Abstract
Bradyrhizobium sp. Ai1a-2 is is an aerobic, motile, Gram-negative, non-spore-forming rod that was isolated from an effective nitrogen fixing root nodule of Andira inermis collected from Tres Piedras in Costa Rica. In this report we describe, for the first time, the genome sequence information and annotation of this legume microsymbiont. The 9,029,266 bp genome has a GC content of 62.56% with 247 contigs arranged into 246 scaffolds. The assembled genome contains 8,482 protein-coding genes and 102 RNA-only encoding genes. This rhizobial genome was sequenced as part of the DOE Joint Genome Institute 2010 Genomic Encyclopedia for Bacteria and Archaea-Root Nodule Bacteria (GEBA-RNB) project proposal.
Electronic supplementary material
The online version of this article (doi:10.1186/s40793-015-0007-z) contains supplementary material, which is available to authorized users.
Keywords: Root-nodule bacteria, Nitrogen fixation, Symbiosis, Alphaproteobacteria, GEBA-RNB
Introduction
Bradyrhizobium sp. strain Ai1a.2 is a representative of a distinctive lineage affiliated with the Bradyrhizobium elkanii superclade [1]. The B. elkanii superclade is one of the three main branches of the genus, together with the B. japonicum/B. diazoefficiens superclade [2,3], and the group encompassing photosynthetic Aeschynomene symbionts [4].
Members of the lineage represented by strain Ai1a.2 are readily diagnosed because they share a distinctive length variant in helix 9 within the 5′ intervening sequence region of the 23S rRNA gene [5]. Strain Ai1a.2 and its relatives have an insertion of 16 nucleotides in this region in comparison to B. elkanii USDA76, which can be identified by a straightforward PCR assay [6]. In a survey of 420 Bradyrhizobium strains from 25 countries [1], only 2% of the strains had this 23S rRNA length variant. These strains all clustered together into a strongly supported clade based on concatenated data for 23S rRNA and five protein-coding genes [1].
This clade was placed as the most basally diverging lineage within the B. elkanii superclade, and it included strains from three locations: Central America, the Caribbean, and South Africa. Strain Ai1a.2 was sampled in Costa Rica from the tree Andira inermis [6], and highly similar strains are also known to occur as symbionts of the same host legume in Panama [7]. Parker and Rousteau [8] also detected strains from this group in nodule samples from the beach legume Canavalia rosea in two Caribbean locations (Guadeloupe and Puerto Rico). Two Bradyrhizobium strains from distantly related legume hosts (Leobordea spp.) in South Africa (WSM2632, WSM2783) also belong to this clade [9].
Andira inermis, the host of strain Ai1a.2, is a large tree (up to 35 m height) commonly found in riparian habitats from southern Mexico through northern South America [10]. Andira was traditionally considered to be an early-diverging lineage within the Tribe Dalbergieae [11], but more recent phylogenetic analyses have suggested that it forms a separate lineage with unclear relationship to dalbergioid legumes [12]. Here we provide an analysis of the high-quality permanent draft genome sequence of Bradyrhizobium strain Ai1a.1. The fact that the genome of its close relative WSM2783 has also been sequenced as part of the Genomic Encyclopedia for Bacteria and Archaea-Root Nodule Bacteria (GEBA-RNB) project [13] will enable detailed comparative analysis of this group.
Organism information
Classification and features
Bradyrhizobium sp. Ai1a-2 is a motile, non-sporulating, non-encapsulated, Gram-negative strain in the order Rhizobiales of the class Alphaproteobacteria. The rod shaped form has dimensions of approximately 0.5 μm in width and 1.5-2.0 μm in length (Figure 1 Left and Center). It is relatively slow growing, forming colonies after 6–7 days when grown on half strength Lupin Agar (½LA) [14], tryptone-yeast extract agar (TY) [15] or a modified yeast-mannitol agar (YMA) [16] at 28°C. Colonies on ½LA are opaque, slightly domed and moderately mucoid with smooth margins (Figure 1 Right).
Figure 1.
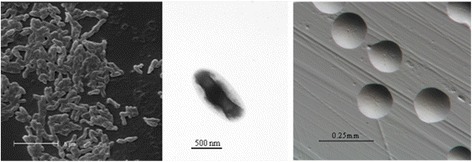
Images of Bradyrhizobium sp. Ai1a-2 using scanning (Left) and transmission (Center) electron microscopy as well as light microscopy to visualize colony morphology on solid media (Right).
Figure 2 shows the phylogenetic relationship of Bradyrhizobium sp. Ai1a-2 in a 16S rRNA gene sequence based tree. The 16S rRNA gene sequence of Aia1-2 (using a 1,370 bp intragenic sequence) is identical to that of Bradyrhizobium sp. WSM2783. Bradyrhizobium sp. Ai1a-2 is also closely related to Bradyrhizobium sp. Cp5.3 and Bradyrhizobium sp. Th.b2 with 16S rRNA gene sequence identities of 99.77% and 99.23%, respectively, as determined using NCBI BLAST analysis [17]. The highest identity (99.16%) of the 16S rRNA gene sequence of strain Ai1a-2 to type strain sequences occurs with Bradyrhizobium icense LMTR 13T and Bradyrhizobium paxllaeri LMTR 21T based on alignment using the EzTaxon-e server [18,19].
Figure 2.
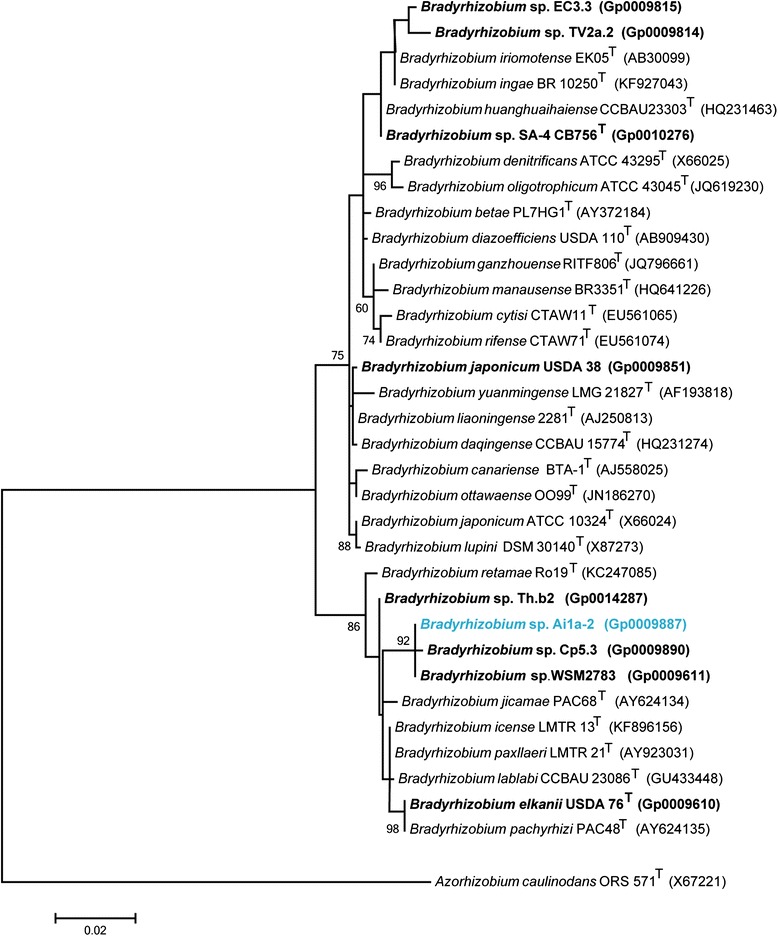
Phylogenetic tree showing the relationship of Bradyrhizobium sp. Ai1a-2 (shown in blue print) relative to other type and non-type strains in the Bradyrhizobium genus using a 1,310 bp intragenic sequence of the 16S rRNA gene. Azorhizobium caulinodans ORS 571T sequence was used as an outgroup. All sites were informative and there were no gap-containing sites. Phylogenetic analyses were performed using MEGA, version 5.05 [37]. The tree was built using the maximum likelihood method with the General Time Reversible model. Bootstrap analysis with 500 replicates was performed to assess the support of the clusters. Type strains are indicated with a superscript T. Strains with a genome sequencing project registered in GOLD [20] have the GOLD ID mentioned after the strain number and are represented in bold, otherwise the NCBI accession number is provided.
Minimum Information about the Genome Sequence (MIGS) is provided in Table 1 and Additional file 1: Table S1.
Table 1.
Classification and general features of Bradyrhizobium sp. Ai1a-2 in accordance with the MIGS recommendations [38] published by the Genome Standards Consortium [39]
| MIGS ID | Property | Term | Evidence code |
|---|---|---|---|
| Classification | Domain Bacteria | TAS [40] | |
| Phylum Proteobacteria | TAS [41,42] | ||
| Class Alphaproteobacteria | TAS [42,43] | ||
| Order Rhizobiales | TAS [44] | ||
| Family Bradyrhizobiaceae | TAS [45] | ||
| Genus Bradyrhizobium | TAS [46] | ||
| Species Bradyrhizobium sp. | IDA | ||
| Gram stain | Negative | IDA | |
| Cell shape | Rod | IDA | |
| Motility | Motile | IDA | |
| Sporulation | Non-sporulating | NAS | |
| Temperature range | Unknown | NAS | |
| Optimum temperature | 28°C | NAS | |
| pH range; Optimum | Unknown | NAS | |
| Carbon source | Varied | NAS | |
| Energy source | Chemoorganotroph | NAS | |
| MIGS-6 | Habitat | Soil, root nodule, host | TAS [6] |
| MIGS-6.3 | Salinity | Non-halophile | NAS |
| MIGS-22 | Oxygen requirement | Aerobic | NAS |
| MIGS-15 | Biotic relationship | Free living, symbiotic | TAS [6] |
| MIGS-14 | Pathogenicity | Non-pathogenic | NAS |
| Biosafety level | 1 | TAS [47] | |
| Isolation | Root nodule of Andira inermis | TAS [6] | |
| MIGS-4 | Geographic location | Tres Piedras, Costa Rica | TAS [6] |
| MIGS-5 | Sample collection | July 14, 2000 | IDA |
| MIGS-4.1 | Latitude | 9.2835 | IDA |
| MIGS-4.2 | Longitude | −83.8533 | IDA |
| MIGS-4.3 | Depth | 5 cm | IDA |
| MIGS-4.4 | Altitude | 50 m | IDA |
Evidence codes – IDA: Inferred from Direct Assay; TAS: Traceable Author Statement (i.e., a direct report exists in the literature); NAS: Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence). Evidence codes are from the Gene Ontology project [48,49].
Symbiotaxonomy
Strain Ai1a.2 was isolated from the tree Andira inermis , Costa Rica [6]. The authentication of the symbiotic ability could not be performed using this host because seeds could not be accessed. The symbiotic capability of strain Ai1a.2 was tested on Macroptilium atropurpureum and this strain was able to nodulate this host. Acetylene reduction assays showed established nodules contained active nitrogenase, indicating an effective symbiosis with this host [6].
Genome sequencing information
Genome project history
This organism was selected for sequencing on the basis of its environmental and agricultural relevance to issues in global carbon cycling, alternative energy production, and biogeochemical importance, and is part of the Genomic Encyclopedia of Bacteria and Archaea, Root Nodulating Bacteria (GEBA-RNB) project at the U.S. Department of Energy, Joint Genome Institute (JGI). The genome project is deposited in the Genomes OnLine Database [20] and a high-quality permanent draft genome sequence in IMG [21]. Sequencing, finishing and annotation were performed by the JGI using state of the art sequencing technology [22]. A summary of the project information is shown in Table 2.
Table 2.
Project information
| MIGS ID | Property | Term |
|---|---|---|
| MIGS-31 | Finishing quality | High-quality permanent draft |
| MIGS-28 | Libraries used | Illumina Standard PE |
| MIGS-29 | Sequencing platforms | Illumina HiSeq2000 |
| MIGS-31.2 | Fold coverage | Illumina, 119.7x |
| MIGS-30 | Assemblers | Velvet version 1.1.04; Allpaths-LG version r42328 |
| MIGS-32 | Gene calling method | Prodigal 1.4 |
| Locus Tag | K288 | |
| GenBank ID | AUEZ00000000 | |
| GenBank release date | June 12, 2014 | |
| GOLD ID | Gp0009887 [50] | |
| BIOPROJECT | 195749 | |
| MIGS-13 | Source Material Identifier | Ai1a-2 |
| Project relevance | Symbiotic nitrogen fixation, agriculture |
Growth conditions and genomic DNA preparation
Bradyrhizobium sp. Ai1a-2 was cultured to mid logarithmic phase in 60 ml of TY rich media on a gyratory shaker at 28°C [23]. DNA was isolated from the cells using a CTAB (Cetyl trimethyl ammonium bromide) bacterial genomic DNA isolation method [24].
Genome sequencing and assembly
The draft genome of Bradyrhizobium sp. Ai1a–2 was generated at the DOE Joint Genome Institute (JGI) using the Illumina technology [25]. An Illumina standard shotgun library was constructed and sequenced using the Illumina HiSeq 2000 platform which generated 21,669,974 reads totaling 3,250.5 Mbp. All general aspects of library construction and sequencing were performed at the JGI and details can be found on the JGI website [26]. All raw Illumina sequence data was passed through DUK, a filtering program developed at JGI, which removes known Illumina sequencing and library preparation artifacts (Mingkun L, Copeland A, Han J, Unpublished). Following steps were then performed for assembly: (1) filtered Illumina reads were assembled using Velvet (version 1.1.04) [27], (2) 1–3 Kbp simulated paired end reads were created from Velvet contigs using wgsim [28], (3) Illumina reads were assembled with simulated read pairs using Allpaths–LG (version r42328) [29]. Parameters for assembly steps were: 1) Velvet (velveth: 63 –shortPaired and velvetg: −very_clean yes –exportFiltered yes –min_contig_lgth 500 –scaffolding no –cov_cutoff 10) 2) wgsim (−e 0 –1 100 –2 100 –r 0 –R 0 –X 0) 3) Allpaths–LG (PrepareAllpathsInputs: PHRED_64 = 1 PLOIDY = 1 FRAG_COVERAGE = 125 JUMP_COVERAGE = 25 LONG_JUMP_COV = 50, RunAllpathsLG: THREADS = 8 RUN = std_shredpairs TARGETS = standard VAPI_WARN_ONLY = True OVERWRITE = True). The final draft assembly contained 247 contigs in 246 scaffolds. The total size of the genome is 9.0 Mbp and the final assembly is based on 1,081.2 Mbp of Illumina data, which provides an average 119.7X coverage of the genome.
Genome annotation
Genes were identified using Prodigal [30], as part of the DOE-JGI genome annotation pipeline [31,32]. The predicted CDSs were translated and used to search the National Center for Biotechnology Information (NCBI) non-redundant database, UniProt, TIGRFam, Pfam, KEGG, COG, and InterPro databases. The tRNAScanSE tool [33] was used to find tRNA genes, whereas ribosomal RNA genes were found by searches against models of the ribosomal RNA genes built from SILVA [34]. Other non–coding RNAs such as the RNA components of the protein secretion complex and the RNase P were identified by searching the genome for the corresponding Rfam profiles using INFERNAL [35]. Additional gene prediction analysis and manual functional annotation was performed within the Integrated Microbial Genomes-Expert Review (IMG-ER) system [36] developed by the Joint Genome Institute, Walnut Creek, CA, USA.
Genome properties
The genome is 9,029,266 nucleotides with 62.56% GC content (Table 3) and comprised of 246 scaffolds. From a total of 8,584 genes, 8,482 were protein encoding and 102 RNA only encoding genes. The majority of genes (75.10%) were assigned a putative function whilst the remaining genes were annotated as hypothetical. The distribution of genes into COGs functional categories is presented in Table 4.
Table 3.
Genome statistics for Bradyrhizobium sp. Ai1a-2
| Attribute | Value | % of total |
|---|---|---|
| Genome size (bp) | 9,029,266 | 100.00 |
| DNA coding (bp) | 7,683,922 | 85.10 |
| DNA G + C (bp) | 5,648,849 | 62.56 |
| DNA scaffolds | 246 | 100 |
| Total genes | 8,584 | 100.00 |
| Protein coding genes | 8,482 | 98.81 |
| RNA genes | 102 | 1.19 |
| Pseudo genes | 0 | 0.00 |
| Genes in internal clusters | 837 | 9.75 |
| Genes with function prediction | 6,447 | 75.10 |
| Genes assigned to COGs | 5,111 | 59.54 |
| Genes with Pfam domains | 6,590 | 76.77 |
| Genes with signal peptides | 837 | 9.75 |
| Genes with transmembrane helices | 1,914 | 22.30 |
| CRISPR repeats | 0 | 0.00 |
Table 4.
Number of genes associated with the general COG functional categories
| Code | Value | % of total (5,698) | COG category |
|---|---|---|---|
| J | 185 | 3.25 | Translation, ribosomal structure and biogenesis |
| A | 0 | 0.00 | RNA processing and modification |
| K | 412 | 7.23 | Transcription |
| L | 223 | 3.91 | Replication, recombination and repair |
| B | 2 | 0.04 | Chromatin structure and dynamics |
| D | 33 | 0.58 | Cell cycle control, cell division, chromosome partitioning |
| V | 89 | 1.56 | Defense mechanisms |
| T | 234 | 4.11 | Signal transduction mechanisms |
| M | 277 | 4.86 | Cell wall/membrane/envelope biogenesis |
| N | 94 | 1.65 | Cell motility |
| U | 128 | 2.25 | Intracellular trafficking, secretion, and vesicular transport |
| O | 191 | 3.35 | Posttranslational modification, protein turnover, chaperones |
| C | 435 | 7.63 | Energy production and conversion |
| G | 340 | 5.97 | Carbohydrate transport and metabolism |
| E | 587 | 10.30 | Amino acid transport and metabolism |
| F | 77 | 1.35 | Nucleotide transport and metabolism |
| H | 198 | 3.47 | Coenzyme transport and metabolism |
| I | 311 | 5.46 | Lipid transport and metabolism |
| P | 364 | 6.39 | Inorganic ion transport and metabolism |
| Q | 256 | 4.49 | Secondary metabolite biosynthesis, transport and catabolism |
| R | 696 | 12.21 | General function prediction only |
| S | 566 | 9.93 | Function unknown |
| - | 3,473 | 40.46 | Not in COGS |
Conclusions
Bradyrhizobium sp. Ai1a-2 is a member of a widely distributed Bradyrhizobium lineage, isolated from diverse legume hosts in North, Central and South America and South Africa. Little is currently known of the symbiotic associations of its host Andira inermis, apart from the discovery that the Puerto Rican isolate Bradyrhizobium sp. EC3.3 can also establish a symbiosis with this host [8]. The Costa Rican isolate Aia1-2 16S rRNA gene sequence is distinct to that of EC3.3 but identical to the 16S rRNA sequence of South African isolate Bradyrhizobium sp. WSM2783. Phylogentically, Ai1a-2 is closely related to Bradyrhizobium sp. Cp5.3 and Bradyrhizobium sp. Th.b2 from Panama and USA, respectively. The genome of Bradyrhizobium 1a-2 and Ai sp.WSM2783 were sequenced along with 23 other Bradyrhizobium genomes as a part of the GEBA-RNB project. Of these 25 sequenced strains, the Bradyrhizobium spp. Ai1a-2, WSM2783, Cp5.3, Th.b2 and B. elkanii USDA76T are affiliated with the Bradyrhizobium elkanii superclade. The Bradyrhizobium Ai1a-2 genome has the 2nd lowest genome size (9 Mbp), gene count (8,584) and signal peptide percentage (9.75%) among these five strains. Comparing the genome attributes of Bradyrhizobium sp. Ai1a-2 along with other sequenced Bradyrhizobium genomes will be important for the understanding of the biogeography of Bradyrhizobium spp. interactions required for the successful establishments of effective symbioses with their diverse hosts.
Acknowledgements
This work was performed under the auspices of the US Department of Energy’s Office of Science, Biological and Environmental Research Program, and by the University of California, Lawrence Berkeley National Laboratory under contract No. DE-AC02-05CH11231. We thank Gordon Thompson (Murdoch University) for the preparation of SEM and TEM photos. We would also like to thank the Center of Nanotechnology at King Abdulaziz University for their support.
Abbreviations
- GEBA-RNB
Genomic Encyclopedia for Bacteria and Archaea-Root Nodule Bacteria
- JGI
Joint Genome Institute
- ½LA
Half strength Lupin Agar
- TY
Tryptone yeast
- YMA
Yeast mannitol agar
- CTAB
Cetyl trimethyl ammonium bromide
Additional file
Associated MIGS record. Table S1. Associated MIGS record for Bradyhizobium sp. Ai1a-2.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MP supplied the strain and background information for this project and the DNA to the JGI, TR performed all imaging, TR and WR drafted the paper, MNB and NAB provided financial support and all other authors were involved in sequencing the genome and/or editing the final paper. All authors read and approved the final manuscript.
Contributor Information
Rui Tian, Email: protein17@yahoo.com.sg.
Matthew Parker, Email: mparker@binghamton.edu.
Rekha Seshadri, Email: rseshadri@lbl.gov.
TBK Reddy, Email: TBReddy@lbl.gov.
Victor Markowitz, Email: VMMarkowitz@lbl.gov.
Natalia Ivanova, Email: NNIvanova@lbl.gov.
Amrita Pati, Email: APati@lbl.gov.
Tanja Woyke, Email: twoyke@lbl.gov.
Mohammed Baeshen, Email: mnbaeshen@kau.edu.sa.
Nabih Baeshen, Email: nabih_baeshen@hotmail.com.
Nikos Kyrpides, Email: nckyrpides@lbl.gov.
Wayne Reeve, Email: W.Reeve@murdoch.edu.au.
References
- 1.Parker MA. The spread of Bradyrhizobium lineages across host legume clades: from Abarema to Zygia. Microb Ecol. 2014;69:630–640. [DOI] [PubMed]
- 2.Willems A, Coopman R, Gillis M. Phylogenetic and DNA-DNA hybridization analyses of Bradyrhizobium species. Int J Syst Evol Microbiol. 2001;51:111–7. doi: 10.1099/00207713-51-1-111. [DOI] [PubMed] [Google Scholar]
- 3.Delamuta JR, Ribeiro RA, Ormeno-Orrillo E, Melo IS, Martinez-Romero E, Hungria M. Polyphasic evidence supporting the reclassification of Bradyrhizobium japonicum group Ia strains as Bradyrhizobium diazoefficiens sp. nov. Int J Syst Evol Microbiol. 2013;63:3342–51. doi: 10.1099/ijs.0.049130-0. [DOI] [PubMed] [Google Scholar]
- 4.Chaintreuil C, Arrighi JF, Giraud E, Miche L, Moulin L, Dreyfus B, et al. Evolution of symbiosis in the legume genus Aeschynomene. New Phytol. 2013;200:1247–59. doi: 10.1111/nph.12424. [DOI] [PubMed] [Google Scholar]
- 5.Evguenieva-Hackenberg E, Klug G. RNase III processing of intervening sequences found in helix 9 of 23S rRNA in the alpha subclass of Proteobacteria. J Bacteriol. 2000;182:4719–29. doi: 10.1128/JB.182.17.4719-4729.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker MA. rRNA and dnaK relationships of Bradyrhizobium sp. nodule bacteria from four papilionoid legume trees in Costa Rica. Syst Appl Microbiol. 2004;27:334–42. doi: 10.1078/0723-2020-00266. [DOI] [PubMed] [Google Scholar]
- 7.Parker MA. Symbiotic relationships of legumes and nodule bacteria on Barro Colorado Island, Panama: a review. Microb Ecol. 2008;55:662–72. doi: 10.1007/s00248-007-9309-z. [DOI] [PubMed] [Google Scholar]
- 8.Parker MA, Rousteau A. Mosaic origins of Bradyrhizobium legume symbionts on the Caribbean island of Guadeloupe. Mol Phylogenet Evol. 2014;77:110–5. doi: 10.1016/j.ympev.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Ardley JK, Reeve WG, O’Hara GW, Yates RJ, Dilworth MJ, Howieson JG. Nodule morphology, symbiotic specificity and association with unusual rhizobia are distinguishing features of the genus Listia within the Southern African crotalarioid clade Lotononis s.l. Ann Bot. 2013;112:1–15. doi: 10.1093/aob/mct095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver PL. Andira inermis (W. Wright) DC. Silvics of forest trees of the American tropics. SO-ITF-SM-20. New Orleans, LA: USDA Forest Service, Southern Forest Experiment Station; 1989. p. 7. [Google Scholar]
- 11.Lewis G SB, Mackinder B, Lock M. Legumes of the world Royal Botanic Gardens, Kew, UK; 2005.
- 12.Cardoso D PR, de Queiroz LP, Boatwright JS, Van Wyk BE, Wojciechowski MF, Lavin M. Reconstructing the deep-branching relationships of the papilionoid legumes. South Afr J Bot. 2013;89:58–75
- 13.Reeve W, Ardley J, Tian R, Eshragi L, Yoon JW, Ngamwisetkun P, et al. A genomic encyclopedia of the root nodule bacteria: assessing genetic diversity through a systematic biogeographic survey. Stand Genomic Sci. 2015;10:14. doi: 10.1186/1944-3277-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howieson JG, Ewing MA, D’antuono MF. Selection for acid tolerance in Rhizobium meliloti. Plant Soil. 1988;105:179–88. doi: 10.1007/BF02376781. [DOI] [Google Scholar]
- 15.Beringer JE. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974;84:188–98. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- 16.Vincent JM. A manual for the practical study of the root-nodule bacteria. International Biological Programme. UK: Blackwell Scientific Publications, Oxford; 1970. [Google Scholar]
- 17.NCBI BLAST [http://blast.ncbi.nlm.nih.gov/Blast.cgi]
- 18.Kim O-S, Cho Y-J, Lee K, Yoon S-H, Kim M, Na H, et al. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012;62:716–21. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- 19.EZTaxon [http://eztaxon-e.ezbiocloud.net/]
- 20.Pagani I, Liolios K, Jansson J, Chen IM, Smirnova T, Nosrat B, et al. The Genomes OnLine Database (GOLD) v. 4: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Res. 2012;40:D571–9. doi: 10.1093/nar/gkr1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markowitz VM, Chen I-MA, Palaniappan K, Chu K, Szeto E, Pillay M, et al. IMG 4 version of the integrated microbial genomes comparative analysis system. Nucleic Acids Res. 2014;42:D560–7. doi: 10.1093/nar/gkt963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mavromatis K, Land ML, Brettin TS, Quest DJ, Copeland A, Clum A, et al. The fast changing landscape of sequencing technologies and their impact on microbial genome assemblies and annotation. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeve WG, Tiwari RP, Worsley PS, Dilworth MJ, Glenn AR, Howieson JG. Constructs for insertional mutagenesis, transcriptional signal localization and gene regulation studies in root nodule and other bacteria. Microbiology. 1999;145:1307–16. doi: 10.1099/13500872-145-6-1307. [DOI] [PubMed] [Google Scholar]
- 24.Protocols and sample preparation information [http://jgi.doe.gov/collaborate-with-jgi/pmo-overview/protocols-sample-preparation-information/]
- 25.Bennett S. Solexa Ltd. Pharmacogenomics. 2004;5:433–8. doi: 10.1517/14622416.5.4.433. [DOI] [PubMed] [Google Scholar]
- 26.JGI:Joint Genome Institute [http://www.jgi.doe.gov]
- 27.Zerbino D, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–9. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reads simulator wgsim [https://github.com/lh3/wgsim]
- 29.Validation of publication of new names and new combinations previously effectively published outside the IJSEM. List no. 106. Int J Syst Evol Microbiol. 2005;55:2235–38. [DOI] [PubMed]
- 30.Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mavromatis K, Ivanova NN, Chen IM, Szeto E, Markowitz VM, Kyrpides NC. The DOE-JGI standard operating procedure for the annotations of microbial genomes. Stand Genomic Sci. 2009;1:63–7. doi: 10.4056/sigs.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen IM, Markowitz VM, Chu K, Anderson I, Mavromatis K, Kyrpides NC, et al. Improving microbial genome annotations in an integrated database context. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–64. doi: 10.1093/nar/25.5.0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188–96. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Infernal: inference of RNA alignments [http://infernal.janelia.org/]
- 36.Markowitz VM, Mavromatis K, Ivanova NN, Chen IM, Chu K, Kyrpides NC. IMG ER: a system for microbial genome annotation expert review and curation. Bioinformatics. 2009;25:2271–8. doi: 10.1093/bioinformatics/btp393. [DOI] [PubMed] [Google Scholar]
- 37.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, et al. Towards a richer description of our complete collection of genomes and metagenomes “Minimum Information about a Genome Sequence” (MIGS) specification. Nature Biotechnol. 2008;26:541–7. doi: 10.1038/nbt1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Field D, Amaral-Zettler L, Cochrane G, Cole JR, Dawyndt P, Garrity GM, et al. The Genomic Standards Consortium. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. P Natl A Sci USA. 1990;87:4576–9. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrity GM, Bell JA, Lilburn T. hylum XIV. Proteobacteria phyl. nov. In: Garrity GM, Brenner DJ, Kreig NR, Staley JT, editors. Bergey’s manual of systematic bacteriology. Volume 2. Second. New York: Springer - Verlag; 2005. p. 1. [Google Scholar]
- 42.Gnerre S, MacCallum I, Przybylski D, Ribeiro FJ, Burton JN, Walker BJ, et al. High-quality draft assemblies of mammalian genomes from massively parallel sequence data. P Natl A Sci. 2011;108:1513–8. doi: 10.1073/pnas.1017351108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrity GM, Bell JA, Lilburn T. Class I. Alphaproteobacteria class. In: Garrity GM, Brenner DJ, Kreig NR, Staley JT, editors. Bergey’s manual of systematic bacteriology. Volume 2. Second. New York: Springer - Verlag; 2005. p. 1. [Google Scholar]
- 44.Kuykendall LD. Order VI. Rhizobiales ord. nov. In: Garrity GM, Brenner DJ, Kreig NR, Staley JT, editors. Bergey’s manual of systematic bacteriology. Second. New York: Springer - Verlag; 2005. p. 324. [Google Scholar]
- 45.Garrity GM, Bell JA, Lilburn T. Family VII. Bradyrhizobiaceae fam. nov. In Bergey’s Manual of Systematic Bacteriology. Volume 2. edition. Edited by Brenn DJ. New York: Springer - Verlag; 2005: 438
- 46.Jordan DC. Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. nov., a genus of slow-growing, root nodule bacteria from leguminous plants. Int J Syst Bacteriol. 1982;32:136–9. doi: 10.1099/00207713-32-1-136. [DOI] [Google Scholar]
- 47.Biological Agents: Technical rules for biological agents. TRBA:466.
- 48.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat Genet. 2000;25:25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guide to GO evidence codes [http://www.geneontology.org/GO.evidence.shtml]
- 50.GOLD ID for Bradyrhizobium elkanii WSM1741 [https://gold.jgi-psf.org/projects?id=9846]


