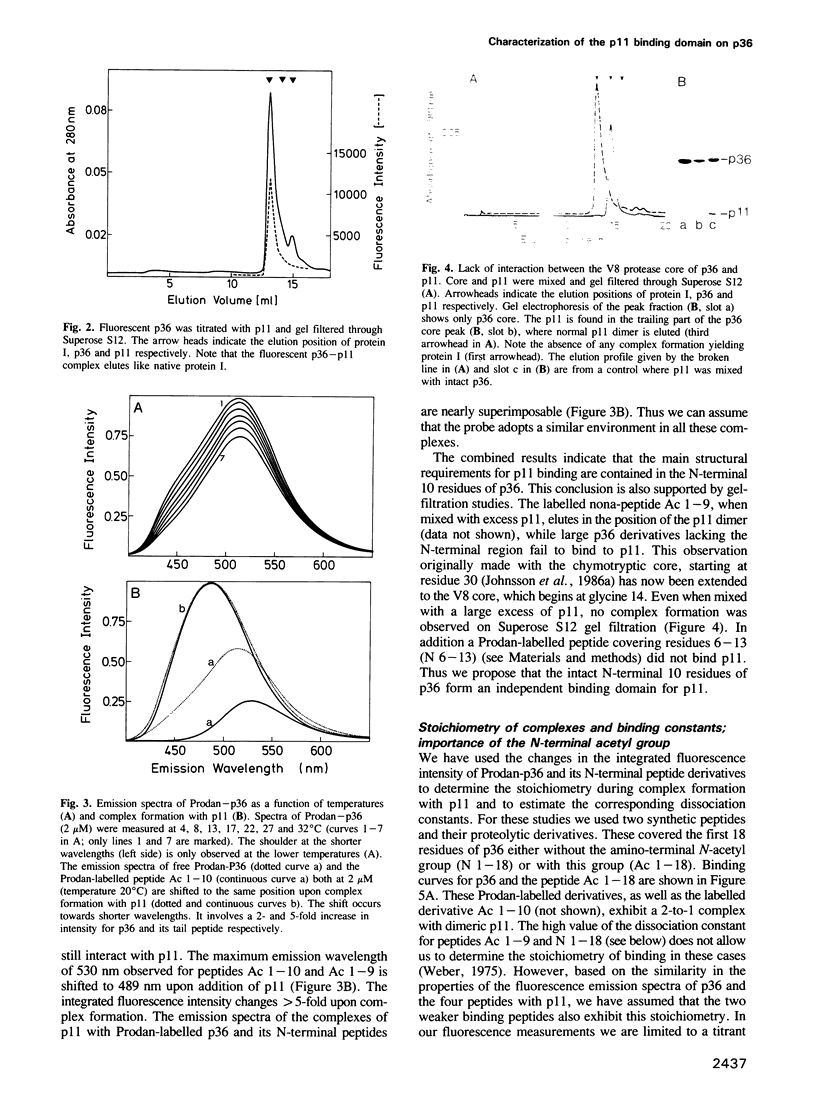
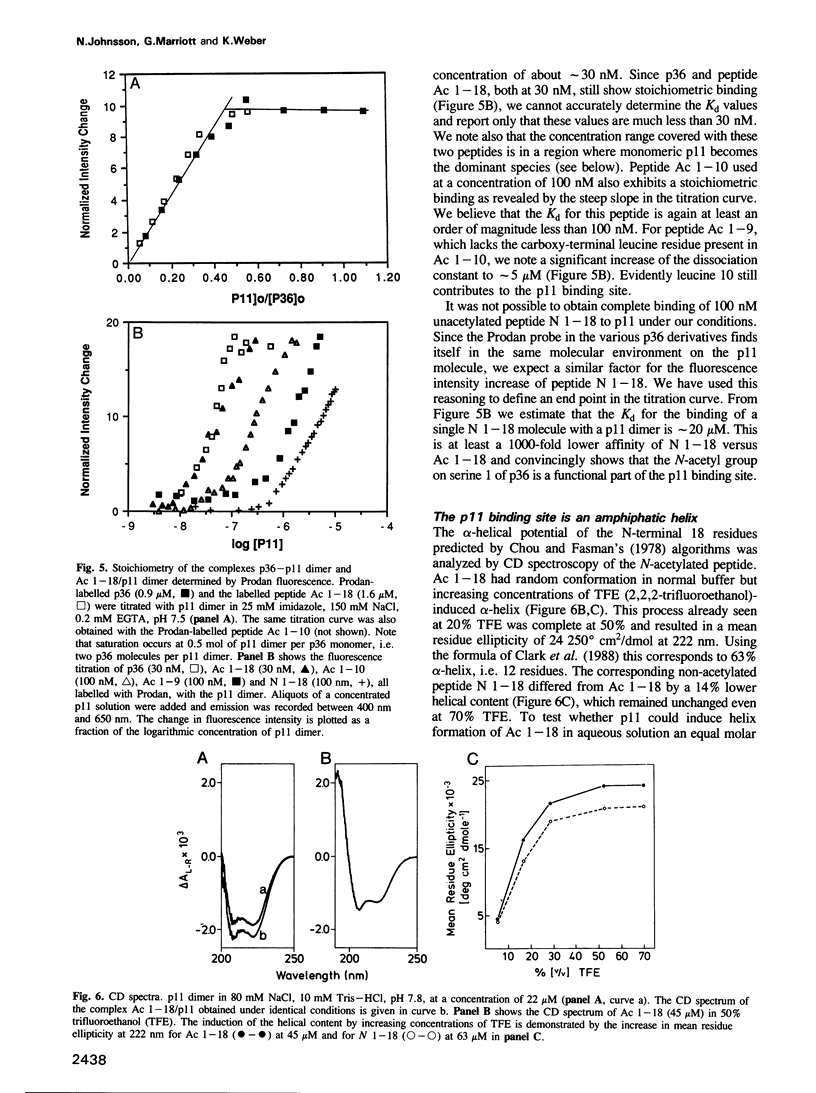
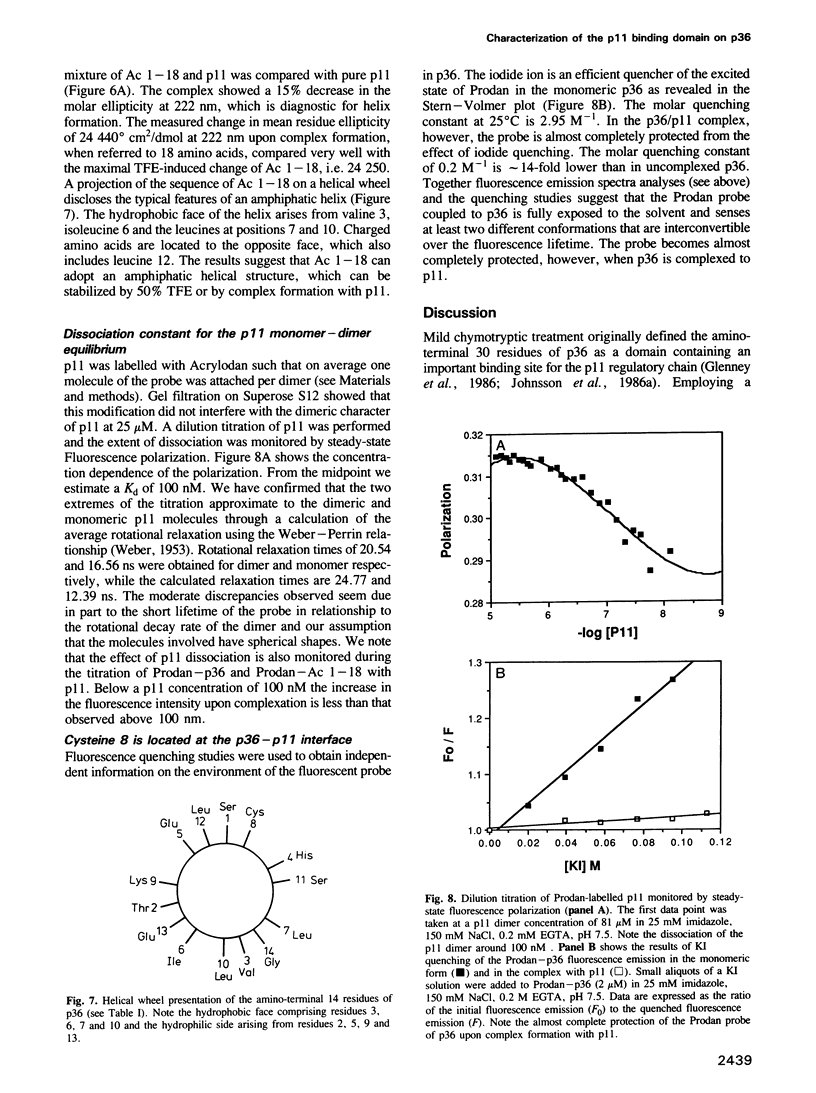
- Johnsson N., Nguyen Van P., Söling H. D., Weber K. Functionally distinct serine phosphorylation sites of p36, the cellular substrate of retroviral protein kinase; differential inhibition of reassociation with p11. EMBO J. 1986 Dec 20;5(13):3455–3460. doi: 10.1002/j.1460-2075.1986.tb04669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson N., Vandekerckhove J., Van Damme J., Weber K. Binding sites for calcium, lipid and p11 on p36, the substrate of retroviral tyrosine-specific protein kinases. FEBS Lett. 1986 Mar 31;198(2):361–364. doi: 10.1016/0014-5793(86)80437-9. [DOI] [PubMed] [Google Scholar]
- Lukas T. J., Burgess W. H., Prendergast F. G., Lau W., Watterson D. M. Calmodulin binding domains: characterization of a phosphorylation and calmodulin binding site from myosin light chain kinase. Biochemistry. 1986 Mar 25;25(6):1458–1464. doi: 10.1021/bi00354a041. [DOI] [PubMed] [Google Scholar]
- Macgregor R. B., Weber G. Estimation of the polarity of the protein interior by optical spectroscopy. Nature. 1986 Jan 2;319(6048):70–73. doi: 10.1038/319070a0. [DOI] [PubMed] [Google Scholar]
- Marqusee S., Baldwin R. L. Helix stabilization by Glu-...Lys+ salt bridges in short peptides of de novo design. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8898–8902. doi: 10.1073/pnas.84.24.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R., Scheraga H. A. The effect of neighboring charges on the helix forming ability of charged amino acids in proteins. Macromolecules. 1975 Jul-Aug;8(4):491–493. doi: 10.1021/ma60046a022. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Dedman J. R. Calcium-dependent protein binding to phenothiazine columns. J Biol Chem. 1982 Aug 25;257(16):9663–9667. [PubMed] [Google Scholar]
- O'Neil K. T., Wolfe H. R., Jr, Erickson-Viitanen S., DeGrado W. F. Fluorescence properties of calmodulin-binding peptides reflect alpha-helical periodicity. Science. 1987 Jun 12;236(4807):1454–1456. doi: 10.1126/science.3589665. [DOI] [PubMed] [Google Scholar]
- Osborn M., Johnsson N., Wehland J., Weber K. The submembranous location of p11 and its interaction with the p36 substrate of pp60 src kinase in situ. Exp Cell Res. 1988 Mar;175(1):81–96. doi: 10.1016/0014-4827(88)90257-1. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Sinclair L. K. Epidermal growth factor-dependent phosphorylation of lipocortin. Nature. 1986 May 1;321(6065):81–84. doi: 10.1038/321081a0. [DOI] [PubMed] [Google Scholar]
- Powell M. A., Glenney J. R. Regulation of calpactin I phospholipid binding by calpactin I light-chain binding and phosphorylation by p60v-src. Biochem J. 1987 Oct 15;247(2):321–328. doi: 10.1042/bj2470321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast F. G., Meyer M., Carlson G. L., Iida S., Potter J. D. Synthesis, spectral properties, and use of 6-acryloyl-2-dimethylaminonaphthalene (Acrylodan). A thiol-selective, polarity-sensitive fluorescent probe. J Biol Chem. 1983 Jun 25;258(12):7541–7544. [PubMed] [Google Scholar]
- Saris C. J., Kristensen T., D'Eustachio P., Hicks L. J., Noonan D. J., Hunter T., Tack B. F. cDNA sequence and tissue distribution of the mRNA for bovine and murine p11, the S100-related light chain of the protein-tyrosine kinase substrate p36 (calpactin I). J Biol Chem. 1987 Aug 5;262(22):10663–10671. [PubMed] [Google Scholar]
- Saris C. J., Tack B. F., Kristensen T., Glenney J. R., Jr, Hunter T. The cDNA sequence for the protein-tyrosine kinase substrate p36 (calpactin I heavy chain) reveals a multidomain protein with internal repeats. Cell. 1986 Jul 18;46(2):201–212. doi: 10.1016/0092-8674(86)90737-3. [DOI] [PubMed] [Google Scholar]
- Shadle P. J., Gerke V., Weber K. Three Ca2+-binding proteins from porcine liver and intestine differ immunologically and physicochemically and are distinct in Ca2+ affinities. J Biol Chem. 1985 Dec 25;260(30):16354–16360. [PubMed] [Google Scholar]
- Südhof T. C., Ebbecke M., Walker J. H., Fritsche U., Boustead C. Isolation of mammalian calelectrins: a new class of ubiquitous Ca2+-regulated proteins. Biochemistry. 1984 Mar 13;23(6):1103–1109. doi: 10.1021/bi00301a010. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Slaughter C. A., Leznicki I., Barjon P., Reynolds G. A. Human 67-kDa calelectrin contains a duplication of four repeats found in 35-kDa lipocortins. Proc Natl Acad Sci U S A. 1988 Feb;85(3):664–668. doi: 10.1073/pnas.85.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER G. Polarization of the fluorescence of macromolecules. I. Theory and experimental method. Biochem J. 1952 May;51(2):145–155. doi: 10.1042/bj0510145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner B. P., Mattaliano R. J., Hession C., Cate R. L., Tizard R., Sinclair L. K., Foeller C., Chow E. P., Browing J. L., Ramachandran K. L. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986 Mar 6;320(6057):77–81. doi: 10.1038/320077a0. [DOI] [PubMed] [Google Scholar]
- Weber G. Energetics of ligand binding to proteins. Adv Protein Chem. 1975;29:1–83. doi: 10.1016/s0065-3233(08)60410-6. [DOI] [PubMed] [Google Scholar]
- Weber G., Farris F. J. Synthesis and spectral properties of a hydrophobic fluorescent probe: 6-propionyl-2-(dimethylamino)naphthalene. Biochemistry. 1979 Jul 10;18(14):3075–3078. doi: 10.1021/bi00581a025. [DOI] [PubMed] [Google Scholar]
- Weber K., Johnsson N., Plessmann U., Van P. N., Söling H. D., Ampe C., Vandekerckhove J. The amino acid sequence of protein II and its phosphorylation site for protein kinase C; the domain structure Ca2+-modulated lipid binding proteins. EMBO J. 1987 Jun;6(6):1599–1604. doi: 10.1002/j.1460-2075.1987.tb02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Johnsson N. Repeating sequence homologies in the p36 target protein of retroviral protein kinases and lipocortin, the p37 inhibitor of phospholipase A2. FEBS Lett. 1986 Jul 14;203(1):95–98. doi: 10.1016/0014-5793(86)81444-2. [DOI] [PubMed] [Google Scholar]
- Zokas L., Glenney J. R., Jr The calpactin light chain is tightly linked to the cytoskeletal form of calpactin I: studies using monoclonal antibodies to calpactin subunits. J Cell Biol. 1987 Nov;105(5):2111–2121. doi: 10.1083/jcb.105.5.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]





