Abstract
In this article, the pathophysiology of left ventricular failure is reviewed. By contrast, the paucity of information about pulmonary arterial hypertension and right ventricular failure is acknowledged. The potential mechanisms whereby renal sodium and water retention in right ventricular failure secondary to pulmonary arterial hypertension can occur, despite normal left ventricular function, are discussed. With right ventricular failure as the primary cause of death in patients with pulmonary hypertension, more information about the mechanisms of renal sodium and water retention in these patients is direly needed. Specifically, studies to examine the activation of the neurohumoral axis at various stages of pulmonary arterial hypertension and right ventricular failure, including inhibition of mineralocorticoid and V2 vasopressin receptors, are indicated.
The renal sodium and water retention that occurs with advanced left ventricular failure is associated with substantial morbidity and mortality. This sodium and water retention, which can lead to pulmonary edema, pleural effusion, and peripheral edema, occurs despite an increase in total blood volume. In normal individuals, a rise in total blood volume increases renal sodium and water excretion. The kidney is intrinsically intact with left ventricular failure, because the renal sodium and water retention does not persist after a successful heart transplant.
This seeming paradox of increased blood volume yet renal sodium and water retention in cardiac failure has been explained by the body fluid volume regulation hypothesis (1–3). This hypothesis proposes that the kidney does not respond to changes in total blood volume but rather responds to what has been termed effective arterial blood volume. In general terms, approximately 85% of circulating blood volume is in the low-pressure venous side of the circulation, whereas only 15% is in the high-pressure arterial circulation. The integrity of the arterial circulation depends on cardiac output and systemic vascular resistance and is modulated by arterial stretch baroreceptors in the carotid sinus, aortic arch, and afferent arteriole of the glomerulus (4). Thus, despite an increase in total blood volume, arterial underfilling can occur secondary to a decrease in cardiac output in low-output heart failure or decreased systemic vascular resistance in high-output heart failure. With arterial underfilling secondary to either condition, arterial baroreceptor–mediated activation of the neurohumoral axis occurs. The resultant increase in renin-angiotensin-aldosterone system (RAAS) leads to sodium retention, and the increase in the nonosmotic release of arginine vasopressin (AVP) is associated with water retention and hyponatremia in advanced left ventricular failure, a known risk factor for increased mortality (5). This water retention is due to AVP activation of the V2 vasopressin receptors on the basolateral surface of the principal cells of the collecting duct, which increases aquaporin 2 water channel expression and trafficking to the apical membrane of the collecting duct (6–8).
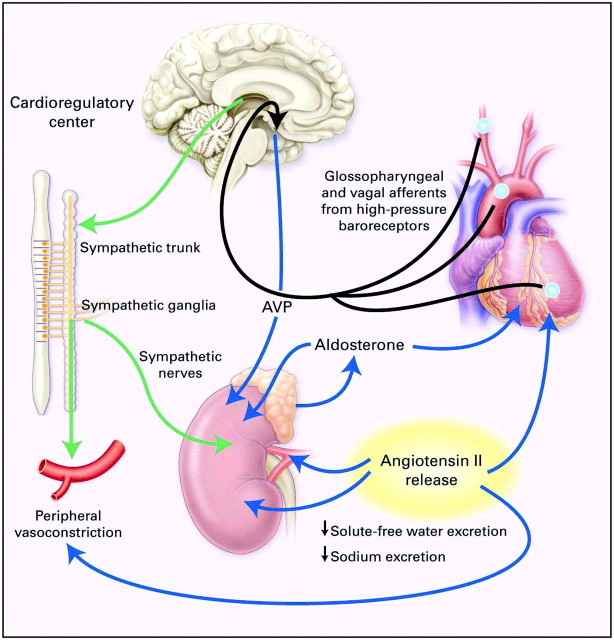
There is also evidence that increased plasma AVP concentration with left ventricular failure stimulates V1 vasopressin receptors on blood vessels, which contributes, along with angiotensin II and the sympathetic nervous system, to increasing systemic vascular resistance in low-cardiac output failure (9). This arterial baroreceptor–mediated neurohumoral activation maintains arterial pressure but at the expense of renal vasoconstriction and sodium and water retention. The pathophysiology of left ventricular cardiac failure is shown in Figure 1.
Figure 1.
Unloading of high-pressure baroceptors (blue circles) in the left ventricle, carotid sinus, and aortic arch generates afferent signals (black) that stimulate cardioregulatory centers in the brain, resulting in the activation of efferent pathways in the sympathetic nervous system (green). The sympathetic nervous system seems to be the primary integrator of the neurohumoral vasoconstrictor response to arterial underfilling. Activation of renal sympathetic nerves stimulates the release of renin and angiotensin II, thereby activating the renin-angiotensin-aldosterone system (RAAS). Concomitantly, sympathetic stimulation of the supraoptic and paraventricular nuclei in the hypothalamus results in the nonosmotic release of arginine vasopressin (AVP). Sympathetic activation also causes peripheral and renal vasoconstriction, as does angiotensin II. Angiotensin II constricts blood vessels and stimulates the release of aldosterone from the adrenal gland, and it also increases tubular sodium reabsorption and causes remodeling of cardiac myocytes. Aldosterone may also have direct cardiac effects on fibrosis, in addition to increasing the reabsorption of sodium and the secretion of potassium and hydrogen ions in the collecting duct. The blue lines designate circulating hormones. Reprinted from reference 2 (Schrier RW, Abraham WT: Hormones and hemodynamics in heart failure. N Engl J Med 341: 577–585, 1999), with permission. Copyright © 1999 Massachusetts Medical Society. All rights reserved.
These arterial baroreceptor pathways seem to override any of the low-pressure reflexes in the atria during left ventricular failure. An increase in transmural atrial pressure normally suppresses AVP and stimulates atrial natriuretic peptide (ANP), which leads to increased sodium and water excretion (10). With advanced left ventricular failure, however, left atrial pressure rises, yet sodium and water retention occurs. This suggests that activation of the arterial stretch receptors in cardiac failure predominates over any atrial pressure receptor reflex. There is also evidence that these normal atrial reflexes are blunted in patients with left ventricular failure (11).
An increase in the ventricular synthesis of brain natriuretic peptide (BNP) and, thus, circulatory BNP concentration also occurs in left ventricular failure and may attenuate the degree of renal sodium and water retention. BNP may decrease the edema formation by both suppressing the RAAS and inhibiting tubular sodium reabsorption (12).
Right Ventricular Failure
What then occurs with pulmonary hypertension (PH) and isolated right ventricular failure? In this setting, left ventricular function is normal as may occur with primary pulmonary hypertension, chronic obstructive pulmonary disease (COPD), and connective tissue diseases.
PH was previously categorized into primary PH or secondary PH depending on the absence or presence of identifiable causes or risk factors. Subsequently World Health Organization classified PH into five groups on the basis of mechanisms, rather than associated conditions. Pulmonary arterial hypertension is group 1 and is defined as a sustained elevation of pulmonary arterial pressure to >25 mmHg at rest or to >30 mmHg with exercise, with a mean pulmonary-capillary wedge pressure and left ventricular end-diastolic pressure of <15 mmHg (13). Pulmonary arterial hypertension comprises idiopathic pulmonary arterial hypertension (formerly primary pulmonary hypertension); pulmonary arterial hypertension in the setting of collagen vascular disease (e.g., in localized cutaneous systemic sclerosis, also known as the CREST syndrome [calcinosis cutis, Raynaud's phenomenon, esophageal dysfunction, sclerodactyly, and telangiectasia]), portal hypertension, congenital left-to-right intracardiac shunts, and infection with the HIV; and persistent PH of the newborn. The histologic appearance of lung tissue in each of these conditions is similar: intimal fibrosis, increased medial thickness, pulmonary arteriolar occlusion, and plexiform lesions predominate (14). PH associated with left ventricular dysfunction comprises of group 2, and PH associated with hypoxemia such as COPD, interstitial lung diseases, and sleep-ventilation disorders constitutes group 3. Group 4 includes PH associated with chronic thromboembolic diseases, and group 5 encompasses miscellaneous disorders such as sarcoidosis, pulmonary Langerhans cell histiocytosis, and lymphangiomatosis. Among these PH groups, groups 1 and 3 are conditions in which right ventricular failure is associated with intrinsically normal left ventricular function.
There is evidence that in patients with COPD with right ventricular failure (group 3 PH), the RAAS axis is stimulated (15,16). This effect may relate to arterial underfilling associated with a decrease in systemic vascular resistance. One theory is that carbon dioxide, commonly elevated in patients with COPD, is a potent vasodilator and lowers the systemic vascular resistance and increases the arterial capacitance. Consequently, arterial underfilling occurs, leading to stimulation of the neurohormonal axis (norepinephrine, RAAS, and AVP) and, hence, sodium and water retention (15). Elevated Paco2 also may lead to sodium retention through increased Na-H exchange in the renal tubules (17). Moreover, hypoxemia associated with hypercapnia may influence urinary sodium excretion. In one study, Reihman et al. (18) demonstrated a significant fall in urinary sodium output on withdrawal of supplemental oxygen. In another study by Mannix et al. (19), oxygen supplementation resulted in enhanced natriuresis, independent of hypercapnia.
Elevated plasma volume has been demonstrated in patients with pulmonary arterial hypertension and found to be associated with poor outcome (20); however, the mechanisms that cause sodium and water retention have not been explained in these patients, because hypercapnia was not present. The neurohumoral axis has been rarely studied in pulmonary arterial hypertension with right ventricular failure in humans. Induction of early experimental models of right ventricular failure by graded valvular damage showed a decrease in renal blood flow, preserved GFR, and intense salt and water retention (21). The underlying neurohormonal state in this model was not examined. Despite the presence of pulmonary artery baroreceptors (22,23), other investigators concluded that when cardiac output is kept constant, pulmonary arterial distention has no direct effect on renal hemodynamics (24,25). The renal hemodynamic changes and the retention of sodium and water observed in patients with pulmonary arterial hypertension therefore may be mediated by systemic, rather than pulmonary, arterial baroreceptors, as has been shown in other edematous states (1,4). One study of patients with pulmonary arterial hypertension showed increases in plasma endothelin, ANP, and norepinephrine concentrations but not in the plasma renin levels. Plasma aldosterone and AVP concentrations were not assessed (26).
In contrast to left ventricular failure (27,28), the role of aldosterone and vasopressin in renal sodium and water retention in patients with pulmonary arterial hypertension has not been examined, even though mineralocorticoid and V2 vasopressin receptor antagonists are clinically available. Mineralocorticoid antagonists have been shown to afford cardiovascular protection in patients who had left-sided heart failure receiving angiotensin-converting enzyme inhibitors. In the Randomized Aldactone Evaluation Study (RALES), improved survival in patients with left ventricular failure was demonstrated using dosages of spironolactone (25 to 50 mg/d), which did not alter urinary sodium excretion (29). Whether the results of this trial are applicable to patients with pulmonary arterial hypertension and intact left ventricular function but right ventricular failure is not known.
There are many other unanswered questions relating to pulmonary arterial hypertension and isolated right ventricular failure. Echocardiogram studies have shown normal left ventricular function and ejection fraction in these patients. Cardiac-renal neural reflexes initiated from the pulmonary arterial circulation and/or right atrial and ventricle have not been well delineated. Plasma ANP and BNP concentrations have been found to be elevated in pulmonary arterial hypertension in proportion to the degree of right ventricular dysfunction (30,31); however, these peptides increase urinary sodium excretion and thus cannot account for the renal sodium and water retention in right ventricular failure. Increased concentrations of these peptides do, however, correlate with mortality in patients with PH (32).
Without sufficient treatment, the natural course of pulmonary arterial hypertension is characterized by a high mortality rate and limited survival. The National Institutes of Health registry (1981 through 1987) included patients with idiopathic/familial PH and reported a median survival of 2.8 yr with 1-yr survival rate of 68% and a 5-yr survival rate of 34% before the availability of sufficient treatment options (33). In a recent single-center cohort, the 1- and 5-yr survival rates were 84 and 58%, respectively, and the median survival time was 3.6 yr with currently available treatments (34). Although newer treatments for pulmonary arterial hypertension have emerged, including prostacyclins, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors, only modest functional improvement with minimal change in hemodynamic measurements at cardiac catheterization have been achieved (35).
Survival in pulmonary arterial hypertension correlates inversely with hemodynamic parameters such as mean pulmonary arterial pressure, right atrial pressure, cardiac index, and right ventricular failure (36). A recent study demonstrated that, similar to left heart failure, hyponatremia was associated with advanced right heart failure and dramatically reduced survival in patients with pulmonary arterial hypertension as compared with individuals with normal serum sodium. Hyponatremia predicted death even after adjustment for hemodynamic, echocardiographic, and clinical variables of known prognostic importance in PAH (37). Peripheral edema and ascites are common in advanced pulmonary arterial hypertension, and resistance to diuretics often occurs as the disease progresses. Patients eventually die from right ventricular failure. Conversely, except for chronic obstructive pulmonary disease, there is scant knowledge about the pathophysiology of hyponatremia, volume overload, neurohumoral axis, particularly the RAAS, in patients with pulmonary arterial hypertension with right ventricular failure. If increased activation of the RAAS does not occur, then it could be due to several factors. First, arterial underfilling, as a result of either a decrease in cardiac output or systemic arterial vasodilation, may not be present with pulmonary arterial hypertension. This is compatible with the observed normal left ventricular function, normal ejection fraction (1), and normal plasma renin activity (26). The question, then, is which factors mediate renal sodium and water retention with pulmonary arterial hypertension and right ventricular failure? ANP and BNP are known to exert an inhibitory effect on the RAAS and catecholamines, but this observation would not explain the renal sodium and water retention in patients with pulmonary arterial hypertension.
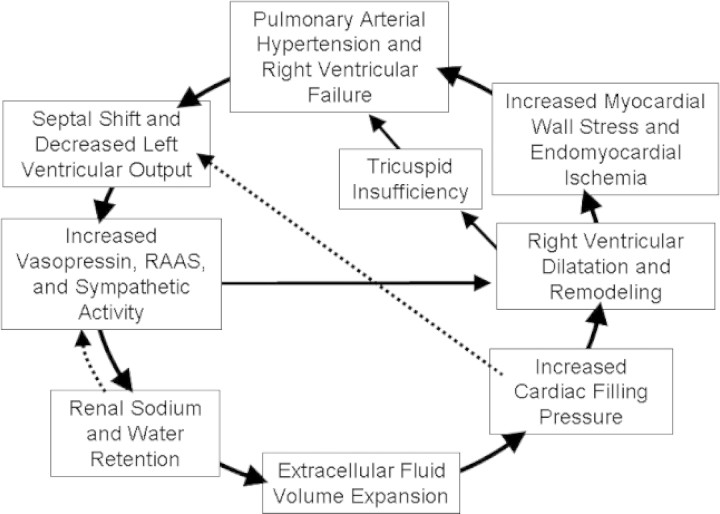
Studies of conscious dogs with pulmonary stenosis, which might bear on the renal sodium and water retention with primary PH, were undertaken more than three decades ago (38). In those studies, there was an initial decrease in cardiac output, a lower mean arterial pressure, and activation of the RAAS. Over a period of time, however, sodium and water retention occurred, and cardiac output and mean arterial pressure returned to baseline, as did the RAAS. These compensatory responses, however, occurred at the expense of substantial edema formation. Because patients with PH are frequently seen for only a snapshot in time, this sequence of compensatory events cannot be excluded. This potential sequence of compensatory events that could lead to sodium and water retention and return of cardiac output and RAAS to the normal range are shown in Figure 2. Follow-up studies from the early to late stages of pulmonary arterial hypertension will be necessary to explore whether this sequence of events occurs. These patients could have normal left ventricular function and ejection fraction when seen but earlier in their disease may have activated the neurohumoral axis secondary to a decrease in cardiac output. In this latter setting, an apparent normal RAAS in patients with pulmonary arterial hypertension could be relatively increased given the positive sodium and water balance. An increase in renal venous pressure with right ventricular failure also could decrease GFR and contribute to sodium and water retention.
Figure 2.
Feedback mechanisms for normalizing neurohormones and cardiac index with myocardial injury. The dashed lines indicate the compensatory responses that could return cardiac index and the RAAS to within normal range.
Other potential mechanisms for decreased left ventricular output in the setting of normal ejection fraction in pulmonary arterial hypertension with isolated right heart failure could be explained by interventricular asynchrony and/or pericardium-mediated right ventricle–left ventricle interaction. Synchronous right and left ventricular pressure measurements in patients with pulmonary arterial hypertension demonstrated a significant right-to-left transseptal pressure gradient at the time of maximal leftward septal displacement measured by magnetic resonance imaging (39). The mechanism behind this asynchrony is that right ventricular pressure overload leads to prolonged contraction of the right ventricular free wall (40). At the time that the left ventricle has entered its early diastolic phase, right ventricular pressure exceeds left ventricular pressure. As a result, a transseptal pressure gradient leads to paradoxic septum movement (41). The consequence of this leftward septal bowing is not only ineffective right ventricular end-systolic contraction but also impaired left ventricular early diastolic filling (41). A decreased left ventricular end-diastolic volume directly impairs left ventricular output according to the Frank-Starling mechanism (42,43).
Next, because both ventricles share the same pericardial space, dilation of the right ventricle will be accompanied by increased pericardial stretch. This inward directed force may impair left ventricular filling. An effect of such pericardial constraint on left ventricular filling has been demonstrated in an acute model of right ventricular pressure overload in dogs (44). Opening of the canine pericardium facilitated left ventricular filling and consequently improved cardiac output (44,45). The consequences of pericardial constraint in chronic pressure overload are less clear (46). Pericardial constraint and impaired left ventricular filling may influence the perfusion of the right coronary artery and thus oxygen supply to the left ventricle.
Conclusions
In summary, patients with pulmonary arterial hypertension and right ventricular dysfunction may have decreased cardiac output, activation of the neurohormonal axis, and renal retention of sodium and water. The analysis of systemic and renal hemodynamics and activation of the neurohormonal axis (norepinephrine, RAAS, and AVP) in patients with right ventricular failure secondary to pulmonary arterial hypertension has not been undertaken. Moreover, the response to mineralocorticoid antagonist and vasopressin receptor antagonist has not been studied in patients with right ventricular failure. Studies at various stages of pulmonary arterial hypertension need to be undertaken to examine sodium and water balance, neurohumoral axis, particularly the RAAS, and the response to V2 vasopressin receptor and mineralocorticoid antagonists.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Schrier RW: Decreased effective blood volume in edematous disorders: What does this mean? J Am Soc Nephrol 18 :2028– 2031,2007 [DOI] [PubMed] [Google Scholar]
- 2.Schrier RW, Abraham WT: Hormones and hemodynamics in heart failure. N Engl J Med 341 :577– 585,1999 [DOI] [PubMed] [Google Scholar]
- 3.Schrier RW: Water and sodium retention in edematous disorders: Role of vasopressin and aldosterone. Am J Med 119[ Suppl 1]:S47– S53,2006 [DOI] [PubMed] [Google Scholar]
- 4.Schrier RW: Body fluid volume regulation in health and disease: A unifying hypothesis. Ann Intern Med 113 :155– 159,1990 [DOI] [PubMed] [Google Scholar]
- 5.Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O'Connor CM, She L, Yancy CW, Young J, Fonarow GC: Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: An analysis from the OPTIMIZE-HF registry. Eur Heart J 28 :980– 988,2007 [DOI] [PubMed] [Google Scholar]
- 6.Nielsen S, Terris J, Andersen D, Ecelbarger C, Frokiaer J, Jonassen T, Marples D, Knepper MA, Petersen JS: Congestive heart failure in rats is associated with increased expression and targeting of aquaporin-2 water channel in collecting duct. Proc Natl Acad Sci U S A 94 :5450– 5455,1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA: Aquaporins in the kidney: From molecules to medicine. Physiol Rev 82 :205– 244,2002 [DOI] [PubMed] [Google Scholar]
- 8.Schrier RW, Cadnapaphornchai MA, Ohara M: Water retention and aquaporins in heart failure, liver disease and pregnancy. J R Soc Med 94 :265– 269,2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liard JF, Spadone JC: Hemodynamic effects of antagonists of the vasoconstrictor action of vasopressin in conscious dogs. J Cardiovasc Pharmacol 6 :713– 719,1984 [DOI] [PubMed] [Google Scholar]
- 10.Schrier RW: Role of diminished renal function in cardiovascular mortality: Marker or pathogenetic factor? J Am Coll Cardiol 47 :1– 8,2006 [DOI] [PubMed] [Google Scholar]
- 11.Koepke JP, DiBona GF: Blunted natriuresis to atrial natriuretic peptide in chronic sodium-retaining disorders. Am J Physiol 252 :F865– F871,1987 [DOI] [PubMed] [Google Scholar]
- 12.Abraham WT, Lowes BD, Ferguson DA, Odom J, Kim JK, Robertson AD, Bristow MR, Schrier RW: Systemic hemodynamic, neurohormonal, and renal effects of a steady-state infusion of human brain natriuretic peptide in patients with hemodynamically decompensated heart failure. J Card Fail 4 :37– 44,1998 [DOI] [PubMed] [Google Scholar]
- 13.Gaine SP, Rubin LJ: Primary pulmonary hypertension. Lancet 352 :719– 725,1998 [DOI] [PubMed] [Google Scholar]
- 14.Rubin LJ: Primary pulmonary hypertension. N Engl J Med 336 :111– 117,1997 [DOI] [PubMed] [Google Scholar]
- 15.Anand IS, ChandrashekharY, Ferrari R, Sarma R, Guleria R, Jindal SK, Wahi PL, Poole-Wilson PA, Harris P: Pathogenesis of congestive state in chronic obstructive pulmonary disease: Studies of body water and sodium, renal function, hemodynamics, and plasma hormones during edema and after recovery. Circulation 86 :12– 21,1992 [DOI] [PubMed] [Google Scholar]
- 16.Farber MO, Roberts LR, Weinberger MH, Robertson GL, Fineberg NS, Manfredi F: Abnormalities of sodium and H2O handling in chronic obstructive lung disease. Arch Intern Med 142 :1326– 1330,1982 [PubMed] [Google Scholar]
- 17.Palange P: Renal and hormonal abnormalities in chronic obstructive pulmonary disease (COPD). Thorax 53 :989– 991,1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reihman DH, Farber MO, Weinberger MH, Henry DP, Fineberg NS, Dowdeswell IR, BurtRW, Manfredi F: Effect of hypoxemia on sodium and water excretion in chronic obstructive lung disease. Am J Med 78 :87– 94,1985 [DOI] [PubMed] [Google Scholar]
- 19.Mannix ET, Dowdeswell IR, Carlone S, Palange P, Aronoff GR, Farber MO: The effect of oxygen on sodium excretion in hypoxemic patients with chronic obstructive lung disease. Chest 97 :840– 844,1990 [DOI] [PubMed] [Google Scholar]
- 20.James KB, Stelmach K, Armstrong R, Young JB, Fouad-Tarazi F: Plasma volume and outcome in pulmonary hypertension. Tex Heart Inst J 30 :305– 307,2003 [PMC free article] [PubMed] [Google Scholar]
- 21.Barger AC, Yates FE, Rudolph AM: Renal hemodynamics and sodium excretion in dogs with graded valvular damage, and in congestive failure. Am J Physiol 200 :601– 608,1961 [DOI] [PubMed] [Google Scholar]
- 22.Coleridge JC, Kidd C, Sharp JA: The distribution, connexions and histology of baroreceptors in the pulmonary artery, with some observations on the sensory innervation of the ductus arteriosus. J Physiol 156 :591– 602,1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleridge JC, Kidd C: Electrophysiological evidence of baroreceptors in the pulmonary artery of the dog. J Physiol 150 :319– 331,1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ledsome JR, Kan WO: Reflex changes in hindlimb and renal vascular resistance in response to distention of the isolated pulmonary arteries of the dog. Circ Res 40 :64– 72,1977 [DOI] [PubMed] [Google Scholar]
- 25.Coleridge JC, Kidd C: Reflex effects of stimulating baroreceptors in the pulmonary artery. J Physiol 166 :197– 210,1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nootens M, Kaufmann E, Rector T, Toher C, Judd D, Francis GS, Rich S: Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: Relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol 26 :1581– 1585,1995 [DOI] [PubMed] [Google Scholar]
- 27.Hensen J, Abraham WT, Durr JA, Schrier RW: Aldosterone in congestive heart failure: Analysis of determinants and role in sodium retention. Am J Nephrol 11 :441– 446,1991 [DOI] [PubMed] [Google Scholar]
- 28.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C: Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355 :2099– 2112,2006 [DOI] [PubMed] [Google Scholar]
- 29.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J: The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341 :709– 717,1999 [DOI] [PubMed] [Google Scholar]
- 30.Adnot S, Chabrier PE, Andrivet P, Viossat I, Piquet J, Brun-Buisson C, Gutkowska Y, Braquet P: Atrial natriuretic peptide concentrations and pulmonary hemodynamics in patients with pulmonary artery hypertension. Am Rev Respir Dis 136 :951– 956,1987 [DOI] [PubMed] [Google Scholar]
- 31.Leuchte HH, Holzapfel M, BaumgartnerRA, Ding I, Neurohr C, Vogeser M, Kolbe T, Schwaiblmair M, Behr J: Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol 43 :764– 770,2004 [DOI] [PubMed] [Google Scholar]
- 32.Nagaya N, Nishikimi T, Okano Y, Uematsu M, Satoh T, Kyotani S, Kuribayashi S, Hamada S, Kakishita M, Nakanishi N, Takamiya M, Kunieda T, Matsuo H, Kangawa K: Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol 31 :202– 208,1998 [DOI] [PubMed] [Google Scholar]
- 33.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT: Survival in patients with primary pulmonary hypertension: Results from a national prospective registry. Ann Intern Med 115 :343– 349,1991 [DOI] [PubMed] [Google Scholar]
- 34.Thenappan T, Shah SJ, Rich S, Gomberg-Maitland M: A united states-based registry for pulmonary arterial hypertension: 1982–2006. Eur Respir J 30 :1103– 1110,2007 [DOI] [PubMed] [Google Scholar]
- 35.Chin KM, Rubin LJ: Pulmonary arterial hypertension. J Am Coll Cardiol 51 :1527– 1538,2008 [DOI] [PubMed] [Google Scholar]
- 36.McLaughlin VV, Presberg KW, Doyle RL, Abman SH, McCrory DC, Fortin T, and Ahearn G: Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest 126[ Suppl]:78S– 92S,2004 [DOI] [PubMed] [Google Scholar]
- 37.Forfia PR, Mathai SC, Fisher MR, Housten-Harris T, Hemnes AR, Champion HC, Girgis RE, Hassoun PM: Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 177 :1364– 1369,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watkins L, Burton JA, Haber E, Cant JR, Smith FW, Barger AC: The renin-angiotensin-aldosterone system in congestive failure in conscious dogs. J Clin Invest 57 :1606– 1617,1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roeleveld RJ, Marcus JT, Faes TJ, Gan TJ, Boonstra A, Postmus PE, Vonk-Noordegraaf A: Interventricular septal configuration at MR imaging and pulmonary arterial pressure in pulmonary hypertension. Radiology 234 :710– 717,2005 [DOI] [PubMed] [Google Scholar]
- 40.Vonk-Noordegraaf A, Marcus JT, Gan CT, Boonstra A, Postmus PE: Interventricular mechanical asynchrony due to right ventricular pressure overload in pulmonary hypertension plays an important role in impaired left ventricular filling. Chest 128[ Suppl]:628S– 630S,2005 [DOI] [PubMed] [Google Scholar]
- 41.Dong SJ, Smith ER, Tyberg JV: Changes in the radius of curvature of the ventricular septum at end diastole during pulmonary arterial and aortic constrictions in the dog. Circulation 86 :1280– 1290,1992 [DOI] [PubMed] [Google Scholar]
- 42.Gan CT, Lankhaar JW, Marcus JT, Westerhof N, Marques KM, Bronzwaer JG, Boonstra A, Postmus PE, Vonk-Noordegraaf A: Impaired left ventricular filling due to right-to-left ventricular interaction in patients with pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 290 :H1528– H1533,2006 [DOI] [PubMed] [Google Scholar]
- 43.Marcus JT, Vonk Noordegraaf A, Roeleveld RJ, Postmus PE, Heethaar RM, Van Rossum AC, Boonstra A: Impaired left ventricular filling due to right ventricular pressure overload in primary pulmonary hypertension: Noninvasive monitoring using MRI. Chest 119 :1761– 1765,2001 [DOI] [PubMed] [Google Scholar]
- 44.Baker AE, Dani R, Smith ER, Tyberg JV, Belenkie I: Quantitative assessment of independent contributions of pericardium and septum to direct ventricular interaction. Am J Physiol 275 :H476– H483,1998 [DOI] [PubMed] [Google Scholar]
- 45.Belenkie I, Sas R, Mitchell J, Smith ER, Tyberg JV: Opening the pericardium during pulmonary artery constriction improves cardiac function. J Appl Physiol 96 :917– 922,2004 [DOI] [PubMed] [Google Scholar]
- 46.Blanchard DG, Dittrich HC: Pericardial adaptation in severe chronic pulmonary hypertension: An intraoperative transesophageal echocardiographic study. Circulation 85 :1414– 1422,1992 [DOI] [PubMed] [Google Scholar]