Abstract
Abnormalities in vitamin D metabolism play a major role in the pathogenesis of secondary hyperparathyroidism in chronic kidney disease. The gradual and progressive decline in 1,25-dihydroxyvitamin D in the course of chronic kidney disease is the result of several mechanisms that limit the ability of the failing kidney to maintain the levels of 1,25-dihydroxyvitamin D despite increasing levels of parathyroid hormone. Recent observations have indicated that chronic kidney disease seems to be associated with a high incidence of nutritional vitamin D insufficiency or deficiency as manifested by decreased levels of 25-hydroxyvitamin D. This contributes to the inability to maintain the levels of 1,25-dihydroxyvitamin D; therefore, current practice guidelines suggest repleting vitamin D status by the administration of native vitamin D as a first step in the therapy of the abnormalities of bone and mineral metabolism in chronic kidney disease. The efficacy of this therapy is extremely variable, and active vitamin D sterols may be required, especially as kidney disease progresses. The importance of the abnormal vitamin D metabolism is being investigated vigorously in view of the observations that vitamin D may have important biologic actions in many tissues in addition to bone and parathyroid. Thus, observational data have suggested potential survival benefits of vitamin D sterol administration in this clinical setting, and experimental data have suggested a potential beneficial effect of vitamin D sterols on the progression of kidney disease. Further work is required to define the mechanisms involved and to examine the effects of vitamin D therapy on outcomes in randomized, controlled trials.
It has been well established that secondary hyperparathyroidism begins relatively early in the course of chronic kidney disease (CKD) and steadily progresses as GFR declines. The pathogenetic factors that contribute to the development and maintenance of secondary hyperparathyroidism are multiple but principally involve the closely related consequences of phosphate retention and abnormalities in vitamin D metabolism. In the course of CKD, there is a slow progressive decrease in the levels of 1,25-dihydroxyvitamin D (calcitriol) as kidney function declines. The role of the decreasing levels of 1,25-dihydroxyvitamin D in the pathogenesis of secondary hyperparathyroidism is supported by the demonstration of the negative effects of 1,25-dihydroxyvitamin D on parathyroid hormone (PTH) gene transcription and the observations that administration of calcitriol can ameliorate the development of hyperparathyroidism and can effectively suppress the secretion of PTH when hyperparathyroidism has been established.
Vitamin D is either synthesized in the skin or ingested in the diet and is transported to the liver, where it is hydroxylated in the 25 position to yield 25-hydroxyvitamin D, which is the main storage form of vitamin D and the vitamin D metabolite to be measured to assess vitamin D nutrition. 25-Hydroxyvitamin D is further hydroxylated by the enzyme 1-α-hydroxylase in the kidney, to yield 1,25-dihyroxyvitamin D, which is the active form of vitamin D, the major endocrine form of vitamin D, and this metabolite is responsible for the effects of vitamin D on calcium and phosphorus metabolism, bone health, and the regulation of parathyroid function.
In recent years, it has been demonstrated that many tissues not only express the vitamin D receptor (VDR) but also may possess 1-α-hydroxylase and are therefore capable of the production of 1, 25-dihydroxyvitamin D, which may act locally. Such tissues include the prostate; the colon; the breast; macrophages; and cells of the vasculature, pancreas, and potentially other sites. The role of this extrarenal 1-α-hydroxylase, with the production of 1,25-dihydroxyvitamin D in these tissues, is not well understood, but a variety of in vitro studies indicate that this process may be involved in the regulation of cell growth and differentiation (1).
Mechanisms of Altered Vitamin D Metabolism in Kidney Disease
There seem to be several mechanisms involved in the decreased levels of 1,25-dihydroxyvitamin D that occur in the course of kidney disease (Figure 1). Thus, a decrease in renal mass will obviously limit the quantities of 1-α-hydroxylase that are available for production of the active vitamin D metabolite. A reduction in GFR may limit delivery of substrate to the 1-α-hydroxylase, which may also limit the ability of the kidney to produce 1,25-dihydroxyvitamin D. The importance of a declining GFR in limiting the ability of the kidney to produce 1 to 25-dihydroxyvitamin D was illustrated by the work of Nykjaer et al. (2–6), who demonstrated that glomerular filtration of 25-hydroxyvitamin D, bound to vitamin D–binding protein, undergoes glomerular filtration and uptake into the proximal tubule cell by the receptor megalin and was the rate-limiting step in the delivery of 25-hydroxyvitamin D to the 1-α-hydroxylase enzyme. Accordingly, as GFR declines, there is a limitation of substrate delivery that can compromise the ability of the failing kidney to produce 1,25-dihydroxyvitamin D (7). This may be compounded by the decreased levels of 25-hydroxyvitamin D that seem to be common in patients with kidney disease (vide infra).
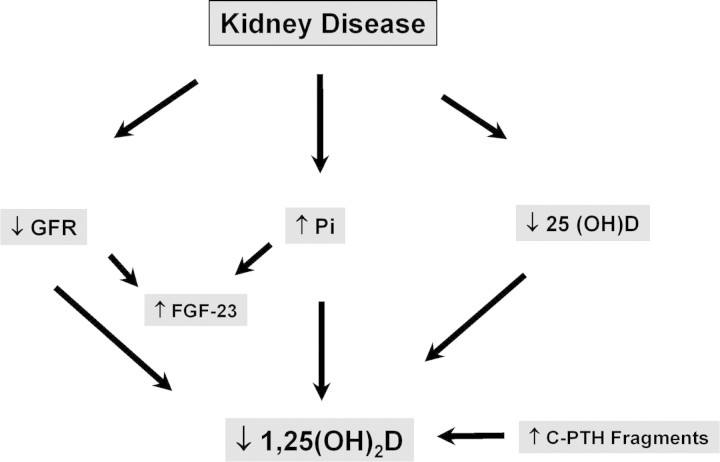
Figure 1.
Diagram of the mechanisms involved in limiting the ability of the kidney to maintain the levels of 1,25-dihydroxyvitamin D in chronic kidney disease (CKD). C-PTH, C-terminal parathyroid hormone; FGF-23, fibroblast growth factor-23; Pi, phosphate.
The recent discovery that fibroblast growth factor-23 (FGF-23), which increases in the course of kidney disease, can directly suppress 1-α-hydroxylase may be an additional contributing factor that limits the ability of the failing kidney to maintain levels of 1,25-dihyroxyvitamin D as kidney disease progresses. Shimada et al. (8) demonstrated that FGF-23 could decrease the levels of 1,25-dihydroxyvitamin D and decrease mRNA for 1-α-hydroxylase. Perwad et al. (9) also showed that FGF-23 induced a dosage-dependent decrease in 1-α-hydroxylase mRNA. In addition, the activity of 1-α-hydroxylase may be directly suppressed by phosphate retention and hyperphosphatemia (1). An additional factor that may be involved is the potential for N-terminally truncated PTH fragments or C-terminal PTH fragments to decrease activity of 1-α-hydroxylase (10).
Vitamin D Deficiency in CKD
Recent observations have demonstrated that kidney disease seems to be associated with a high incidence of vitamin D insufficiency or deficiency (11). Studies by Gonzalez et al. (12) demonstrated that 25-hydroxyvitamin D values are <30 ng/ml, believed be the lower limit of normal, in the majority of patients with CKD. Patients who are severely proteinuric have the lowest values. These investigators have shown that virtually all of the secondary hyperparathyroidism that occurs in the course of CKD is associated with 25-hydroxyvitamin D values that are <30 ng/ml. It is interesting to note that in this patient group, there is a positive relationship between 25-hydroxyvitamin D levels and 1, 25-dihydroxyvitamin D levels, in contrast to what is seen in normal individuals. Thus, when 25-hydroxyvitamin D levels are increased by therapy, one would anticipate an increase in the levels in the 1,25-dihydroxyvitamin D. It is not clear whether this is a contribution of renal 1-α-hydroxylase or the 1-α-hydroxylase at extrarenal sites; however, because of the association of low levels of 25-hydroxyvitamin D with hyperparathyroidism in the course of CKD, it is recommended that in patients with CKD, if hyperparathyroidism is detected, then 25-hydroxyvitamin D should be measured, and if found to be <30 ng/ml, then the initial step in the therapy should be to try to correct this abnormality, as the first step in the control of hyperparathyroidism.
Effects of Therapy with Ergocalciferol
Zisman et al. (13) evaluated the current therapeutic guidelines to raise 25-hydroxyvitamin D by the administration of ergocalciferol. These investigators showed that in 52 patients with stages 3 and 4 CKD, the concentration of 25-hydroxyvitamin D could be raised slightly above 30 ng/ml, and such therapy was associated with a relatively small decrease in the levels of intact PTH, only in the patients with stage 3 CKD and not in those with stage 4 CKD. Chandra et al. (14) evaluated cholecalciferol therapy, 50,000 U/wk for 12 wk, in a randomized, controlled trial of stages 3 and 4 CKD and successfully raised the geometric mean value for 25-hydroxyvitamin D to almost 50 ng/ml and showed a 31% decrease in PTH in the treated group compared with 7% decrease in the placebo group, but this was NS because of high variability in PTH values.
Studies by Al-Aly et al. (15) were similar in design to the studies of Zisman et al. (13), although the conclusions are somewhat different and may shed light on the results of Chandra et al. (14). These investigators showed that only approximately 50% of patients with CKD successfully incremented the levels of 25-hydroxyvitamin D in response to the standard treatment regimens, whereas the remainder did not increment the levels of 25-hydroxyvitamin D. It is interesting that in patients who did increase their levels of 25-hydroxyvitamin D, PTH levels declined, whereas PTH levels did not change substantially in patients who did not respond. Similar findings were also demonstrated in patients with stage 4 CKD. Accordingly, additional studies need to be performed to understand the reasons that 25-hydroxyvitamin D levels were not successfully incremented in half of the patients, to assess the efficacy of this maneuver on the control of hyperparathyroidism.
This issue assumes particular importance in light of the observations that the administration of active vitamin D therapy to patients who are on dialysis seems to be associated with a survival advantage, compared with patients who did not receive any vitamin D (16). These observations are confirmed by other investigators, including Young et al. (17), using the Dialysis Outcomes and Practice Patterns Study (DOPPS) database, and Kalantar-Zadeh et al. (18) in a different cohort of patients, raising the need to understand the mechanisms involved in this apparent survival benefit associated with vitamin D therapy. In these studies, it seems that this apparent survival benefit is seen regardless of calcium, phosphorus, or the levels of PTH, suggesting that this may be an effect of vitamin D that is independent of the effects of vitamin D on bone and mineral metabolism. These observations have also been further extended to vitamin D analogs; the analog paricalcitol has been shown to have an improved survival advantage over the native vitamin D sterol calcitriol (19). Similarly, 1-α-hydroxyvitamin D2 has also been associated with an apparent improved survival advantage over the native hormone calcitriol (20). Again, in these studies, the apparent survival benefit seems to be independent of levels of calcium, phosphorus, or PTH. Accordingly, it seems important to understand the potential mechanisms involved in this apparent survival benefit, with the objective that this can be potentially exploited to improve survival in this patient group.
London et al. (21) evaluated 52 patients who were on hemodialysis in a cross-sectional study for possible relationships of aortic stiffness, brachial artery distensibility, and arterial calcification scores with 25(OH)D3 and 1,25(OH)2D3 serum levels. These investigators noted that these values were negatively correlated with aortic pulse wave velocity and positively correlated with brachial artery distensibility and flow-mediated dilation. Whether vitamin D supplementation will improve arteriosclerosis and endothelial dysfunction in patients who are on hemodialysis needs to be further evaluated in the future (21).
Because there seems to be only a single VDR, it is difficult to understand how vitamin D analogs may differ from the effect of the native hormone, but this indeed seems to be the case. In studies in vitro in vascular smooth muscle cells, calcitriol seems to be a growth factor for vascular smooth muscle cells, whereas the analog, paricalcitol, is not (22). Furthermore, in studies in experimental animals in vivo, vitamin D sterols seem to have a different effect on vascular calcification, in that 1-α-hydroxyvitamin D2 or calcitriol seems to be associated with greater vascular calcification than is seen with paricalcitol, despite equivalent suppression of PTH in these animal models (23). Similar studies have been reported by Wu-Wong et al. (24,25), and other investigators have shown similar results in studies of calcitriol, compared with 22-oxacalcitriol (26). Further studies are clearly needed to understand the potential mechanisms involved for these differential effects.
The pleiotropic effects of vitamin D beyond the control of parathyroid function or mineral metabolism may extend to other potential areas in the course of CKD. Thus, in the course of clinical studies with oral paricalcitol for control of hyperparathyroidism in CKD, it was noted that patients who received paricalcitol seemed to have a reduction in proteinuria, even patients who were treated with angiotensin-converting enzyme inhibitor or angiotensin receptor blocker (27). In those studies, Agarwal et al. (27) demonstrated that the antiproteinuric effect of oral paricalcitol in CKD was shown by a reduction in proteinuria in 29 (51%) of 57 in the paricalcitol group compared with 15 (25%) of 61 in the placebo group with a P = 0.004. This was regardless of age, gender, race, diabetes, hypertension, or use of angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors.
These observations raise the possibility or the consideration that paricalcitol therapy may be associated with or may have the potential to alter the progression of CKD. Some evidence exists to support this possibility. Thus, whereas the role of the renin-angiotensin system (RAS) has been well described in the progression of kidney disease, the vitamin D system has been shown to be involved in the regulation of the RAS by Li et al. (28). In experimental circumstances, there is evidence that vitamin D therapy may favorably affect the progression of CKD. Thus, there are data to show that glomerulosclerosis may be decreased in a model of five-sixths nephrectomy (29). The involvement of the VDR in the suppression of the RAS and the reduction in glomerular growth, cell differentiation, and fibrosis is potentially crucial in the mechanism of CKD progression. Similar effects have also been shown in models of glomerulonephritis, and in additional studies, effects on the widely known factors that have been identified to affect the progression of kidney disease, such as podocyte hypertrophy (30), expression of TGF-β (31,32), expression of monocyte chemoattractant protein-1 (33,34), and the invasion of the remnant kidneys with macrophage-like cells, all have been shown to be potentially modified by vitamin D therapy, such that there is potential for this to affect the progression of kidney disease (35). In addition, Zhang et al. (36) showed that diabetic VDR knockout mice developed more severe albuminuria and glomerulosclerosis and expressed more fibronectin and less nephrin compared with diabetic wild-type animals. In vitro, 1,25-dihydroxyvitamin D inhibited glucose-induced fibronectin production in mesangial cells and increased nephrin in podocytes. Thus, vitamin D has the potential to have a favorable impact in diabetic nephropathy. All of these experimental observations need to be addressed and tested in patients, and studies are in progress or in development to test this idea directly.
Vitamin D may also affect the myocardium directly and play a role in the regulation of myocyte hypertrophy (37). Bodyak et al. (38) showed that paricalcitol attenuates left ventricular abnormalities in Dahl salt-sensitive hypertensive rats. Thus, it is interesting to speculate that such effects of vitamin D on the RAS or on the heart may potentially have an impact on cardiovascular events that are a common cause of death in this patient group.
Recommendations for Vitamin D Therapy in the Course of CKD
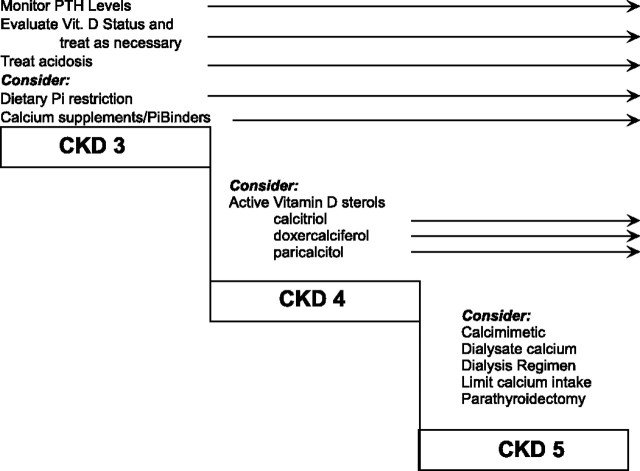
A reasonable approach to the therapy of disorders of bone and mineral metabolism in CKD, on the basis of current Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines and clinical and experimental data, are proposed in Figure 2. Future guidelines may modify this approach as they become available and additional data are obtained, such as the forthcoming Kidney Disease: Improving Global Outcomes (KDIGO) guidelines.
Figure 2.
A suggested scheme for the assessment and therapy of disorders of bone and mineral metabolism throughout the course of CKD. Reprinted from reference (39), with permission.
Disturbances in bone and mineral metabolism should be evaluated early in the course of CKD by the measurement of intact PTH, and interventions should be undertaken using a “stepped care” approach as illustrated in Figure 2 (39). Thus, in stage 2 or 3 CKD, if PTH is elevated, then vitamin D status should be evaluated by measurement of 25-hydroxyvitamin D, and if <30 ng/ml, then treatment should be undertaken according to current practice guidelines, which recommend the administration of a vitamin D preparation such as ergocalciferol in sufficient dosage to raise 25-hydroxyvitamin D levels >30 ng/ml. Vitamin D, as either D3 or D2, does not have significant biologic activity and must be metabolized within the body to the hormonally active form 1,25 dihydroxyvitamin D. The difference between ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3) with regard to the degree of response of 25-hydroxyvitamin D levels has not been studied in comparative trials in patients with CKD.
Dietary restriction of phosphorus may be used in early CKD to assist in the control of the developing hyperparathyroidism, although protein restriction should be modest to avoid malnutrition. Other measures that have been shown to be successful include calcium supplementation and the use of phosphate binders and the use of vitamin D sterols such as calcitriol (40), the vitamin D prohormones alfacalcidol (41) and doxercalciferol (42), and the vitamin D analog paricalcitol (43). In advanced kidney disease, the use of active vitamin D sterols is extremely effective in the control of hyperparathyroidism (Table 1). The vitamin D prohormones 1-α-hydroxyvitamin D3 and 1-α-hydroxyvitamin D2 undergo 25-hydroxylation in the liver and become 1 to 25-dihydroxyvitamin D3 and 1 to 25-dihydroxyvitamin D2, respectively. Vitamin D analogs are the active vitamin D molecules with side chain modification or A ring alterations that have been introduced with a goal of suppression of PTH while minimizing the effects on calcium and phosphorus levels. Three such analogs have been introduced: 19-nor-1,25-dihydroxyvitamin D2 (44), 22-oxacalcitriol (45), and 26,27-hexafluorocalcitriol (46). 19-Nor-1,25-dihydroxyvitamin D2 is widely used in the United States and has been effective with somewhat lesser toxicity than the native sterol calcitriol (47). The oral form can be used in stages 3 and 4 CKD (43). There are no comparative studies of the different vitamin D analogs with regard to safety and efficacy in patients with CKD.
Table 1.
Vitamin D sterols used in chronic kidney disease
| Native vitamin D | |
| vitamin D2 | Ergocalciferol |
| vitamin D3 | Cholecalciferol |
| Vitamin D prohormones | |
| 1-α-(OH) D3 | Alfacalcidol |
| 1-α-(OH) D2 | Doxercalciferol |
| Active vitamin D sterols | |
| 1-α-25- (OH)2 D3 | Calcitriol |
| 22-oxa-1, 25(OH)2 D3 | Maxicalcitol |
| 1,25(OH)2-26, 27-F6-D3 | Falecalcitriol |
| 19-Nor-1, 25(OH)2 D2 | Paricalcitol |
Further studies are required regarding the importance of therapy with vitamin D sterols in the course of CKD in view of recent observations by Wolf et al. (48). These investigators examined the correlation of vitamin D levels and early mortality in a retrospective cohort cross-sectional analysis of 825 incident hemodialysis population. The team measured the blood level of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D at baseline, and 90-d mortality was evaluated. A total of 78% of the patient population was vitamin D deficient, and 18% were severely deficient, and low levels of 25-hydroxyvitamin D were associated with increased mortality (48). Intervention with active vitamin D seemed to be associated with improved outcome. These important results will need confirmation and extension by randomized, placebo-controlled trials, and additional studies will be needed to evaluate the effects of vitamin D sterols on the progression of kidney disease.
Conclusions
In-depth research in the past two decades has uncovered many of the potential mechanisms in which vitamin D is involved in the initiation and maintenance of the disturbances of bone and mineral metabolism in the CKD population. This has been reproduced in bench and bedside clinical observations. The currently available therapeutic strategies for vitamin D deficiency are important beyond the management and control of the complications of disturbed mineral metabolism. Further clinical research is needed to answer questions including such issues as the optimal time to screen for vitamin D deficiency irrespective of PTH levels; the effects of treatment of vitamin D deficiency and insufficiency, perhaps irrespective of PTH levels; potential differences between vitamin D2 and D3 sterols; and studies to define the optimal dosage, duration, goals, and outcomes of treatment of vitamin D deficiency.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Dusso AS, Brown AJ, Slatopolsky E: Vitamin D. Am J Physiol Renal Physiol 289 :F8– F28,2005 [DOI] [PubMed] [Google Scholar]
- 2.Hilpert J, Wogensen L, Thykjaer T, Wellner M, Schlichting U, Orntoft TF, Bachmann S, Nykjaer A, Willnow TE: Expression profiling confirms the role of endocytic receptor megalin in renal vitamin D3 metabolism. Kidney Int 62 :1672– 1681,2002 [DOI] [PubMed] [Google Scholar]
- 3.Willnow TE, Nykjaer A: Pathways for kidney-specific uptake of the steroid hormone 25-hydroxyvitamin D3. Curr Opin Lipidol 13 :255– 260,2002 [DOI] [PubMed] [Google Scholar]
- 4.Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, Ray R, Gliemann J, Willnow TE, Christensen EI: Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3). Proc Natl Acad Sci U S A 98 :13895– 13900,2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, Jacobsen C, Aucouturier P, Moskaug JO, Otto A, Christensen EI, Willnow TE: Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol 155 :1361– 1370,1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE: An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96 :507– 515,1999 [DOI] [PubMed] [Google Scholar]
- 7.Andress DL: Vitamin D in chronic kidney disease: A systemic role for selective vitamin D receptor activation. Kidney Int 69 :33– 43,2006 [DOI] [PubMed] [Google Scholar]
- 8.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T: FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19 :429– 435,2004 [DOI] [PubMed] [Google Scholar]
- 9.Perwad F, Zhang MY, Tenenhouse HS, Portale AA: Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol 293 :F1577– F1583,2007 [DOI] [PubMed] [Google Scholar]
- 10.Usatii M, Rousseau L, Demers C, Petit JL, Brossard JH, Gascon-Barre M, Lavigne JR, Zahradnik RJ, Nemeth EF, D'Amour P: Parathyroid hormone fragments inhibit active hormone and hypocalcemia-induced 1,25(OH)2D synthesis. Kidney Int 72 :1330– 1335,2007 [DOI] [PubMed] [Google Scholar]
- 11.LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, Graves KL, Moe SM: Prevalence of calcidiol deficiency in CKD: A cross-sectional study across latitudes in the United States. Am J Kidney Dis 45 :1026– 1033,2005 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez EA, Sachdeva A, Oliver DA, Martin KJ: Vitamin D insufficiency and deficiency in chronic kidney disease: A single center observational study. Am J Nephrol 24 :503– 510,2004 [DOI] [PubMed] [Google Scholar]
- 13.Zisman AL, Hristova M, Ho LT, Sprague SM: Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol 27 :36– 43,2007 [DOI] [PubMed] [Google Scholar]
- 14.Chandra P, Binongo JN, Ziegler TR, Schlanger LE, Wang W, Someren JT, Tangpricha V: Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: A randomized controlled pilot study. Endocr Pract 14 :10– 17,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Aly Z, Qazi RA, Gonzalez EA, Zeringue A, Martin KJ: Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis 50 :59– 68,2007 [DOI] [PubMed] [Google Scholar]
- 16.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA Jr, Thadhani R: Activated injectable vitamin D and hemodialysis survival: A historical cohort study. J Am Soc Nephrol 16 :1115– 1125,2005 [DOI] [PubMed] [Google Scholar]
- 17.Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK: Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 67 :1179– 1187,2005 [DOI] [PubMed] [Google Scholar]
- 18.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70 :771– 780,2006 [DOI] [PubMed] [Google Scholar]
- 19.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R: Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 349 :446– 456,2003 [DOI] [PubMed] [Google Scholar]
- 20.Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, Johnson HK, Zager PG: Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int 70 :1858– 1865,2006 [DOI] [PubMed] [Google Scholar]
- 21.London GM, Guerin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Metivier F: Mineral metabolism and arterial functions in end-stage renal disease: Potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol 18 :613– 620,2007 [DOI] [PubMed] [Google Scholar]
- 22.Cardus A, Panizo S, Parisi E, Fernandez E, Valdivielso JM: Differential effects of vitamin D analogs on vascular calcification. J Bone Miner Res 22 :860– 866,2007 [DOI] [PubMed] [Google Scholar]
- 23.Mizobuchi M, Finch JL, Martin DR, Slatopolsky E: Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int 72 :709– 715,2007 [DOI] [PubMed] [Google Scholar]
- 24.Wu-Wong JR, Nakane M, Ma J, Ruan X, Kroeger PE: Effects of Vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis 186 :20– 28,2006 [DOI] [PubMed] [Google Scholar]
- 25.Wu-Wong JR, Nakane M, Ma J, Ruan X, Kroeger PE: VDR-mediated gene expression patterns in resting human coronary artery smooth muscle cells. J Cell Biochem 100 :1395– 1405,2007 [DOI] [PubMed] [Google Scholar]
- 26.Hirata M, Katsumata K, Endo K, Fukushima N, Ohkawa H, Fukagawa M: In subtotally nephrectomized rats 22-oxacalcitriol suppresses parathyroid hormone with less risk of cardiovascular calcification or deterioration of residual renal function than 1,25(OH)2 vitamin D3. Nephrol Dial Transplant 18 :1770– 1776,2003 [DOI] [PubMed] [Google Scholar]
- 27.Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D: Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int 68 :2823– 2828,2005 [DOI] [PubMed] [Google Scholar]
- 28.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J: Vitamin D: A negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol 89–90 :387– 392,2004 [DOI] [PubMed] [Google Scholar]
- 29.Schwarz U, Amann K, Orth SR, Simonaviciene A, Wessels S, Ritz E: Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int 53 :1696– 1705,1998 [DOI] [PubMed] [Google Scholar]
- 30.Kuhlmann A, Haas CS, Gross ML, Reulbach U, Holzinger M, Schwarz U, Ritz E, Amann K: 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol 286 :F526– F533,2004 [DOI] [PubMed] [Google Scholar]
- 31.Aschenbrenner JK, Sollinger HW, Becker BN, Hullett DA: 1,25-(OH(2))D(3) alters the transforming growth factor beta signaling pathway in renal tissue. J Surg Res 100 :171– 175,2001 [DOI] [PubMed] [Google Scholar]
- 32.Subramaniam N, Leong GM, Cock TA, Flanagan JL, Fong C, Eisman JA, Kouzmenko AP: Cross-talk between 1,25-dihydroxyvitamin D3 and transforming growth factor-beta signaling requires binding of VDR and Smad3 proteins to their cognate DNA recognition elements. J Biol Chem 276 :15741– 15746,2001 [DOI] [PubMed] [Google Scholar]
- 33.Kruger S, Kreft B: 1,25-dihydroxyvitamin D3 differentially regulates IL-1alpha-stimulated IL-8 and MCP-1 mRNA expression and chemokine secretion by human primary proximal tubular epithelial cells. Exp Nephrol 9 :223– 228,2001 [DOI] [PubMed] [Google Scholar]
- 34.Zhu KJ, Shen QY, Zheng M, Mrowietz U: Effects of calcitriol and its analogues on interaction of MCP-1 and monocyte derived dendritic cells in vitro. Acta Pharmacol Sin 22 :62– 65,2001 [PubMed] [Google Scholar]
- 35.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH: Cutting edge: 1,25-Dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173 :2909– 2912,2004 [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, Sun L, Wang Y, Ning G, Minto AW, Kong J, Quigg RJ, Li YC: Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int 73 :163– 171,2008 [DOI] [PubMed] [Google Scholar]
- 37.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC: Cardiac hypertrophy in vitamin D receptor knockout mice: Role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab 288 :E125– E132,2005 [DOI] [PubMed] [Google Scholar]
- 38.Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, Rigor DL, Stillman I, Tamez H, Kroeger PE, Wu-Wong RR, Karumanchi SA, Thadhani R, Kang PM: Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci U S A 104 :16810– 16815,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin KJ, Gonzalez EA: Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol 18 :875– 885,2007 [DOI] [PubMed] [Google Scholar]
- 40.Ritz E, Kuster S, Schmidt-Gayk H, Stein G, Scholz C, Kraatz G, Heidland A: Low-dose calcitriol prevents the rise in 1,84-iPTH without affecting serum calcium and phosphate in patients with moderate renal failure (prospective placebo-controlled multicentre trial). Nephrol Dial Transplant 10 :2228– 2234,1995 [DOI] [PubMed] [Google Scholar]
- 41.Brandi L, Nielsen PK, Bro S, Daugaard H, Olgaard K: Long-term effects of intermittent oral alphacalcidol, calcium carbonate and low-calcium dialysis (1.25 mmol L-1) on secondary hyperparathyroidism in patients on continuous ambulatory peritoneal dialysis. J Intern Med 244 :121– 131,1998 [DOI] [PubMed] [Google Scholar]
- 42.Coburn JW, Maung HM, Elangovan L, Germain MJ, Lindberg JS, Sprague SM, Williams ME, Bishop CW: Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis 43 :877– 890,2004 [DOI] [PubMed] [Google Scholar]
- 43.Coyne D, Acharya M, Qiu P, Abboud H, Batlle D, Rosansky S, Fadem S, Levine B, Williams L, Andress DL, Sprague SM: Paricalcitol capsule for the treatment of secondary hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis 47 :263– 276,2006 [DOI] [PubMed] [Google Scholar]
- 44.Martin KJ, Gonzalez EA, Gellens M, Hamm LL, Abboud H, Lindberg J: 19-Nor-1-alpha-25-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol 9 :1427– 1432,1998 [DOI] [PubMed] [Google Scholar]
- 45.Tsukamoto Y, Hanaoka M, Matsuo T, Saruta T, Nomura M, Takahashi Y: Effect of 22-oxacalcitriol on bone histology of hemodialyzed patients with severe secondary hyperparathyroidism. Am J Kidney Dis 35 :458– 464,2000 [DOI] [PubMed] [Google Scholar]
- 46.Akiba T, Marumo F, Owada A, Kurihara S, Inoue A, Chida Y, Ando R, Shinoda T, Ishida Y, Ohashi Y: Controlled trial of falecalcitriol versus alfacalcidol in suppression of parathyroid hormone in hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis 32 :238– 246,1998 [DOI] [PubMed] [Google Scholar]
- 47.Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D: Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int 63 :1483– 1490,2003 [DOI] [PubMed] [Google Scholar]
- 48.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA Jr, Tonelli M, Thadhani R: Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72 :1004– 1013,2007 [DOI] [PubMed] [Google Scholar]