Abstract
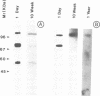
The asymmetric (20S) form of acetylcholinesterase (AChE) in 1-day-old chick muscle is a hybrid enzyme containing both AChE (110 kd) and butyrylcholinesterase (BuChE, 72 kd) catalytic subunits. However, we now report that the asymmetric AChE extracted or immunopurified from older adult chicken muscles, where it is the endplate form, shows a progressive developmental loss of the BuChE subunit and its activities, centred around 4 weeks of age, while the AChE and collagenous subunits remain. In confirmation, using differential labelling and co-sedimentation it was shown that the hybrid 20S AChE/BuChE form of 1-day chick muscle is gradually and completely replaced during muscle maturation by a 21.3S form, also collagen-tailed but otherwise homogeneous in AChE catalytic subunits. Two other changes occur concomitantly. Firstly, the AChE catalytic subunit of the adult form has a lower apparent mol. wt in gel electrophoresis, by 5 kd, than the same subunit in the 1-day hybrid enzyme; this difference does not reside in the carbohydrate attachments. Secondly, the collagen tail changes, in that some conformation-dependent epitopes on it disappear in the same period. Hence, a major reorganization of the asymmetric AChE, involving all three types of subunit, occurs in the course of muscle development.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allemand P., Bon S., Massoulié J., Vigny M. The quaternary structure of chicken acetylcholinesterase and butyrylcholinesterase; effect of collagenase and trypsin. J Neurochem. 1981 Mar;36(3):860–867. doi: 10.1111/j.1471-4159.1981.tb01673.x. [DOI] [PubMed] [Google Scholar]
- Bandman E., Matsuda R., Micou-Eastwood J., Strohman R. In vitro translation of RNA from embryonic and from adult chicken pectoralis muscle produces different myosin heavy chains. FEBS Lett. 1981 Dec 28;136(2):301–305. doi: 10.1016/0014-5793(81)80640-0. [DOI] [PubMed] [Google Scholar]
- Berman H. A., Decker M. M., Jo S. Reciprocal regulation of acetylcholinesterase and butyrylcholinesterase in mammalian skeletal muscle. Dev Biol. 1987 Mar;120(1):154–161. doi: 10.1016/0012-1606(87)90113-8. [DOI] [PubMed] [Google Scholar]
- Brimijoin S. Molecular forms of acetylcholinesterase in brain, nerve and muscle: nature, localization and dynamics. Prog Neurobiol. 1983;21(4):291–322. doi: 10.1016/0301-0082(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Carson S., Bon S., Vigny M., Massoulié J., Fardeau M. Distribution of acetylcholinesterase molecular forms in neural and non-neural sections of human muscle. FEBS Lett. 1979 Jan 15;97(2):348–352. doi: 10.1016/0014-5793(79)80119-2. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Jedrzejczyk J., Silman I., Lyles J. M., Barnard E. A. Molecular forms of the cholinesterases inside and outside muscle endplates. Biosci Rep. 1981 Jan;1(1):45–51. doi: 10.1007/BF01115148. [DOI] [PubMed] [Google Scholar]
- Lai J., Jedrzejczyk J., Pizzey J. A., Green D., Barnard E. A. Neural control of the forms of acetylcholinesterase in slow mammalian muscles. Nature. 1986 May 1;321(6065):72–74. doi: 10.1038/321072a0. [DOI] [PubMed] [Google Scholar]
- Layer P. G., Alber R., Sporns O. Quantitative development and molecular forms of acetyl- and butyrylcholinesterase during morphogenesis and synaptogenesis of chick brain and retina. J Neurochem. 1987 Jul;49(1):175–182. doi: 10.1111/j.1471-4159.1987.tb03411.x. [DOI] [PubMed] [Google Scholar]
- Lyles J. M., Silman I., Barnard E. A. Developmental changes in levels and forms of cholinesterases in muscles of normal and dystrophic chickens. J Neurochem. 1979 Sep;33(3):727–738. doi: 10.1111/j.1471-4159.1979.tb05218.x. [DOI] [PubMed] [Google Scholar]
- Lyles J. M., Silman I., Di Giamberardino L., Couraud J. Y., Barnard E. A. Comparison of the molecular forms of the cholinesterases in tissues of normal and dystrophic chickens. J Neurochem. 1982 Apr;38(4):1007–1021. doi: 10.1111/j.1471-4159.1982.tb05342.x. [DOI] [PubMed] [Google Scholar]
- Massoulié J., Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annu Rev Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Randall W. R., Tsim K. W., Lai J., Barnard E. A. Monoclonal antibodies against chicken brain acetylcholinesterase. Their use in immunopurification and immunochemistry to demonstrate allelic variants of the enzyme. Eur J Biochem. 1987 Apr 1;164(1):95–102. doi: 10.1111/j.1432-1033.1987.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Rieger F., Powell J. A., Pinçon-Raymond M. Extensive nerve overgrowth and paucity of the tailed asymmetric form (16 S) of acetylcholinesterase in the developing skeletal neuromuscular system of the dysgenic (mdg/mdg) mouse. Dev Biol. 1984 Jan;101(1):181–191. doi: 10.1016/0012-1606(84)90128-3. [DOI] [PubMed] [Google Scholar]
- Rotundo R. L., Fambrough D. M. Molecular forms of chicken embryo acetylcholinesterase in vitro and in vivo. Isolation and characterization. J Biol Chem. 1979 Jun 10;254(11):4790–4799. [PubMed] [Google Scholar]
- Rotundo R. L. Purification and properties of the membrane-bound form of acetylcholinesterase from chicken brain. Evidence for two distinct polypeptide chains. J Biol Chem. 1984 Nov 10;259(21):13186–13194. [PubMed] [Google Scholar]
- Silman I., di Giamberardino L., Lyles L., Couraud J. Y., Barnard E. A. Parallel regulation of acetylcholinesterase and pseudocholinesterase in normal, denervated and dystrophic chicken skeletal muscle. Nature. 1979 Jul 12;280(5718):160–162. doi: 10.1038/280160a0. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Coen D. M., Rich A. Tissue-specific forms of actin in the developing chick. Cell. 1976 Aug;8(4):521–527. doi: 10.1016/0092-8674(76)90220-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsim K. W., Randall W. R., Barnard E. A. An asymmetric form of muscle acetylcholinesterase contains three subunit types and two enzymic activities in one molecule. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1262–1266. doi: 10.1073/pnas.85.4.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsim K. W., Randall W. R., Barnard E. A. Identification of a 17 S asymmetric butyrylcholinesterase in chick muscle by monoclonal antibodies. Neurosci Lett. 1988 Mar 31;86(2):245–249. doi: 10.1016/0304-3940(88)90579-4. [DOI] [PubMed] [Google Scholar]
- Vigny M., Gisiger V., Massoulié J. "Nonspecific" cholinesterase and acetylcholinesterase in rat tissues: molecular forms, structural and catalytic properties, and significance of the two enzyme systems. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2588–2592. doi: 10.1073/pnas.75.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigny M., Koenig J., Rieger F. The motor end-plate specific form of acetylcholinesterase: appearance during embryogenesis and re-innervation of rat muscle. J Neurochem. 1976 Dec;27(6):1347–1353. doi: 10.1111/j.1471-4159.1976.tb02614.x. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Protein synthesis and actin heterogeneity in calf muscle cells in culture. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2018–2022. doi: 10.1073/pnas.73.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younkin S. G., Rosenstein C., Collins P. L., Rosenberry T. L. Cellular localization of the molecular forms of acetylcholinesterase in rat diaphragm. J Biol Chem. 1982 Nov 25;257(22):13630–13637. [PubMed] [Google Scholar]