Abstract
This scientific commentary refers to ‘Cav1.3 channels control D2-autoreceptor responses via NCS-1 in substantia nigra dopamine neurons', by Dragicevic et al. (doi:10.1093/brain/awu131).
This scientific commentary refers to ‘Cav1.3 channels control D2 autoreceptor responses via NCS1 in substantia nigra dopamine neurons’ by Dragicevic et al. (doi:10.1093/brain/awu131).
Appropriate activity of substantia nigra dopamine neurons is required for proper motor function, habit formation and motivation, and degeneration of these neurons in Parkinson’s disease leads to disrupted control of voluntary movement. In this issue of Brain, Dragicevic et al. unite two previously separate lines of research on the regulation of substantia nigra dopamine neuron activity—one based on L-type calcium channels and the other on D2 autoreceptors—and suggest that these mechanisms converge in a previously unsuspected way in Parkinson’s disease (Dragicevic et al., 2014).
One of the two lines of research stems from decades of work to define how the activity of midbrain dopamine neurons is controlled. These neurons alternate between a relatively slow, baseline, pacemaking activity (∼4 Hz) that presumably supplies the striatum with tonic low levels of extracellular dopamine, and bursts of activity of variable duration and only slightly higher frequency (∼15 Hz) (Grace et al., 2007). The resulting ‘bandwidth’ is not large: in contrast, activities of cortical output neurons can range from silent states to firing at frequencies of 20 Hz or more. While questions such as how salient sensory stimuli cause bursting are active areas of research, the pacemaking activity—which is autonomous, occurring even in cultured substantia nigra neurons—is fairly well elucidated, albeit subject to continuing elaboration in papers such as the one being discussed here.
In contrast to most other tonically active CNS neurons, which depend on monovalent cation channels to generate spontaneous action potentials, depolarization of mature pacemaking substantia nigra dopamine neurons may involve the opening of L-type calcium Cav1.3 channels, together with hyperpolarization-activated, cyclic nucleotide-gated (HCN) sodium channels (Puopolo et al., 2013). The large calcium conductance through Cav1.3 channels has been suggested to underlie the specific vulnerability of substantia nigra (as well as locus coeruleus and dorsal motor nucleus of the vagus) neurons to cell death in Parkinson’s disease (Surmeier and Schumacker, 2013). These neurons, moreover, exhibit wide action potentials (>2 ms), giving rise to further calcium entry via voltage-activated channels in the interspike interval (Puopolo et al., 2013). Substantia nigra dopamine neurons also lack significant calcium buffering by proteins such as parvalbumin and calbindin—the latter of which is more highly expressed in ventral tegmental area dopamine neurons, which are relatively spared in Parkinson’s disease.
Studies by James Surmeier and collaborators demonstrate that the high intracellular calcium load in substantia nigra dopamine neurons causes mitochondrial and oxidative stress, and others have provided evidence that high calcium can exacerbate neurodegeneration through the accumulation of neurotoxic levels of cytosolic catecholamines (Mosharov et al., 2009). Inhibition of L-type calcium channels with dihydropyridines protects substantia nigra pars compacta neurons against neurotoxins associated with Parkinson’s disease in a variety of animal studies (Surmeier and Schumacker, 2013). These data suggest that inhibition of Cav1.3 channel activity may be neuroprotective for the remaining substantia nigra pars compacta neurons in patients with Parkinson’s disease, and isradipine, a dihydropyridine L-type calcium channel blocker shown to be effective in mouse models of the disorder, is currently in a clinical trial as a Parkinson’s disease therapy (Parkinson Study Group, 2013).
The second line of research extends from the study of dopamine receptor-mediated auto-inhibition of neuronal activity. In substantia nigra neurons, this is mediated by D2-type receptors, which activate G protein coupled potassium channels (GIRKs) that hyperpolarize neurons and block cell firing (Lacey et al., 1987). The response of substantia nigra neurons to dopamine is highly regulated, with chronic loss of dopamine leading to receptor sensitization (Schultz and Ungerstedt, 1978), a phenomenon strongly implicated in Parkinson’s disease and its animal models. Work by John Williams and collaborators has shown that somatodendritic dopamine release drives rapid D2 receptor-mediated hyperpolarization of neighbouring dopaminergic neurons (Beckstead et al., 2004). It may be, therefore, that dopamine autoreceptor activation inhibits the voltage and activity-dependent calcium-mediated stress associated with Parkinson’s disease, and it is further possible that this is another advantage of clinical treatment with l-DOPA and dopamine agonists, although this has not been directly addressed.
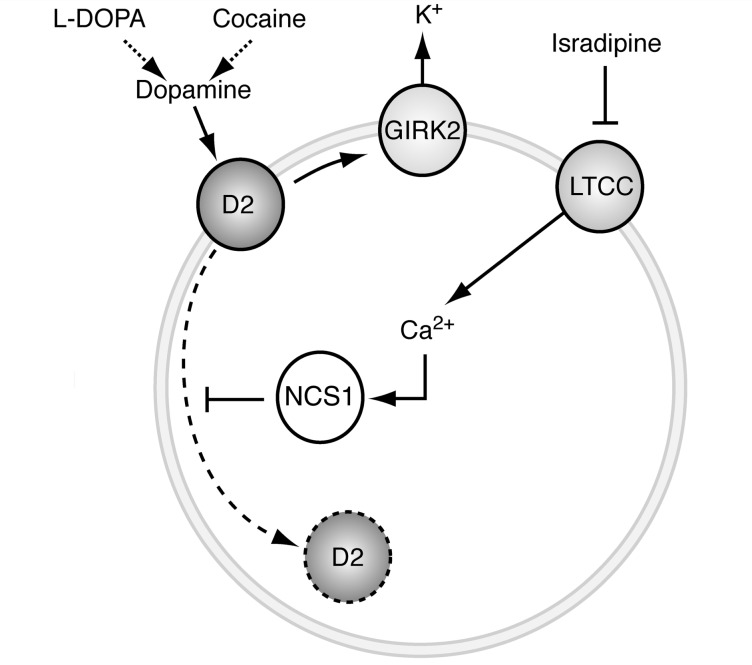
In their new study, Dragicevic et al. connect these two lines of research by demonstrating that L-type calcium channels can promote D2 receptor function in juvenile substantia nigra pars compacta dopamine neurons (in contrast to the above D2 receptor-mediated inhibition of calcium currents). They show that this occurs after in vivo high dopamine states (induced by l-DOPA or cocaine) via Cav1.3-mediated interactions between the D2 receptor and a neuronal calcium sensor protein known as NCS1. In response to increased intracellular calcium, NCS1 prevents the internalization of plasma membrane D2 receptors, thus blocking receptor desensitization. Blocking L-type calcium channels consistently prevented the development of non-desensitized responses in these juvenile neurons (Fig. 1).
Figure 1.
Proposed mechanism by which L-type calcium channels stabilize autoreceptor function in dopamine neurons. Dopamine D2 receptors are prototypical autoreceptors that control the firing rate of pacemaking substantia nigra dopamine neurons via activation of inhibitory GIRK channels. In response to stimulation by dopamine or indirect ligands such as cocaine, the inhibitory effect on dopamine neuron firing is reduced due to internalization of D2 receptors. This D2-desensitization is prevented by the neuronal calcium sensor protein NCS1, which is regulated by calcium supplied via Cav1.3 L-type calcium channels active during autonomous firing of dopamine neurons. The L-type calcium channel (LTCC) blocker isradipine, which is currently being evaluated as a neuroprotective therapy for Parkinson’s disease, facilitates desensitization of the D2 receptor and disrupts the ability of dopamine to inhibit neuronal activity.
Thus, the high calcium load in substantia nigra neurons might provide a protective stress response, in which mature neurons do not undergo autoreceptor desensitization: this might block further calcium-related damage that could otherwise lead to Parkinson’s disease. Remarkably, the authors report that NCS1 expression was increased in surviving dopamine neurons in the brains of patients with Parkinson’s disease, suggesting that D2 receptors may indeed have been driven to a non-desensitized state; while this clearly did not prevent the disease, it may have slowed its progression. An important point to note, however, is that these patients had almost certainly been treated with L-DOPA and/or dopamine agonists, which may have contributed to the increased NCS1 expression and reduced autoreceptor desensitization.
The introduction of this novel pathway—calcium entry → NCS1 activation → inhibition of D2 receptor desensitization—raises numerous questions. One raised by Dragicevic et al. themselves is that dihydropyridines, by exacerbating D2 receptor desensitization, could (perversely) lead to excitotoxicity, although there is so far no evidence for this possibility. Another issue to explore is the importance of this response in mature substantia nigra neurons; while the authors report that desensitization of dopamine responses occurred selectively in juvenile substantia nigra pars compacta neurons, it is not clear if it also occurs in adult cells. Indeed, Dragicevic et al. studied a knockout line that did not express the Cav1.3 channel during development, and in contrast to the expected autoreceptor desensitization, they detected non-desensitized receptors in substantia nigra dopamine neurons in both juvenile and adult mice. Further questions are raised by studies from Mark Brodie and colleagues (Nimitvilai et al., 2012) demonstrating that desensitization of D2 receptors in ventral tegmental area neurons is enhanced by calcium signalling, in a manner that is independent of L-type calcium channels—what underlies these apparent differences? Irrespective of how these issues are eventually resolved, Dragicevic et al. have introduced a new pathway: and, as seems to be the usual pattern in our field, they have revealed that brain activity is more complex and interactive than we thought. This may make our job of deciphering neural function more challenging, but it is precisely these complexities that provide us with the ability to recognize these patterns at all.
References
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–46. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Dragicevic E, Poetschke C, Duda J, Schlaudraff F, Lammel S, Schiemann J, et al. Cav1.3 channels control D2-autoreceptor responses via NCS-1 Substantia NIgra dopamine neurons. Brain. 2014 doi: 10.1093/brain/awu131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–7. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–29. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, Arora DS, Brodie MS. Reversal of dopamine inhibition of dopaminergic neurons of the ventral tegmental area is mediated by protein kinase C. Neuropsychopharmacology. 2012;37:543–56. doi: 10.1038/npp.2011.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson Study Group. Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson's disease (STEADY-PD) Mov Disord. 2013;28:1823–31. doi: 10.1002/mds.25639. [DOI] [PubMed] [Google Scholar]
- Puopolo M, Binshtok AM, Yao GL, Oh SB, Woolf CJ, Bean BP. Permeation and block of TRPV1 channels by the cationic lidocaine derivative QX-314. J Neurophysiol. 2013;109:1704–12. doi: 10.1152/jn.00012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Ungerstedt U. Striatal cell supersensitivity to apomorphine in dopamine-lesioned rats correlated to behaviour. Neuropharmacology. 1978;17:349–53. doi: 10.1016/0028-3908(78)90005-9. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Schumacker PT. Calcium, bioenergetics, and neuronal vulnerability in Parkinson's disease. J Biol Chem. 2013;288:10736–41. doi: 10.1074/jbc.R112.410530. [DOI] [PMC free article] [PubMed] [Google Scholar]