Abstract
Despite the huge amount of studies looking for candidate genes, the ACE gene remains the unique, well-characterized locus clearly associated with pathogenesis and progression of chronic kidney disease, and with response to treatment with drugs that directly interfere with the renin angiotensin system (RAS), such as angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor antagonists (ARA). The II genotype is protective against development and progression of type I and type II nephropathy and is associated with a slower progression of nondiabetic proteinuric kidney disease. ACE inhibitors are particularly effective at the stage of normoalbuminuria or microalbuminuria in both type I and type II diabetics with the II genotype, whereas the DD genotype is associated with a better response to ARA therapy in overt nephropathy of type II diabetes and to ACE inhibitors in male patients with nondiabetic proteinuric nephropathies. The role of other RAS or non-RAS polymorphisms and their possible interactions with different ACE I/D genotypes are less clearly defined. Thus, evaluating the ACE I/D polymorphism is a reliable tool to identify patients at risk and those who may benefit the most of renoprotective therapy with ACE inhibitors or ARA. This may guide pharmacologic therapy in individual patients and help design clinical trials in progressive nephropathies. Moreover, it might help optimize prevention and intervention strategies at population levels, in particular, in countries where resources are extremely limited and 1 million patients continue to die every year of cardiovascular or renal disease.
More than one hundred years have elapsed since 1898, when Tigerstedt and Bergman at Karolinska Institute observed that injection of a crude extract of rabbit kidney raised the blood pressure (BP) in dogs (1). The extract contained a substance with long-lasting pressure effects that they named renin. Curiously, however, this seminal observation remained almost unnoticed until 1934, when Goldblatt showed that clamping of a kidney artery induced hypertension in the dog, an effect associated with the release of a vasopressor substance in the ipsilateral renal vein (2). Demonstrating an enhanced release of pressure substances from damaged kidneys into the circulation provided a reasonable pathophysiologic explanation for the well-known association between kidney disease and arterial hypertension and revived the seminal intuition of Claude Bernard that an endocrine mechanism, the milieu interior, is involved in the regulation of the systemic BP. A few years later, the concept of a system specifically involved in BP control came to the light with discovery by Page and Braun-Menendez that renin is a peptidase that produces the vasoconstrictor peptide angiotensin II from the precursor angiotensinogen (3). Since then, multidisciplinary research involving clinical scientists, pathologists, biochemists, physiologists, molecular biologists, and, more recently, geneticists, allowed identifying and characterizing many other components of the renin-angiotensin system (RAS), including the angiotensin-converting-enzyme (ACE), and specific receptors for the main RAS effectors, such as angiotensin II and aldosterone (4–10). Even more important, it soon became evident that RAS, in addition to controlling the systemic BP, may also have many other effects. Navar and Rosivall showed that angiotensin II enhances the tone of the postglomerular arteriole (11), which induces glomerular hypertension and increases the filtration fraction (12), hemodynamic adaptations that in the long-term may cause glomerular damage and dysfunction (13). Angiotensin II may also induce mesangial and vascular cell hypertrophy (14) and sustain chronic inflammation, ischemia, and fibrosis in proteinuric kidneys (15).
The awareness that chronic RAS activation could have a key role in widely diffused, highly invalidating and often fatal diseases, boosted research efforts aimed to better characterize the system and to guide the development of intervention strategies aimed to limit its effects on vessels and tissues. Along this line, a major contribution came from novel molecular biologic techniques that, since the early 1980s, allowed to clone the genes encoding for various components of the system, such as renin, ACE and angiotensinogen (AGT), as well as for angiotensin II receptors type 1 (AT1R) and type 2 (AT2R) DNA cloning allowed also to detect gene polymorphisms of several components of the RAS, which helped geneticists to better understand the complex relationships between RAS and disease. In 1990, Rigat et al. (16) first described the insertion (I)/ deletion (D) polymorphism of the ACE gene, a major locus that accounts for approximately 50% of the total phenotypic variance of circulating and tissue ACE. Subjects carrying the D allele were found to have increased systemic and renal ACE levels, whereas those with the II genotype had the lowest ACE expression (17). Evidence that ACE is a limiting factor for angiotensin II synthesis, and therefore for most of the systemic and renal effects of the RAS, pushed researchers to focus a large part of genetic analyses of the RAS on the study of ACE I/D polymorphism. Thus, over the last decade, several studies converged to indicate that risk for development and progression of diabetic (18) and nondiabetic (19–21) chronic renal disease, as well as of related cardiovascular complications (22), varies with different I/D genotypes.
With the development of captopril, the ancestor of a new class of antihypertensive agents, the ACE inhibitors, developed with the specific objective of limiting uncontrolled RAS activation in renovascular disease, Ondetti et al. (23) paved the way to a series of pharmacologic studies that provided new and largely unexpected insights on the concert of RAS effects. Studies found that RAS inhibition may lower the BP in patients with ischemic kidney disease and in a large proportion of those with essential hypertension as well, including patients with low plasma renin activity (24). Moreover, with time, the area of application of RAS inhibitor therapy extended from arterial hypertension to left ventricular hypertrophy and congestive heart failure, prevention of stroke, atherosclerosis, atrial fibrillation, and prevention and treatment of chronic kidney disease. Evidence that RAS inhibitors can be effective even in patients without signs of activity of the system is a major challenge to explore the possibility that the pathophysiology of cardiovascular and renal disease(s) are somehow linked to the function of renin angiotensin axis. Better understanding of the complex interactions of the RAS with hemodynamic and metabolic abnormalities of patients with hypertension, diabetes or renal disease, as well as with genetic variants of other pathways possibly involved in target organ damage, will hopefully open novel perspective of therapy for these high-risk patients. Evaluating these interactions, however, is difficult: large and well-characterized patient populations with homogeneous genetic backgrounds and univocally defined and clinically relevant outcomes are needed to give enough power to the analyses and limit the risk of random data fluctuations. Moreover, studies must take into consideration the role of acquired and environmental factors that may vary from one experimental setting to the other and may confound data interpretation. Unfortunately, most of the studies performed so far failed to satisfy the above requirements, largely because of the too small sample size or the heterogeneity of studied populations; and this may explain why data on the role of the RAS, and in particular of the ACE gene, in human disease were often inconclusive and sometimes even conflicting between different studies.
Thus, our major task was to provide a critical overview of available evidence, trying to focus on most robust findings and, where possible, clean available information from unreliable or misleading data. We also discussed less solid data on other genetic factors that, combined with different ACE I/D genotypes, may contribute to the large interindividual variability in disease susceptibility and treatment response. Patients with diabetic and nondiabetic renal disease were treated separately to assess whether the same genetic factors may play a similar or different role in these two clinical settings. On the contrary, dissecting data on type I from those on type II diabetics was often difficult because many studies considered these patients together and only occasionally provided aggregate data in the two populations analyzed separately. We focused on papers considering microalbuminuria or macroalbuminuria, proteinuria, kidney function, and end-stage kidney disease (ESKD) as outcome variables. Relevant studies were retrieved by a literature search performed in MEDLINE (from 1996 to most recent) and EMBASE (from 1980 to most recent) by using specific key words according to considered genetic (RAS polymorphisms), disease (diabetic nephropathy, nondiabetic nephropathy) and drug categories (ACE inhibitors, angiotensin II receptor antagonism [ARA]). Randomized clinical trials were identified and selected for the meta-analysis according to the search strategy developed for the Cochrane Collaboration. Data on renal transplant patients were not considered.
Onset and Progression
Diabetic Renal Disease
A meta-analysis of 8663 type I and type II diabetics with incipient or overt nephropathy (defined, respectively, by the presence of microalbuminuria or macroalbuminuria/proteinuria, with or without renal insufficiency) and 6064 diabetic controls with no evidence of renal disease (defined as a urinary albumin excretion below the threshold for microalbuminuria) included in 47 studies published from 1994 to 2004, showed that those with the II ACE polymorphism had a 22% lower risk for nephropathy than homozygous or heterozygous carriers of the D allele (odds ratio [OR] = 0.78; 95% confidence interval [CI] = 0.69 to 0.88) (18). This analysis, however, considered together data in type II Asians and in type I and type II white. When these subgroups were considered separately, the II genotype was associated with a remarkably lower (35%) risk in type II Asians (OR = 0.65; 95% CI = 0.51 to 0.83) but had only a marginal impact on disease onset in type I (OR = 0.83; 95% CI = 0.65 to 1.07) and type II (OR = 0.90; 95% CI = 0.78 to 1.04) whites. A closer look at the data showed that risk for microalbuminuria was only marginally associated with the ACE I/D polymorphism in each subgroup, whereas risk for macroalbuminuria was lower in patients with the II genotype, in particular in type II Asians. A possible explanation is that, unlike macroalbuminuria, microalbuminuria does not necessarily reflect structural kidney damage and may be associated with insulin resistance, endothelial dysfunction, obesity, heart failure, and other clinical conditions that may not be affected by ACE I/D polymorphism or race, and may therefore confound data interpretation by masking (i.e., “diluting”) the association, if any, between II genotype and lower risk for incipient nephropathy.
The association between ACE polymorphism and nephropathy in type II Asian diabetics was subsequently confirmed by an observational study showing that, among 1281 Chinese diabetics, those with the II compared with those with the DD genotype had a threefold lower risk of progression to a renal endpoint (defined as need for chronic dialysis, doubling of baseline serum creatinine, or creatinine increase >500 μmol/L). Of interest, the risk of events was significantly associated with the ACE I/D polymorphism even after adjustments for potential confounders, such as gender, age, and duration of diabetes, but not when serum ACE levels were considered in the model, a finding suggesting that the protective effect of the II genotype against nephropathy could be explained, at least in part, by lower ACE activity (25). The protective effect of this genotype has been recently observed also in a case-control study including 1057 French, Danish, and Finnish type I diabetics with persistent albuminuria and 1127 controls without evidence of renal disease (26), an effect that was significant even after adjustment for gender, smoking status, diabetes duration, and glycosylated hemoglobin concentration.
Altogether, available data provide convincing evidence that the ACE I/D polymorphism is significantly associated with overt nephropathy, being the II genotype protective against the disease both in type I and type II diabetics. A possible explanation is that changes in blood glucose may affect renal hemodynamics to a different extent in different genotypes. Indeed, hyperglycemia does not appreciably affect the glomerular filtration rate (GFR) in diabetics with the II genotype and low plasma ACE levels, whereas in those with the ID and DD genotype it induces a GFR increase that correlates with ACE plasma levels (27). A plausible and intriguing explanation is that vasodilatation of the arterioles afferent to the glomeruli is NO dependent and is amplified by local angiotensin II levels in a dose-dependent manner (28). Thus, in patients with the II genotype, reduced angiotensin II biovailability secondary to decreased ACE activity may limit glucose-induced preglomerular vasodilatation. In carriers of the I allele, this might translate into less severe hyperfiltration, in particular in those with less effective metabolic control who, in the long term, would be to some extent protected from development and progression of nephropathy (25). Data on the association between the ACE genotype and microalbuminuria are less consistent and need to be confirmed in more powerful studies.
Nondiabetic Renal Disease
The role of the ACE I/D polymorphism in nondiabetic chronic kidney disease is far from being definitely established. Possible reasons include the sparseness and heterogeneity of available studies and, conceivably, the different impact the same genetic factors may have in different underlying renal diseases. Most studies evaluated the relationships between ACE I/D and adult polycystic kidney disease (APKD) or IgA nephropathy. These studies were recently reviewed in two comprehensive meta-analyses showing that this polymorphism is not associated with the progression of APKD (20) but may affect both the incidence and progression of IgA nephropathy, although to a different extent in different considered populations (21). Indeed, comparative analyses between 1339 patients with IgA and 1881 healthy controls found that the DD compared with the II and ID genotype was associated with a more than double incidence of IgA nephropathy in Asians, but with a similar risk of disease in whites. On the contrary, comparative analyses between 2706 progressors and 1578 nonprogressors found that the ACE I/D polymorphism remarkably affected the risk of ESKD in both populations, to the extent that Asian and white carriers of the DD genotype had, respectively, a 78% and 90% excess incidence of ESKD compared with carriers of the II or ID genotypes considered together (21).
A wide number of relatively small studies evaluated the influence of ACE I/D polymorphism on the risk of developing idiopathic nephrotic syndrome and eventually progressing to ESKD, but results were largely inconclusive and often contrasting (29–33). This conceivably reflected the limited power of the studies, the heterogeneity of considered populations and the confounding effect of concomitant treatments that, altogether, converged to increase the risk of random fluctuations of final data. One randomized, double-blind, placebo-controlled, clinical trial, the Ramipril Efficacy In Nephropathy (REIN) study (34), was formally designed to test whether glomerular protein traffic and its modification by ACE inhibitor therapy influenced chronic renal disease progression. This study prospectively evaluated the relationships between ACE I/D polymorphism and kidney function loss by serial GFR measurements by gold-standard techniques in patients with different nondiabetic renal diseases (19). Data in 212 genotyped patients showed a similar rate of GFR decline and incidence of ESKD in different ACE I/D subgroups, an effect, however, that was the result of different response to ACE inhibitor and conventional therapy in considered groups. When the analyses were restricted to those on placebo, male patients with the II or ID genotypes were found to have a slower progression to ESKD than those with the DD genotype (35). Similar outcomes were described by a smaller study in 70 patients with moderately advanced renal insufficiency showing a slower progression in II or ID than in DD males (36), a trend that was observed also in females when the analyses were restricted to patients with baseline proteinuria <3.5 g/24 h. Along the same line, van Essen et al. found a slower progression in 64 patients with the ID or II genotype compared with 17 DD patients (37). Consistently, a retrospective analysis evaluating the prevalence of different genotypes in 260 ESKD patients and 327 controls found an association between the II and ID genotype and slower progression among patients with nondiabetic kidney disease (38).
Altogether, these data show a slower progression in patients with the II or ID genotype, a trend that appears to be particularly consistent in the male gender.
ACE I/D Polymorphism and Response to RAS Inhibitor Therapy
Diabetic Renal Disease
ACE Inhibitors.
Data on the interactions between ACE I/D polymorphism and response to ACE inhibitor therapy are quite contrasting in type I and type II diabetics (Table 1). A large study in type I diabetes evaluated the interaction between ACE I/D polymorphism and ACE inhibitor therapy in 530 patients with normoalbuminuria or microalbuminuria included in the EURODIAB Controlled Trial of Lisinopril in IDDM, a prospective, randomized, clinical trial aimed to compare the effects of 2 yr treatment with lisinopril or placebo on urinary albumin excretion rate (39). At study end, the ACE inhibitor compared with placebo reduced albuminuria by 51.3% in those with the II genotype, and by 14.8% or 7.7% in those with the ID or DD genotype, respectively. Finding that in the II group the difference was statistically significant, even after adjustment for gender and baseline and follow-up BP and metabolic control, provided consistent evidence that the I allele compared with the D allele was associated with a better antiproteinuric response to ACE inhibitor therapy.
Table 1.
Main studies evaluating the relationships between ACE I/D genotypes and response to ACE inhibitor therapy in diabetic renal disease
| Penno et al. (39), 1998 | Jacobsen et al. (40), 2003 | Parving et al. (41), 1996 | Jacobsen et al. (42), 1998 | So et al. (44), 2006 | Ha et al. (45), 2000 | |
|---|---|---|---|---|---|---|
| Ethnicity | White | White | White | White | Asian | Asian |
| Type of diabetes | I | I | I | I | II | II |
| No. of patients | 530 | 169 | 35 | 60 | 2089 | 83 |
| Follow-up (yr) | 2 | 6 | 7 | 0.5 | 3.7 | 0.25 |
| Patient characteristics | Normotension, normoalbuminuria (75%), or microalbuminuria (15%) | Persistent microalbuminuria | Macroalbuminuria | Overt nephropathy | Normoalbuminuria (58.6%), microalbuminuria (21.2%), or macroalbuminuria (20.2%) | Overt nephropathy |
| Baseline characteristics of genotype groups | Comparable | Comparable | Comparable (no data on gender distribution) | Comparable | Comparable | Unbalanced albuminuria |
| Treatment in genotype groups | Comparable | Comparable | Comparable | Comparable | Comparable | Comparable |
| Adjustments for relevant covariates | Yes | Yes | Yes | Yes | Yes | Partial |
| Effect of ACE inhibitor therapy | Albuminuria reduction versus placebo: II, 51.3%; ID, 14.8%; DD, 7.7% | Rate ratio of doubling serum creatinine or ESKD for each D allele: 1.81 (95% CI, 1.09-3.03) | More albuminuria reduction and slower GFR decline in II than ID + DD | More albuminuria reduction in II than ID or DD | Hazard ratio for the renal composite endpoint: DD, 0.95; ID, 0.43; II, 0.52 | Albuminuria reduction: DD, 52.6%; ID, 19.2%; II, 24.8% |
Other studies in type I diabetes included patients with incipient or overt nephropathy. All patients were on ACE inhibitor therapy; thus, treatment effect could not be assessed versus placebo. However, comparative analyses between different genotypes consistently showed in the three studies that best outcomes were associated with the I allele. Among the 169 patients with overt nephropathy studied by Jacobsen et al. (40), the I allele was associated with a slower progression to doubling of serum creatinine or ESKD. This confirmed and extended previous evidence from two other albeit smaller studies. Parving et al. (41) showed that GFR decline over time was significantly slower in 24 patients who were heterozygous or homozygous for the I allele than in 11 patients homozygous for the D allele. Jacobsen et al. (42) showed more albuminuria reduction with captopril therapy in II than in ID or DD carriers. In all of the above studies, superior outcomes in the II groups were significant, even after adjustment for baseline characteristic and BP and metabolic control on follow-up, which can be taken to suggest that the I allele increases the responsiveness to the renoprotective effects of ACE inhibition therapy. A possible explanation is that ACE inhibitors reduce glomerular capillary hydraulic pressure more effectively in patients with the II than in those with the DD genotype (43). In II carriers, amelioration of glomerular hypertension might amplify the long-term protective effect of ACE inhibitor therapy against development and progression of nephropathy, in particular in those at increased risk because of poor metabolic control. Whether and to which extent the hemodynamic response to ACE inhibitor therapy depends on ACE plasma levels and/or ACE kidney activity, however, is still unknown. Regardless of the above, evidence that whites with the II genotype are protected from glucose-induced glomerular hyperfiltration and, at the same time, benefit the most of ACE inhibitor therapy, may explain why these patients compared with carriers of the D allele are at remarkably lower risk of nephropathy. No data on the interactions between ACE I/D polymorphism and response to ACE inhibitors are available for Asians or other non-white ethnic groups.
Data in type II diabetes are less clear (Table 1). Consistent with data in type I diabetes, the study by So et al. in 2089 Chinese patients with normoalbuminuria, microalbuminuria, or macroalbuminuria (44) found that over a median period of 44.6 mo, ACE inhibitor therapy decreased mortality, ESKD, or progression to estimated GFR <15 ml/min per 1.73 m2 more effectively in II and ID than in DD carriers. This was the only study in type II diabetes with adequate power and follow-up. In apparent contrast with the above study were the results of a remarkably smaller study of 83 type II diabetics with overt nephropathy showing that 3-mo ACE inhibitor therapy decreased proteinuria more effectively in those with the DD than in those with the II or ID genotype (45). However, it should be considered that in the study by So et al. (44) the favorable effects of the I allele were almost entirely restricted to those with normoalbuminuria or microalbuminuria, whereas in those with macroalbuminuria the effects of ACE inhibitor therapy were similar in different genotypes. Altogether, the findings of the above studies would indicate that the I allele may be associated with an increased benefit of ACE inhibitor therapy in early stages of diabetic nephropathy, whereas in more advanced stages treatment appears to confer consistent renoprotection to all patients regardless of their ACE I/D genotype.
In summary, within the limitation of the heterogeneity of the above data that were generated by studies that largely differed for the characteristics of included patients, severity of kidney disease, design and duration of follow-up, available data suggest that in both type I and type II diabetes early intervention with ACE inhibitor therapy may be particularly effective in patients with the I allele. This may have practical implications for healthcare providers in planning population-based screening and prevention strategies. At the stage of overt nephropathy, all patients without specific contraindications should be offered ACE inhibitor therapy regardless of the type of diabetes and the ACE I/D genotype.
Angiotensin II Receptor Antagonists.
Two relatively small studies by Andersen et al. (46,47) found that the antiproteinuric effect of 30- and 4-mo ARA with losartan was substantially independent of the ACE I/D polymorphism in 54 hypertensive whites with type I diabetes and overt nephropathy (Table 2). Along the same line, Haneda et al. (48) found no associations between treatment-induced proteinuria reduction and I/D polymorphism in 126 Asians with type II diabetes and overt nephropathy. More robust data on the interactions between ACE I/D polymorphism and ARA therapy have been provided by prespecified analyses of the Reduction of Endpoints in NIDDM with the AII Antagonist Losartan study, a double blind, multicenter, prospective, randomized, placebo-controlled clinical trial designed to evaluate the renal effects of losartan in 1513 type II diabetic patients with overt nephropathy (49). Data in the 1435 patients with available ACE I/D data (50) showed a risk reduction for ESKD of 3.1%, 30.5%, and 50.4% in the II, ID, and DD groups, respectively (Table 2). Consistently, the risk of progression to the composite endpoint (doubling of baseline serum creatinine, ESKD, or death) was decreased by 17.5% and by 38.1% in those with the II compared with those with the ID or DD genotype. Findings that differences in both outcomes were statistically significant between considered subgroups even after adjustments for main baseline and follow-up covariates provided convincing enough evidence that in overt nephropathy of type II diabetes the D compared with the I allele is associated with better response to angiotensin II blockade. Whether this may apply also to ACE inhibition is unknown because no adequately powered randomized clinical trial evaluated so far the relationships between ACE I/D polymorphism and the protective effect against ESKD of ACE inhibitor therapy. Vis-à-vis comparative studies evaluating response to ACE inhibitor and angiotensin II blocker therapy in homogeneous patient populations are needed to establish which is the treatment of choice (if any) according to the type of diabetes and the stage of renal involvement.
Table 2.
Main studies evaluating the relationships between ACE I/D genotypes and response to ARA therapy in diabetic renal disease
| Andersen et al. (46), 2003 | Andersen et al. (47), 2002 | Parving et al. (50), 2008 | Haneda et al. (48), 2004 | |
|---|---|---|---|---|
| Ethnicity | White | White | White | Asian |
| Type of diabetes | I | I | II | II |
| No. of patients | 54 | 54 | 1435 | 126 |
| Follow-up (yr) | 3 | 0.33 | 3.4 | 0.25 |
| Patient characteristics | Hypertension with persistent albuminuria | Hypertension with persistent albuminuria | Proteinuria | Proteinuria |
| Baseline characteristics of genotype groups | Comparable | Comparable | Comparable (but BMI and race) | Comparable |
| Treatment in genotype groups | Comparable | Comparable | ? | ? |
| Adjustments for relevant covariates | No | No | Yes | ? |
| Effect of ARA therapy | Comparable proteinuria reduction and GFR decline in II and DD | Comparable albuminuria reduction in II (55%) and DD (46%) | Risk reduction for ESKD: DD, 50.1%; ID, 30.5%; II, 3.1% | No interaction between genotype and albuminuria reduction |
?, insufficient information.
Nondiabetic Renal Disease
ACE Inhibitors.
Several studies evaluated the relationships between ACE I/D polymorphism and response to ACE inhibitor therapy, but many of them provided inconclusive or unreliable information because of the too small sample size or short follow-up (51–54). To address this issue, here we considered only those studies that included at least 30 patients who were followed for a minimum of 1 mo (Table 3). The largest study in whites satisfying these criteria considered 212 patients included in the REIN study and followed prospectively with serial GFR measurements for a median of 30 mo. Data showed that, independent of the underlying disease, ACE inhibition with ramipril compared with conventional, non-RAS inhibiting therapy consistently reduced proteinuria, the rate of GFR decline (ΔGFR), and incidence of ESKD in those with the DD genotype. A similar trend in proteinuria and, even if less consistent, in ΔGFR and events was observed also in patients with the ID genotype, whereas all considered outcomes were virtually identical on ramipril or conventional therapy among patients with the II genotype (Table 3). These different trends were not explained by differences in patient characteristics that were similar between different genotypes. Of note, finding that BP control was also similar between groups suggests that different outcomes reflected a specific effect of the ACE I/D polymorphism on intrinsic kidney responsiveness to ACE inhibitor therapy (19). Consistently, a smaller study in patients with IgA nephropathy found more treatment-induced proteinuria reduction in those with the DD than in those with the ID or II genotype (55). Other studies that, compared with the REIN analysis, included remarkably smaller numbers of patients, had shorter follow-up and, in most cases, did not measure the GFR failed to find a correlation between ACE I/D polymorphism and response to ACE inhibitor treatment (56,57) or even showed a better outcome on ACE inhibitor therapy in carriers of the I allele (37,58). In addition to the limited statistical power of the above studies, a possible interpretation for these findings is that in those with the DD genotype the response to ACE inhibitors was blunted by an excessive dietary intake of sodium (57). Indeed, evidence is available that response to ACE inhibitor therapy is more dependent on concomitant sodium intake in patients with the DD than in those with the ID or II genotype. A stronger gene-environment interaction in those with the DD genotype was originally described by van der Kleij et al. in healthy subjects receiving angiotensin I infusion in combination with high or low sodium intake (59). Of interest, the response to angiotensin II was increased in patients with the DD compared with those with the ID or II genotype during high sodium intake, but no difference between the three ACE I/D genotypes was detectable when subjects were given a low sodium diet. Thus, a confounding effect of unrestricted sodium intake may explain why some studies failed to detect a superior renoprotective effect of ACE inhibitor therapy in DD than in ID or II patients with chronic renal disease (57). A possible confounding effect of gender must also be taken into consideration because, as demonstrated in the REIN study, the DD genotype remarkably increases the response to ACE inhibitors in males, whereas the role of ACE I/D polymorphism looks less consistent in females, that uniformly benefit of ACE inhibitor therapy regardless of their genotype (35).
Table 3.
Main studies evaluating the relationships between ACE I/D genotypes and response to ACE inhibitor therapy in nondiabetic renal disease
| Ruggenenti et al. (35), 2000a | Perna et al. (19), 2000a | Essen et al. (37), 1996 | van der Kleij et al. (56), 1997 | van der Kleij et al. (57), 1997 | Haas et al. (58), 1998 | Yoshida et al. (55), 1995 | Moriyama et al. (54), 1995 | |
|---|---|---|---|---|---|---|---|---|
| Ethnicity | White | White | White | White | White | White | Asian | Asian |
| No. of patients | 352 | 212 | 81 | 61 | 88 | 36 | 53 | 36 |
| Follow-up (yr) | 6 | 4.7 | 3 | 0.2 | 0.2 | 0.5 | 1 | 0.25 |
| Patient characteristics | Persistent proteinuria | Persistent proteinuria | Proteinuria | Stable proteinuria | Stable proteinuria | Protenuric glomerular disease | IgA nephropathy | Proteinuria |
| Baseline characteristics of genotype groups | Comparable | Comparable | Different GFR | Comparable | Comparable | ? | Comparable | ? |
| Treatment in genotype groups | Comparable | Comparable | Comparable | Comparable | Comparable | ? | ? | ? |
| Adjustments for relevant covariates | Yes | Yes | ? | ? | ? | ? | Yes | ? |
| Effect of ACE inhibitor therapy | Slower ΔGFR and reduced ESKD in women and in DD men | Slower ΔGFR and reduced ESKD in DD, non significant effect in ID and II | Proteinuria reduction and slower ΔGFR in ID and II | Similar effects on proteinuria and GFR | Similar proteinuria reductionb | Proteinuria reduction in ID and II | Proteinuria reduction in DD | More reduced proteinuria in ID + II versus DD |
?, insufficient information.
Data retrieved from the Ramipril Efficacy in Nephropathy (REIN) trial.
Less albuminuria reduction in DD with high sodium excretion.
Angiotensin Receptor Antagonists.
Data on ARA treatment are limited to few and small studies (60–62), which does not allow drawing any conclusion on the impact of the ACE I/D polymorphism on response to angiotensin blockade (Table 4).
Table 4.
Main studies evaluating the relationships between ACE I/D genotypes and response to ARA therapy in non diabetic renal disease
| Redon et al. (61), 2005 | Coto et al. (60), 2005a | Park et al. (62), 2006 | |
|---|---|---|---|
| Ethnicity | White | White | Asian |
| No. of patients | 206 | 37 | 99 |
| Follow-up (yr) | 1 | 0.3 | 055 |
| Patient characteristics | Essential hypertension, microalbuminuria | Chronic nephropathy | Overt proteinuria |
| Baseline characteristics of genotype groups | No data on age and gender | ? | Comparable |
| Treatment in genotype groups | ? | ? | ? |
| Adjustments for relevant covariates | ? | ? | ? |
| Effect of ACE inhibitor therapy | Comparable UAE reduction in the 3 genotypes | Comparable UAE reduction in the 3 genotypes | Comparable proteinuria reduction in the 3 genotypes |
?, insufficient information.
Data retrieved from the English abstract.
Altogether, and with the above limitations, available data can be taken to suggest that in diabetic and nondiabetic proteinuric renal disease the D allele is associated with an increased susceptibility to the protective effect of RAS inhibitor therapy against progression to ESKD. To formally test this hypothesis, we performed the meta-analysis described below.
Meta-analysis of Randomized Clinical Trials of RAS Inhibitor Therapy in Diabetic and Nondiabetic Proteinuric Renal Disease
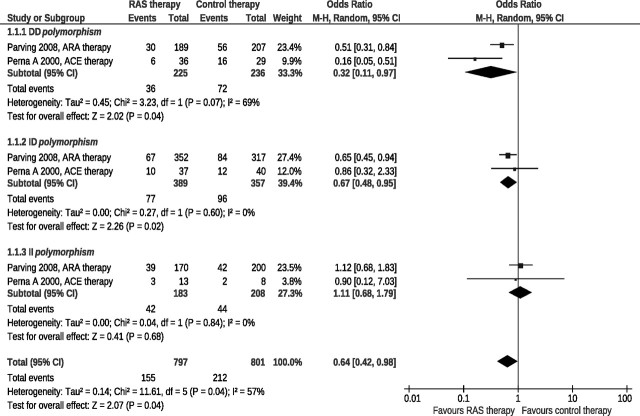
Post hoc analyses of two large prospective, randomized, double blind, placebo-controlled clinical trials of RAS inhibitor therapy in diabetic (50) and nondiabetic (19) proteinuric renal disease reported data on progression to ESKD in DD, ID, or II genotypes considered separately. Both studies satisfied the Cochrane Collaboration criteria for the identification of randomized clinical trials and were considered in the present meta-analysis. ESKD was the primary outcome variable. Results (Figure 1) were expressed as ORs with 95% CIs. Sources of heterogeneity were quantified by I2 statistics.
Figure 1.
Effects of RAS inhibitor therapy compared with placebo according to the DD, ID, or II genotype. A meta-analysis of two prospective, randomized, double-blind, placebo-controlled trials in patients with diabetic or nondiabetic proteinuric nephropathies.
In the study group as a whole, the experimental treatment compared with placebo decreased the risk of progression to ESKD by 36% and the odds ratio was statistically significant (Figure 1). The effect of treatment, however, differed in different ACE I/D subgroups. In patients with the DD and ID genotype, RAS inhibition compared with placebo decreased the risk of ESKD by 68% and 23%, respectively, and the OR was statistically significant in both groups (Figure 1). On the contrary, no treatment effect was observed in the II subgroup. Of note, within each subgroup, patients with diabetic and nondiabetic proteinuric nephropathy showed a similar response to RAS inhibition, with a nonsignificant trend to more risk reduction in those with nondiabetic nephropathy within the DD subgroup.
A strength of this meta-analysis is that both considered studies were randomized, double-blinded and placebo-controlled. A major limitation is that the RAS was inhibited by an ARA in diabetic and by an ACE inhibitor in the nondiabetic population. Thus, it is impossible to establish whether the marginal differences in treatment effect within the DD genotype group are explained by the different underlying pathology (diabetic versus nondiabetic renal disease) or, alternatively, by the different treatment used to block the RAS. Regardless of the above, data consistently show that in both diabetic and nondiabetic proteinuric nephropathy the D allele is associated with a superior protective effect of RAS inhibitor therapy against progression to ESKD. These findings may provide the background for prospective trials formally evaluating the effect of RAS inhibition therapy within patient populations a priori identified on the basis of their genotype. This approach could help address whether RAS inhibition is really poorly effective in patients with the II genotype and whether alternative treatments must be specifically investigated in this population.
Data from randomized trials considering microalbuminuria or macroalbuminuria as outcomes were not sufficient to definitely establish the interactions between ACE I/D polymorphism and RAS inhibition therapy.
Other RAS Polymorphisms and Their Interactions with the ACE I/D Polymorphism
Following the initial description of the I/D polymorphism of the ACE encoding gene, several gene polymorphisms for other components of the RAS, such as angiotensinogen (AGT) and the angiotensin II type I receptor (AT1R), have been reported, and over most recent years a progressively increasing number of studies tried to evaluate their biologic effects and their possible interactions with different ACE genotypes.
Angiotensinogen.
More than 30 AGT genetic variants have been described so far (63,64) and one of them, a T to C base substitution at position 702 on exon 2 with consequent replacement of methionine 235 with threonine (M235T), has been associated with arterial hypertension (63), and with progression of diabetic nephropathy (65,66). Actually, the possibility that different AGT genotypes might result in different AGT bioavailability raised the intriguing hypothesis that RAS activity might be affected not only by ACE I/D genotypes but also by the several variants of the AGT gene. This boosted several studies that, however, failed to demonstrate any consistent relationship of considered AGT polymorphisms with RAS activity and cardiovascular or renal disease. Indeed, after the initial observation that the AGT 235T may be associated with higher AGT levels than the AGT 235M allele (63), subsequent studies showed that the AGT M235T polymorphism may account for no more than 5% of AGT variability (67). Moreover, in the only study showing a relationship between the T allele and increased angiotensin II plasma levels, the association could be detected only in male patients (68). This may explain why studies following the original report by Rogus et al. (66) failed to detect any significant association between AGT variants and diabetic (69–71) or nondiabetic (72,73) chronic kidney disease. Altogether, these data appear to challenge the hypothesis that AGT polymorphism may appreciably affect RAS activity and effects. Another explanation for the above inconsistent findings is that a more complex model than a single functional diallelic variant may explain AGT expression, which could be affected by different and still poorly understood combinations of several AGT gene variants.
Angiotensin II Type 1 Receptor.
In 1994, Bonnardeaux et al. (74), analyzing the entire coding and 3′ untranslated regions of the AT1R encoding gene, identified five silent polymorphisms: T573C, A1062G, A1166C, G1517T, and A1878G. Whites with arterial hypertension (75,76) or type II diabetes (77) were subsequently found to carry the 1166C allele more frequently than healthy controls. The 1166C allele was also associated with an increased risk of microalbuminuria (70) and a faster progression of nephropathy (78) in patients with type II diabetes, findings that, however, were in contrast with data from other studies showing no association between the A1166C polymorphism and diabetic renal disease (79).
A case-control study in ESKD patients found an association between the A1166C and the progression of non-diabetic kidney disease (72). However, studies focusing on specific renal diseases, such as focal segmental glomerulosclerosis, IgA nephropathy, and APKD (33,73,80–83) failed to detect any association between this polymorphism and progression.
Interactions with ACE I/D Polymorphism.
Marrè et al. found that in a large cohort of type I diabetics with proliferative retinopathy and different renal involvement (ranging from no evidence of renal disease to microalbuminuria, macroalbuminuria, or renal insufficiency), the severity of renal disease was associated with the D allele of the ACE I/D polymorphism and, among patients with the ID or DD genotypes, renal involvement increased from AGT MM to TT genotypes (84). They concluded that both polymorphisms are risk factors for the progression of glomerular disease in uncontrolled type 1 diabetic patients. Jacobsen et al. also found that the number of M(M235T)/D(I/D)/A(A1166C) alleles influenced albuminuria and time to doubling of serum creatinine or ESKD in 169 patients with type I diabetes (40). Along the same line, Bantis et al., described a synergistic association of AGT M235T and ACE I/D polymorphisms also with the progression of IgA nephropathy to ESKD (85) and found that those patients who carried more than two ACE-D or AGT-T alleles (that is the DD/TT, DD/MT, or ID/TT genotypes) were the ones with the largest benefit from ACE inhibitor therapy. In contrast with the above findings, however, Osawa et al. (86) failed to detect any significant association of AT1R A1166C, ACE I/D, and AGT M235T polymorphisms with diabetic renal disease. Thus, whether and to what extent the interactions between AGT, and AT1R, and ACE polymorphisms may affect the progression of diabetic and nondiabetic chronic renal disease and response to RAS inhibitor therapy is still unclear.
Other Non-RAS Polymorphisms and Their Interactions with the ACE I/D Polymorphism
Endothelial Nitric Oxide Synthase Polymorphism.
Nitric oxide generated through the endothelial nitric oxide synthase (eNOS) modulates vascular tone by contrasting the effects of several vasoconstrictors, inhibits smooth muscle cell proliferation, and prevents endothelial platelet adhesion, and its inhibition has been associated with increased BP and accelerated progression of kidney disease (87). After Wang et al. (88) described a 27-bp long repeat in the fourth intron of eNOS, the 4a/4b polymorphism, associated with the risk to develop coronary vascular disease in smokers, a progressively increasing number of studies tried to assess the biologic effects of this polymorphism and its possible interactions with the RAS. Again, results of genetic studies were contrasting, with some authors showing an association with the progression of IgA nephropathy (89) that was not confirmed by others (90). Of note, however, in a combined gene analysis of eNOS 4a/4b, ACE I/D, AGT M235T, and AT1R A166C polymorphisms in a large cohort of patients with idiopathic membranous nephropathy, Stratta et al. found that the combination of aa+ba of eNOS 4a/4b genotypes with II+ID ACE I/D or TT+MT AGT M235T genotypes was associated with accelerated renal disease progression, whereas the same eNOS polymorphism had no predictive role when considered alone (91). This finding suggests that in some circumstances a single gene variant may result in appreciable phenotypic changes only upon combination with other polymorphisms that may result in additional or synergistic effects on the same metabolic pathways.
Alpha-adducin.
Studies in Milan hypertensive rats found that hypertension develops in this rat strain because of a primary increase in kidney sodium reabsorption because of a functional point mutation in the gene coding for α-adducin (ADD1) (92) (93) and a point mutation in the ADD1 coding region (G460W) was subsequently associated with hypertension also in humans (94). Pedrinelli et al. (95) found that risk for microalbuminuria was increased in patients with essential hypertension who were GG homozygous for the G460W polymorphism of ADD1. The association between hypertension and albuminuria, however, was significant only in DD carriers of the ACE I/D polymorphism. Consistently, Wang et al. (96) observed that, in 1454 subjects randomly selected from a white population, those with the combined presence of the W and D alleles of the ADD1 G460W and ACE I/D polymorphisms, respectively, were at increased risk of renal disease. The WW genotype was also associated with faster progression in Japanese patients with IgA nephropathy when combined with the II ACE genotype (97).
Thus, the above preliminary findings provide some evidence that polymorphisms of components of non-RAS pathways, in combination with different ACE I/D genotypes, may affect the risk of development and progression of chronic kidney disease.
Strengths and Limitations
The present review was focused on the relationships between ACE gene polymorphism, development and progression of chronic renal disease, and response to RAS inhibitor therapy. Data on possible interactions between the ACE I/D polymorphism and polymorphisms of genes encoding for other components of the RAS, such as angiotensinogen and angiotensin II type 1 receptor, were less extensively discussed. Actually, the review was not intended to consider the entire polymorphic spectra of RAS genes, such as tagging SNPs and/or haplotypes, also because of the limited information about their specific role in disease progression and response to treatment. The review considered only published studies that included at least 30 patients followed for at least 3 mo to limit the risk of confounding data retrieved from studies that were too small or short to reliably assess possible relationships between considered polymorphisms and disease outcome. The meta-analysis considered only prospective, randomized, double blind, placebo-controlled clinical trials that had a similar design and included patients with homogeneous clinical conditions. This limited the risk of bias associated with study heterogeneity. However, only two trials satisfied these characteristics, which limits the generalizability of the findings. Moreover, no trial so far stratified patients according to the DD, ID, or II genotype before randomization to RAS inhibitor therapy YES or NO. Availability of trials with this design would certainly increase the power of analyses aimed at evaluating the relationships between response to RAS inhibition and ACE I/D polymorphism. The exclusion of unpublished studies cannot definitely rule out the possibility of a selection bias, but this very unlikely affected the results of the analysis to any appreciable extent. The analyses of individual data were beyond the purposes of the present work.
Conclusion
Despite the huge amount of studies looking for candidate genes, the ACE gene remains the unique, well-characterized locus clearly associated with pathogenesis and progression of chronic kidney disease. Thus, evaluating the ACE I/D polymorphism is by no means a reliable and cost-effective tool to identify patients at risk and those who may benefit the most of renoprotective therapy with ACE inhibitors or angiotensin II antagonists and, possibly, with other inhibitors of the RAS, such as renin and aldosterone antagonists. In addition to guiding pharmacologic therapy in individual patients, ACE I/D genotyping might help design clinical trials in progressive nephropathies and optimize prevention and intervention strategies at population levels, in particular in countries were resources are extremely limited and 1 million patients continue to die every year of cardiovascular or renal disease (98). Focusing on patients who, on the basis of their ACE I/D genotype, are expected to have a higher rate of events and/or to have events more effectively prevented by a given intervention, would increase the power of analyses aimed to test the advantages of novel, experimental treatments compared with conventional therapy. This would allow detecting a given treatment effect with a reduced number of patients or, alternatively, a smaller effect with the same number of patients. The possibility to limit the size of clinical studies will certainly have major practical implications because, with the improvements in standard of care, the incremental benefits of novel versus preexisting treatments are progressively decreasing and demonstrating these benefits in randomized clinical trials requires a number of patients that is exponentially increasing over time. This already precludes the possibility to realize these trials to most academic institutions and, should this trend continue over time, costs for these trials will soon become unaffordable even for the most powerful and reach pharmaceutical companies. Thus, ACE I/D genotyping, in addition to improving the feasibility of renal and cardiovascular trials, will hopefully offer the academy the chance to regain its central role in an important area of medical research. This approach would also increase the cost-effectiveness of prevention and intervention strategies focusing on subjects a priori predicted to benefit from ACE inhibitors, angiotensin blockers, aldosterone antagonists, or other inhibitors of the RAS that will soon become available for clinical use such as renin antagonists or inhibitors of aldosterone synthesis. On the other hand, the identification of a substantial proportion of subjects that might not benefit from drugs that interfere with the RAS is expected to boost research for novel treatments targeted at other metabolic pathways that may play a role in the pathogenesis and progression of renal and cardiovascular disease. In this perspective, screening for the ACE I/D polymorphism may be an efficient approach to optimize intervention in subjects at increased risk because of diabetes, hypertension, or familial predisposition. In those with proteinuric CKD, genotype-guided ACE inhibitor therapy, in addition to extend freedom from ESKD, may save direct costs for renal replacement therapy (99). Whether ACE I/D genotyping may prove cost-effective also at population level is worth investigating, at least in some specific settings. In this regard, it is worth mentioning that, in parallel with the progressively expiring of patents for ACE inhibitors, costs for ACE inhibitor therapy are expected to remarkably decrease in a few years, which should allow implementation of their intensified use in preventing programs, in particular in less favorable settings (100). In this perspective, the possibility that population-based ACE I/D genotyping to guide ACE inhibitor therapy might increase the efficiency of screening and prevention programs implemented in developing countries, such as the ones by the Commission for the Global Advancement of Nephrology of the International Society of Nephrology (98) might warrant some consideration. Hopefully, more complex pharmacogenomic approaches will help to identify even more effective preventive and therapeutic strategies for renal patients in the future.
Disclosures
None
Acknowledgments
Supported in part by a grant from EU (GENECURE, contract no. 037697 of FP6). The authors thank Dr. Marina Noris for helpful critical appraisal of the manuscript, Ms. Manuela Passera for manuscript preparation, and Ms. Valeria Miglioli for literature retrieval.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Tigerstedt R, Bergman P: Niere und Kreislauf. Skan Arch Physiol 8 :223– 271,1898 [Google Scholar]
- 2.Goldblatt H: Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med 59 :347 ,1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun-Menendez E, Page JH: Suggested revision of nomenclature–angiotensin. Science 127 :242 ,1958 [DOI] [PubMed] [Google Scholar]
- 4.Skeggs LT Jr, Marsh WH, Kahn JR, Shumway NP: The purification of hypertensin I. J Exp Med 100 :363– 370,1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skeggs LT Jr, Marsh WH, Kahn JR, Shumway NP: The existence of two forms of hypertensin. J Exp Med 99 :275– 282,1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skeggs LT Jr, Kahn JR, Shumway NP: The preparation and function of the hypertensin-converting enzyme. J Exp Med 103 :295– 299,1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skeggs LT Jr, Lentz KE, Kahn JR, Shumway NP, Woods KR: The amino acid sequence of hypertensin. II. J Exp Med 104 :193– 197,1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott DF, Peart WS: Amino-acid sequence in a hypertensin. Nature 177 :527– 528,1956 [DOI] [PubMed] [Google Scholar]
- 9.Skeggs LT Jr, Kahn JR, Lentz K, Shumway NP: The preparation, purification, and amino acid sequence of a polypeptide renin substrate. J Exp Med 106 :439– 453,1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodfriend TL, Lin SY: Receptors for angiotensin I and II. Circ Res 27 :163– 174,1970 [PubMed] [Google Scholar]
- 11.Navar LG, Rosivall L: Contribution of the renin-angiotensin system to the control of intrarenal hemodynamics. Kidney Int 25 :857– 868,1984 [DOI] [PubMed] [Google Scholar]
- 12.Raij L, Keane WF: Glomerular mesangium: its function and relationship to angiotensin II. Am J Med 79 :24– 30,1985 [DOI] [PubMed] [Google Scholar]
- 13.Anderson S, Brenner BM: The role of intraglomerular pressure in the initiation and progression of renal disease. J Hypertens 4[Suppl] :236– 238,1986 [PubMed] [Google Scholar]
- 14.Anderson PW, Do YS, Hsueh WA: Angiotensin II causes mesangial cell hypertrophy. Hypertension 21 :29– 35,1993 [DOI] [PubMed] [Google Scholar]
- 15.Remuzzi G, Bertani T: Is glomerulosclerosis a consequence of altered glomerular permeability to macromolecules? Kidney Int 38 :384– 394,1990 [DOI] [PubMed] [Google Scholar]
- 16.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F: An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 86 :1343– 1346,1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobsen PK: Preventing end stage renal disease in diabetic patients: genetic aspect (part I). J Renin Angiotensin Aldosterone Syst 6 :1– 14,2005 [DOI] [PubMed] [Google Scholar]
- 18.Ng DP, Tai BC, Koh D, Tan KW, Chia KS: Angiotensin-I converting enzyme insertion/deletion polymorphism and its association with diabetic nephropathy: a meta-analysis of studies reported between 1994 and 2004 and comprising 14,727 subjects. Diabetologia 48 :1008– 1016,2005 [DOI] [PubMed] [Google Scholar]
- 19.Perna A, Ruggenenti P, Testa A, Spoto B, Benini R, Misefari V, Remuzzi G, Zoccali C: ACE genotype and ACE inhibitors induced renoprotection in chronic proteinuric nephropathies1. Kidney Int 57 :274– 281,2000 [DOI] [PubMed] [Google Scholar]
- 20.Pereira TV, Nunes AC, Rudnicki M, Magistroni R, Albertazzi A, Pereira AC, Krieger JE: Influence of ACE I/D gene polymorphism in the progression of renal failure in autosomal dominant polycystic kidney disease: a meta-analysis. Nephrol Dial Transplant 21 :3155– 3163,2006 [DOI] [PubMed] [Google Scholar]
- 21.Yong D, Qing WQ, Hua L, Kan JJ, Xi CJ, Jin QQ, Chao SH: Association of angiotensin I-converting enzyme gene insertion/deletion polymorphism and IgA nephropathy: a meta-analysis. Am J Nephrol 26 :511– 518,2006 [DOI] [PubMed] [Google Scholar]
- 22.Niu T, Chen X, Xu X: Angiotensin converting enzyme gene insertion/deletion polymorphism and cardiovascular disease: therapeutic implications. Drugs 62 :977– 993,2002 [DOI] [PubMed] [Google Scholar]
- 23.Ondetti MA, Rubin B, Cushman DW: Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science 196 :441– 444,1977 [DOI] [PubMed] [Google Scholar]
- 24.Buhler FR, deLeeuw PW, Doyle AE, Fleckenstein A, Fleckenstein-Grun G, Frishman WH, Zanchetti A: Proceedings of a symposium: calcium metabolism and calcium channel blockers for understanding and treating hypertension. Am J Med 77 :1– 23,1984 [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Ng MC, So WY, Tong PC, Ma RC, Chow CC, Cockram CS, Chan JC: Prognostic effect of insertion/deletion polymorphism of the ace gene on renal and cardiovascular clinical outcomes in Chinese patients with type 2 diabetes. Diabetes Care 28 :348– 354,2005 [DOI] [PubMed] [Google Scholar]
- 26.Hadjadj S, Tarnow L, Forsblom C, Kazeem G, Marre M, Groop PH, Parving HH, Cambien F, Tregouet DA, Gut IG, Theva A, Gauguier D, Farrall M, Cox R, Matsuda F, Lathrop M, Hager-Vionnet N: Association between angiotensin-converting enzyme gene polymorphisms and diabetic nephropathy: case-control, haplotype, and family-based study in three European populations. J Am Soc Nephrol 18 :1284– 1291,2007 [DOI] [PubMed] [Google Scholar]
- 27.Marre M, Bouhanick B, Berrut G, Gallois Y, Le Jeune JJ, Chatellier G, Menard J, Alhenc-Gelas F: Renal changes on hyperglycemia and angiotensin-converting enzyme in type 1 diabetes. Hypertension 33 :775– 780,1999 [DOI] [PubMed] [Google Scholar]
- 28.Ito S, Arima S, Ren YL, Juncos LA, Carretero OA: Endothelium-derived relaxing factor/nitric oxide modulates angiotensin II action in the isolated microperfused rabbit afferent but not efferent arteriole. J Clin Invest 91 :2012– 2019,1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasse B, Hailemariam S, Wuthrich RP, Kemper MJ, Neuhaus TJ: Angiotensin converting enzyme gene polymorphisms do not predict the course of idiopathic nephrotic syndrome in Swiss children. Nephrology (Carlton) 11 :538– 541,2006 [DOI] [PubMed] [Google Scholar]
- 30.Tsai IJ, Yang YH, Lin YH, Wu VC, Tsau YK, Hsieh FJ: Angiotensin-converting enzyme gene polymorphism in children with idiopathic nephrotic syndrome. Am J Nephrol 26 :157– 162,2006 [DOI] [PubMed] [Google Scholar]
- 31.Serdaroglu E, Mir S, Berdeli A, Aksu N, Bak M: ACE gene insertion/deletion polymorphism in childhood idiopathic nephrotic syndrome. Pediatr Nephrol 20 :1738– 1743,2005 [DOI] [PubMed] [Google Scholar]
- 32.Oktem F, Sirin A, Bilge I, Emre S, Agachan B, Ispir T: ACE I/D gene polymorphism in primary FSGS and steroid-sensitive nephrotic syndrome. Pediatr Nephrol 19 :384– 389,2004 [DOI] [PubMed] [Google Scholar]
- 33.Luther Y, Bantis C, Ivens K, Fehsel K, Kolb-Bachhofen V, Heering P: Effects of the genetic polymorphisms of the renin-angiotensin system on focal segmental glomerulosclerosis. Kidney Blood Press Res 26 :333– 337,2003 [DOI] [PubMed] [Google Scholar]
- 34.GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349 :1857– 1863,1997 [PubMed] [Google Scholar]
- 35.Ruggenenti P, Perna A, Zoccali C, Gherardi G, Benini R, Testa A, Remuzzi G: Chronic proteinuric nephropathies: II. Outcomes and response to treatment in a prospective cohort of 352 patients: differences between women and men in relation to the ACE gene polymorphism. Gruppo Italiano di Studi Epidemologici in Nefrologia (GISEN). J Am Soc Nephrol 11 :88– 96,2000 [DOI] [PubMed] [Google Scholar]
- 36.Samuelsson O, Attman PO, Larsson R, Mulec H, Rymo L, Weiss L, Ricksten A: Angiotensin I-converting enzyme gene polymorphism in non-diabetic renal disease. Nephrol Dial Transplant 15 :481– 486,2000 [DOI] [PubMed] [Google Scholar]
- 37.van Essen GG, Rensma PL, de Zeeuw D, Sluiter WJ, Scheffer H, Apperloo AJ, de Jong PE: Association between angiotensin-converting-enzyme gene polymorphism and failure of renoprotective therapy. Lancet 347 :94– 95,1996 [DOI] [PubMed] [Google Scholar]
- 38.Lovati E, Richard A, Frey BM, Frey FJ, Ferrari P: Genetic polymorphisms of the renin-angiotensin-aldosterone system in end-stage renal disease. Kidney Int 60 :46– 54,2001 [DOI] [PubMed] [Google Scholar]
- 39.Penno G, Chaturvedi N, Talmud PJ, Cotroneo P, Manto A, Nannipieri M, Luong LA, Fuller JH: Effect of angiotensin-converting enzyme (ACE) gene polymorphism on progression of renal disease and the influence of ACE inhibition in IDDM patients: findings from the EUCLID Randomized Controlled Trial. EURODIAB Controlled Trial of Lisinopril in IDDM. Diabetes 47 :1507– 1511,1998 [DOI] [PubMed] [Google Scholar]
- 40.Jacobsen P, Tarnow L, Carstensen B, Hovind P, Poirier O, Parving HH: Genetic variation in the renin-angiotensin system and progression of diabetic nephropathy. J Am Soc Nephrol 14 :2843– 2850,2003 [DOI] [PubMed] [Google Scholar]
- 41.Parving HH, Jacobsen P, Tarnow L, Rossing P, Lecerf L, Poirier O, Cambien F: Effect of deletion polymorphism of angiotensin converting enzyme gene on progression of diabetic nephropathy during inhibition of angiotensin converting enzyme: observational follow up study. BMJ 313 :591– 594,1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobsen P, Rossing K, Rossing P, Tarnow L, Mallet C, Poirier O, Cambien F, Parving HH: Angiotensin converting enzyme gene polymorphism and ACE inhibition in diabetic nephropathy. Kidney Int 53 :1002– 1006,1998 [DOI] [PubMed] [Google Scholar]
- 43.Weekers L, Bouhanick B, Hadjadj S, Gallois Y, Roussel R, Pean F, Ankotche A, Chatellier G, Alhenc-Gelas F, Lefebvre PJ, Marre M: Modulation of the renal response to ACE inhibition by ACE insertion/deletion polymorphism during hyperglycemia in normotensive, normoalbuminuric type 1 diabetic patients. Diabetes 54 :2961– 2967,2005 [DOI] [PubMed] [Google Scholar]
- 44.So WY, Ma RC, Ozaki R, Tong PC, Ng MC, Ho CS, Lam CW, Chow CC, Chan WB, Kong AP, Chan JC: Angiotensin-converting enzyme (ACE) inhibition in type 2, diabetic patients: interaction with ACE insertion/deletion polymorphism. Kidney Int 69 :1438– 1443,2006 [DOI] [PubMed] [Google Scholar]
- 45.Ha SK, Yong Lee S, Su Park H, Ho Shin J, Jung Kim S, Hun Kim D, Rae Kim K, Yung Lee H, Suk Han D: ACE DD genotype is more susceptible than ACE II and ID genotypes to the antiproteinuric effect of ACE inhibitors in patients with proteinuric non-insulin-dependent diabetes mellitus. Nephrol Dial Transplant 15 :1617– 1623,2000 [DOI] [PubMed] [Google Scholar]
- 46.Andersen S, Tarnow L, Cambien F, Rossing P, Juhl TR, Deinum J, Parving HH: Long-term renoprotective effects of losartan in diabetic nephropathy: interaction with ACE insertion/deletion genotype? Diabetes Care 26 :1501– 1506,2003 [DOI] [PubMed] [Google Scholar]
- 47.Andersen S, Tarnow L, Cambien F, Rossing P, Juhl TR, Deinum J, Parving HH: Renoprotective effects of losartan in diabetic nephropathy: interaction with ACE insertion/deletion genotype? Kidney Int 62 :192– 198,2002 [DOI] [PubMed] [Google Scholar]
- 48.Haneda M, Kikkawa R, Sakai H, Kawamori R: Antiproteinuric effect of candesartan cilexetil in Japanese subjects with type 2 diabetes and nephropathy. Diabetes Res Clin Pract 66 :87– 95,2004 [DOI] [PubMed] [Google Scholar]
- 49.Brenner BM, Cooper ME, de Zeeuw D, Grunfeld JP, Keane WF, Kurokawa K, McGill JB, Mitch WE, Parving HH, Remuzzi G, Ribeiro AB, Schluchter MD, Snavely D, Zhang Z, Simpson R, Ramjit D, Shahinfar S: The losartan renal protection study: rationale, study design and baseline characteristics of RENAAL (Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan). J Renin Angiotensin Aldosterone Syst 1 :328– 335,2000 [DOI] [PubMed] [Google Scholar]
- 50.Parving HH, de Zeeuw D, Cooper ME, Remuzzi G, Liu N, Lunceford J, Shahinfar S, Wong PH, Lyle PA, Rossing P, Brenner BM: ACE gene polymorphism and losartan treatment in type 2 diabetic patients with nephropathy. J Am Soc Nephrol 19 :771– 779,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han SY, Kwon YJ, Jo SK, Shin JH, Cha DR, Cho WY, Pyo HJ, Kim HK: ACE gene polymorphism and renal responsiveness to ACE inhibitors in IgA nephropathy patients. Korean J Intern Med 15 :13– 18,2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kutyrina IM, Tareeva IE, Nosikov VV, Kamyshova ES, Gorashko NM, Chistiakov DA, Okonova EB, Troepol'skaia OV: [Polymorphism studies of angiotensin converting enzyme gene in chronic glomerulonephritis]. Ter Arkh 71 :30– 34,1999 [PubMed] [Google Scholar]
- 53.Liu J, Wang M, Liu Q, Li Y, Peng B: [Influence of angiotensin-converting enzyme gene polymorphism on the anti-proteinuria efficacy of angiotensin-converting enzyme inhibitor in Han nationality of southern Sichuan province in China]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 22 :204– 205,2005 [PubMed] [Google Scholar]
- 54.Moriyama T, Kitamura H, Ochi S, Izumi M, Yokoyama K, Yamauchi A, Ueda N, Kamada T, Imai E: Association of angiotensin I-converting enzyme gene polymorphism with susceptibility to antiproteinuric effect of angiotensin I-converting enzyme inhibitors in patients with proteinuria. J Am Soc Nephrol 6 :1676– 1678,1995 [DOI] [PubMed] [Google Scholar]
- 55.Yoshida H, Mitarai T, Kawamura T, Kitajima T, Miyazaki Y, Nagasawa R, Kawaguchi Y, Kubo H, Ichikawa I, Sakai O: Role of the deletion of polymorphism of the angiotensin converting enzyme gene in the progression and therapeutic responsiveness of IgA nephropathy. J Clin Invest 96 :2162– 2169,1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Kleij FG, Navis GJ, Gansevoort RT, Heeg JE, Scheffer H, de Zeeuw D, de Jong PE: ACE polymorphism does not determine short-term renal response to ACE-inhibition in proteinuric patients. Nephrol Dial Transplant 12[Suppl 2] :42– 46,1997 [PubMed] [Google Scholar]
- 57.van der Kleij FG, Schmidt A, Navis GJ, Haas M, Yilmaz N, de Jong PE, Mayer G, de Zeeuw D: Angiotensin converting enzyme insertion/deletion polymorphism and short-term renal response to ACE inhibition: role of sodium status. Kidney Int 63[Suppl 2] :23– 26,1997 [PubMed] [Google Scholar]
- 58.Haas M, Yilmaz N, Schmidt A, Neyer U, Arneitz K, Stummvoll HK, Wallner M, Auinger M, Arias I, Schneider B, Mayer G: Angiotensin-converting enzyme gene polymorphism determines the antiproteinuric and systemic hemodynamic effect of enalapril in patients with proteinuric renal disease: Austrian Study Group of the Effects of Enalapril Treatment in Proteinuric Renal Disease. Kidney Blood Press Res 21 :66– 69,1998 [DOI] [PubMed] [Google Scholar]
- 59.van der Kleij FG, de Jong PE, Henning RH, de Zeeuw D, Navis G: Enhanced responses of blood pressure, renal function, and aldosterone to angiotensin I in the DD genotype are blunted by low sodium intake. J Am Soc Nephrol 13 :1025– 1033,2002 [DOI] [PubMed] [Google Scholar]
- 60.Coto E, Marin R, Alvarez V, Praga M, Fernandez Andrade C, Arias M, Poveda R, Valles M, Galceran JM, Luno J, Rivera F, Campistol JM: [Pharmacogenetics of angiotensin system in non diabetic nephropathy]. Nefrologia 25 :381– 386,2005 [PubMed] [Google Scholar]
- 61.Redon J, Luque-Otero M, Martell N, Chaves FJ: Renin-angiotensin system gene polymorphisms: relationship with blood pressure and microalbuminuria in telmisartan-treated hypertensive patients. Pharmacogenomics J 5 :14– 20,2005 [DOI] [PubMed] [Google Scholar]
- 62.Park HC, Choi HY, Kim BS, Kang SW, Choi KH, Ha SK, Lee HY, Han DS: Antiproteinuric effect of losartan in non-diabetic renal disease is not dependent on ACE insertion/deletion polymorphism. Kidney Blood Press Res 29 :216– 224,2006 [DOI] [PubMed] [Google Scholar]
- 63.Jeunemaitre X, Soubrier F, Kotelevtsev YV, Lifton RP, Williams CS, Charru A, Hunt SC, Hopkins PN, Williams RR, Lalouel JM, et al: Molecular basis of human hypertension: role of angiotensinogen. Cell 71 :169– 180,1992 [DOI] [PubMed] [Google Scholar]
- 64.Inoue I, Nakajima T, Williams CS, Quackenbush J, Puryear R, Powers M, Cheng T, Ludwig EH, Sharma AM, Hata A, Jeunemaitre X, Lalouel JM: A nucleotide substitution in the promoter of human angiotensinogen is associated with essential hypertension and affects basal transcription in vitro. J Clin Invest 99 :1786– 1797,1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freire MB, Ji L, Onuma T, Orban T, Warram JH, Krolewski AS: Gender-specific association of M235T polymorphism in angiotensinogen gene and diabetic nephropathy in NIDDM. Hypertension 31 :896– 899,1998 [DOI] [PubMed] [Google Scholar]
- 66.Rogus JJ, Moczulski D, Freire MB, Yang Y, Warram JH, Krolewski AS: Diabetic nephropathy is associated with AGT polymorphism T235: results of a family-based study. Hypertension 31 :627– 631,1998 [DOI] [PubMed] [Google Scholar]
- 67.Brand E, Chatelain N, Paillard F, Tiret L, Visvikis S, Lathrop M, Soubrier F, Demenais F: Detection of putative functional angiotensinogen (AGT) gene variants controlling plasma AGT levels by combined segregation-linkage analysis. Eur J Hum Genet 10 :715– 723,2002 [DOI] [PubMed] [Google Scholar]
- 68.Reyes-Engel A, Morcillo L, Aranda FJ, Ruiz M, Gaitan MJ, Mayor-Olea A, Aranda P, Ferrario CM: Influence of gender and genetic variability on plasma angiotensin peptides. J Renin Angiotensin Aldosterone Syst 7 :92– 97,2006 [DOI] [PubMed] [Google Scholar]
- 69.Zychma MJ, Zukowska-Szczechowska E, Lacka BI, Grzeszczak W: Angiotensinogen M235T and chymase gene CMA/B polymorphisms are not associated with nephropathy in type II diabetes. Nephrol Dial Transplant 15 :1965– 1970,2000 [DOI] [PubMed] [Google Scholar]
- 70.Fradin S, Goulet-Salmon B, Chantepie M, Grandhomme F, Morello R, Jauzac P, Reznik Y: Relationship between polymorphisms in the renin-angiotensin system and nephropathy in type 2 diabetic patients. Diabetes Metab 28 :27– 32,2002 [PubMed] [Google Scholar]
- 71.Hadjadj S, Belloum R, Bouhanick B, Gallois Y, Guilloteau G, Chatellier G, Alhenc-Gelas F, Marre M: Prognostic value of angiotensin-I converting enzyme I/D polymorphism for nephropathy in type 1 diabetes mellitus: a prospective study. J Am Soc Nephrol 12 :541– 549,2001 [DOI] [PubMed] [Google Scholar]
- 72.Buraczynska M, Ksiazek P, Drop A, Zaluska W, Spasiewicz D, Ksiazek A: Genetic polymorphisms of the renin-angiotensin system in end-stage renal disease. Nephrol Dial Transplant 21 :979– 983,2006 [DOI] [PubMed] [Google Scholar]
- 73.Woo KT, Lau YK, Choong LH, Zhao Y, Tan HB, Fook-Chong S, Tan EK, Yap HK, Wong KS: Polymorphism of renin-angiotensin system genes in IgA nephropathy. Nephrology (Carlton) 9 :304– 309,2004 [DOI] [PubMed] [Google Scholar]
- 74.Bonnardeaux A, Davies E, Jeunemaitre X, Fery I, Charru A, Clauser E, Tiret L, Cambien F, Corvol P, Soubrier F: Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension 24 :63– 69,1994 [DOI] [PubMed] [Google Scholar]
- 75.Wang WY, Zee RY, Morris BJ: Association of angiotensin II type 1 receptor gene polymorphism with essential hypertension. Clin Genet 51 :31– 34,1997 [DOI] [PubMed] [Google Scholar]
- 76.Tiret L, Blanc H, Ruidavets JB, Arveiler D, Luc G, Jeunemaitre X, Tichet J, Mallet C, Poirier O, Plouin PF, Cambien F: Gene polymorphisms of the renin-angiotensin system in relation to hypertension and parental history of myocardial infarction and stroke: the PEGASE study. Projet d'Etude des Genes de l'Hypertension Arterielle Severe a moderee Essentielle. J Hypertens 16 :37– 44,1998 [DOI] [PubMed] [Google Scholar]
- 77.Buraczynska M, Ksiazek P, Lopatynski J, Spasiewicz D, Nowicka T, Ksiazek A: [Association of the renin-angiotensin system gene polymorphism with nephropathy in type II diabetes]. Pol Arch Med Wewn 108 :725– 730,2002 [PubMed] [Google Scholar]
- 78.Tomino Y, Makita Y, Shike T, Gohda T, Haneda M, Kikkawa R, Watanabe T, Baba T, Yoshida H: Relationship between polymorphism in the angiotensinogen, angiotensin-converting enzyme or angiotensin II receptor and renal progression in Japanese NIDDM patients. Nephron 82 :139– 144,1999 [DOI] [PubMed] [Google Scholar]
- 79.Yoshida H, Kuriyama S, Atsumi Y, Tomonari H, Mitarai T, Hamaguchi A, Kubo H, Kawaguchi Y, Kon V, Matsuoka K, Ichikawa I, Sakai O: Angiotensin I converting enzyme gene polymorphism in non-insulin dependent diabetes mellitus. Kidney Int 50 :657– 664,1996 [DOI] [PubMed] [Google Scholar]
- 80.Maruyama K, Yoshida M, Nishio H, Shirakawa T, Kawamura T, Tanaka R, Nakamura H, Iijima K, Yoshikawa N: Polymorphisms of renin-angiotensin system genes in childhood IgA nephropathy. Pediatr Nephrol 16 :350– 355,2001 [DOI] [PubMed] [Google Scholar]
- 81.Hunley TE, Julian BA, Phillips JA 3rd, Summar ML, Yoshida H, Horn RG, Brown NJ, Fogo A, Ichikawa I, Kon V: Angiotensin converting enzyme gene polymorphism: potential silencer motif and impact on progression in IgA nephropathy. Kidney Int 49 :571– 577,1996 [DOI] [PubMed] [Google Scholar]
- 82.Konoshita T, Miyagi K, Onoe T, Katano K, Mutoh H, Nomura H, Koni I, Miyamori I, Mabuchi H: Effect of ACE gene polymorphism on age at renal death in polycystic kidney disease in Japan. Am J Kidney Dis 37 :113– 118,2001 [DOI] [PubMed] [Google Scholar]
- 83.Baboolal K, Ravine D, Daniels J, Williams N, Holmans P, Coles GA, Williams JD: Association of the angiotensin I converting enzyme gene deletion polymorphism with early onset of ESRF in PKD1 adult polycystic kidney disease. Kidney Int 52 :607– 613,1997 [DOI] [PubMed] [Google Scholar]
- 84.Marrè M, Jeunemaitre X, Gallois Y, Rodier M, Chatellier G, Sert C, Dusselier L, Kahal Z, Chaillous L, Halimi S, Muller A, Sackmann H, Bauduceau B, Bled F, Passa P, Alhenc-Gelas F: Contribution of genetic polymorphism in the renin-angiotensin system to the development of renal complications in insulin-dependent diabetes: Genetique de la Nephropathie Diabetique (GENEDIAB) study group. J Clin Invest 99 :1585– 1595,1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bantis C, Ivens K, Kreusser W, Koch M, Klein-Vehne N, Grabensee B, Heering P: Influence of genetic polymorphisms of the renin-angiotensin system on IgA nephropathy. Am J Nephrol 24 :258– 267,2004 [DOI] [PubMed] [Google Scholar]
- 86.Osawa N, Koya D, Araki S, Uzu T, Tsunoda T, Kashiwagi A, Nakamura Y, Maeda S: Combinational effect of genes for the renin-angiotensin system in conferring susceptibility to diabetic nephropathy. J Hum Genet 52 :143– 151,2007 [DOI] [PubMed] [Google Scholar]
- 87.Baylis C, Mitruka B, Deng A: Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest 90 :278– 281,1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang XL, Sim AS, Badenhop RF, McCredie RM, Wilcken DE: A smoking-dependent risk of coronary artery disease associated with a polymorphism of the endothelial nitric oxide synthase gene. Nat Med 2 :41– 45,1996 [DOI] [PubMed] [Google Scholar]
- 89.Morita T, Ito H, Suehiro T, Tahara K, Matsumori A, Chikazawa H, Nakauchi Y, Nishiya K, Hashimoto K: Effect of a polymorphism of endothelial nitric oxide synthase gene in Japanese patients with IgA nephropathy. Clin Nephrol 52 :203– 209,1999 [PubMed] [Google Scholar]
- 90.Burg M, Menne J, Ostendorf T, Kliem V, Floege J: Gene-polymorphisms of angiotensin converting enzyme and endothelial nitric oxide synthase in patients with primary glomerulonephritis. Clin Nephrol 48 :205– 211,1997 [PubMed] [Google Scholar]
- 91.Stratta P, Bermond F, Guarrera S, Canavese C, Carturan S, Dall'Omo A, Ciccone G, Bertola L, Mazzola G, Fasano E, Matullo G: Interaction between gene polymorphisms of nitric oxide synthase and renin-angiotensin system in the progression of membranous glomerulonephritis. Nephrol Dial Transplant 19 :587– 595,2004 [DOI] [PubMed] [Google Scholar]
- 92.Salardi S, Saccardo B, Borsani G, Modica R, Ferrandi M, Tripodi MG, Soria M, Ferrari P, Baralle FE, Sidoli A, et al: Erythrocyte adducin differential properties in the normotensive and hypertensive rats of the Milan strain: characterization of spleen adducin mRNA. Am J Hypertens 2 :229– 237,1989 [DOI] [PubMed] [Google Scholar]
- 93.Bianchi G, Tripodi G, Casari G, Salardi S, Barber BR, Garcia R, Leoni P, Torielli L, Cusi D, Ferrandi M, et al: Two point mutations within the adducin genes are involved in blood pressure variation. Proc Natl Acad Sci U S A 91 :3999– 4003,1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Staessen JA, Wang JG, Brand E, Barlassina C, Birkenhager WH, Herrmann SM, Fagard R, Tizzoni L, Bianchi G: Effects of three candidate genes on prevalence and incidence of hypertension in a Caucasian population. J Hypertens 19 :1349– 1358,2001 [DOI] [PubMed] [Google Scholar]
- 95.Pedrinelli R, Dell'Omo G, Penno G, Di Bello V, Pucci L, Fotino C, Lucchesi D, Del Prato S, Dal Fiume C, Barlassina C, Cusi D: Alpha-adducin and angiotensin-converting enzyme polymorphisms in hypertension: evidence for a joint influence on albuminuria. J Hypertens 24 :931– 937,2006 [DOI] [PubMed] [Google Scholar]
- 96.Wang JG, Staessen JA, Tizzoni L, Brand E, Birkenhager WH, Fagard R, Herrmann SM, Bianchi G: Renal function in relation to three candidate genes. Am J Kidney Dis 38 :1158– 1168,2001 [DOI] [PubMed] [Google Scholar]
- 97.Narita I, Goto S, Saito N, Song J, Ajiro J, Sato F, Saga D, Kondo D, Akazawa K, Sakatsume M, Gejyo F: Interaction between ACE and ADD1 gene polymorphisms in the progression of IgA nephropathy in Japanese patients. Hypertension 42 :304– 309,2003 [DOI] [PubMed] [Google Scholar]
- 98.Schieppati A, Perico N, Remuzzi G: Preventing end-stage renal disease: the potential impact of screening and intervention in developing countries. Nephrol Dial Transplant 18 :858– 859,2003. ; discussion 857 [DOI] [PubMed] [Google Scholar]
- 99.Costa-Scharplatz M, van Asselt AD, Bachmann LM, Kessels AG, Severens JL: Cost-effectiveness of pharmacogenetic testing to predict treatment response to angiotensin-converting enzyme inhibitor. Pharmacogenet Genomics 17 :359– 368,2007 [DOI] [PubMed] [Google Scholar]
- 100.Hoy WE, Baker PR, Kelly AM, Wang Z: Reducing premature death and renal failure in Australian aboriginals: a community-based cardiovascular and renal protective program. Med J Aust 172 :473– 478,2000 [PubMed] [Google Scholar]