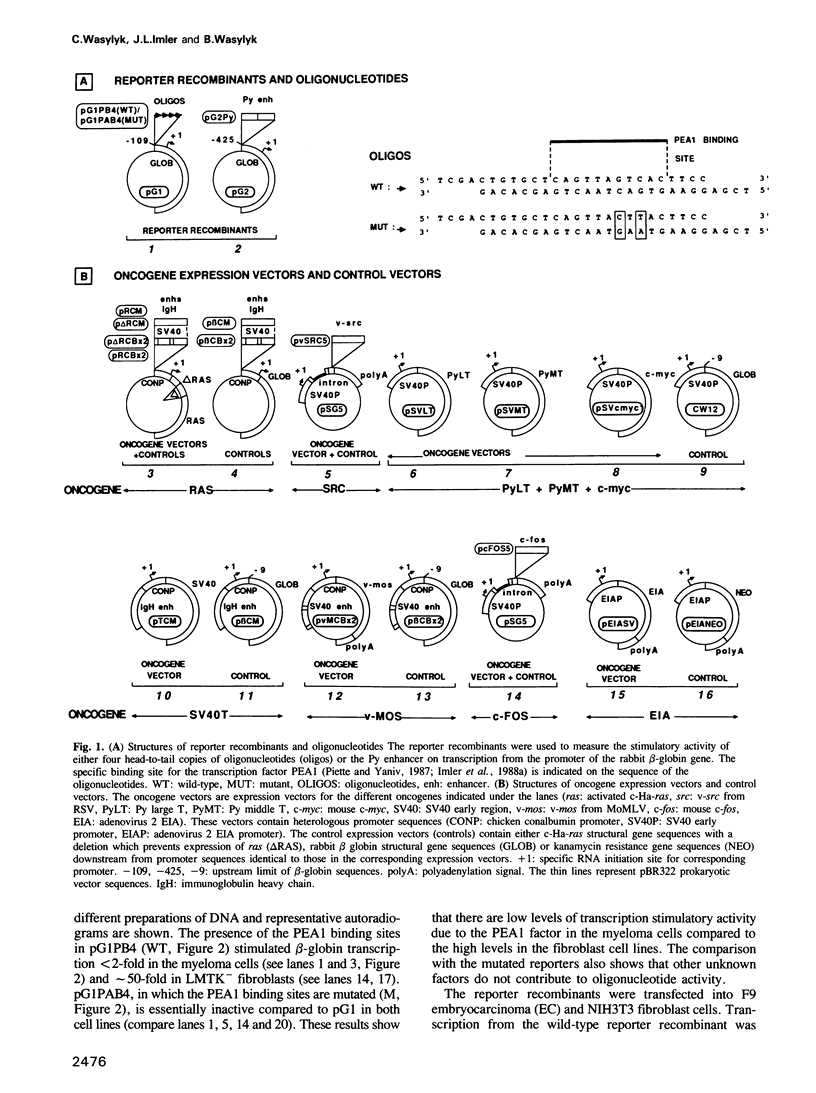
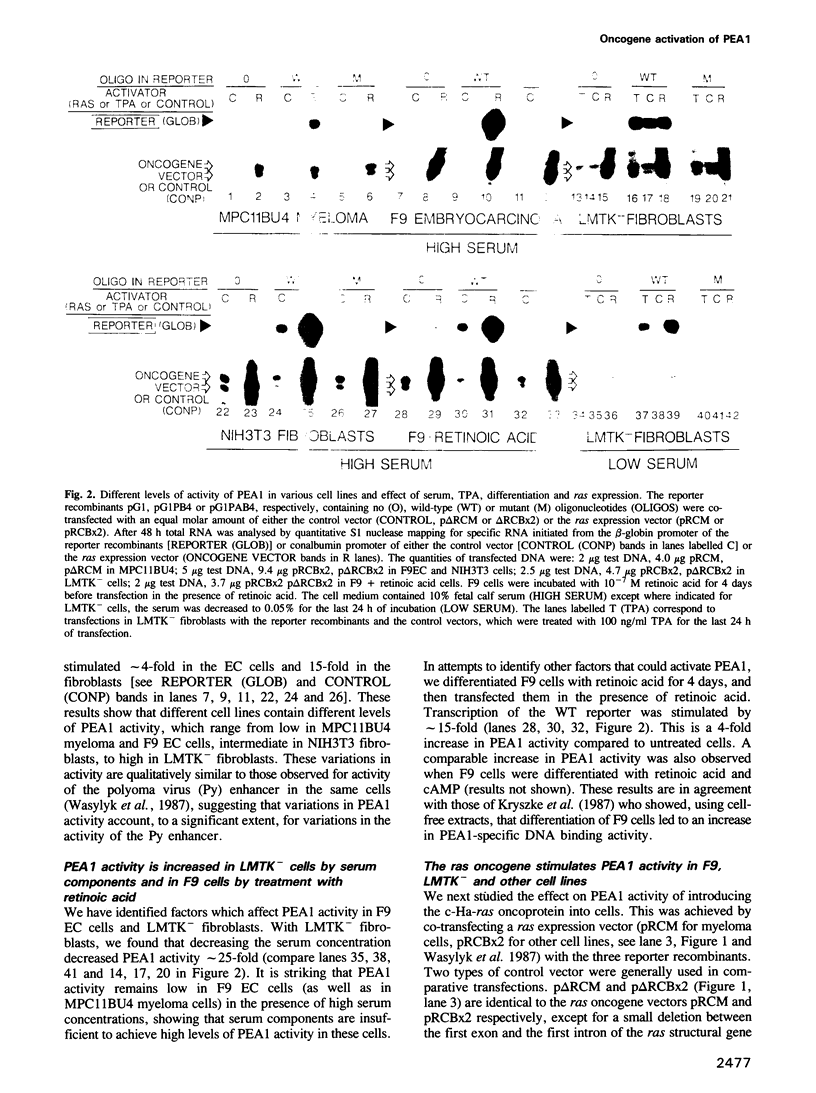
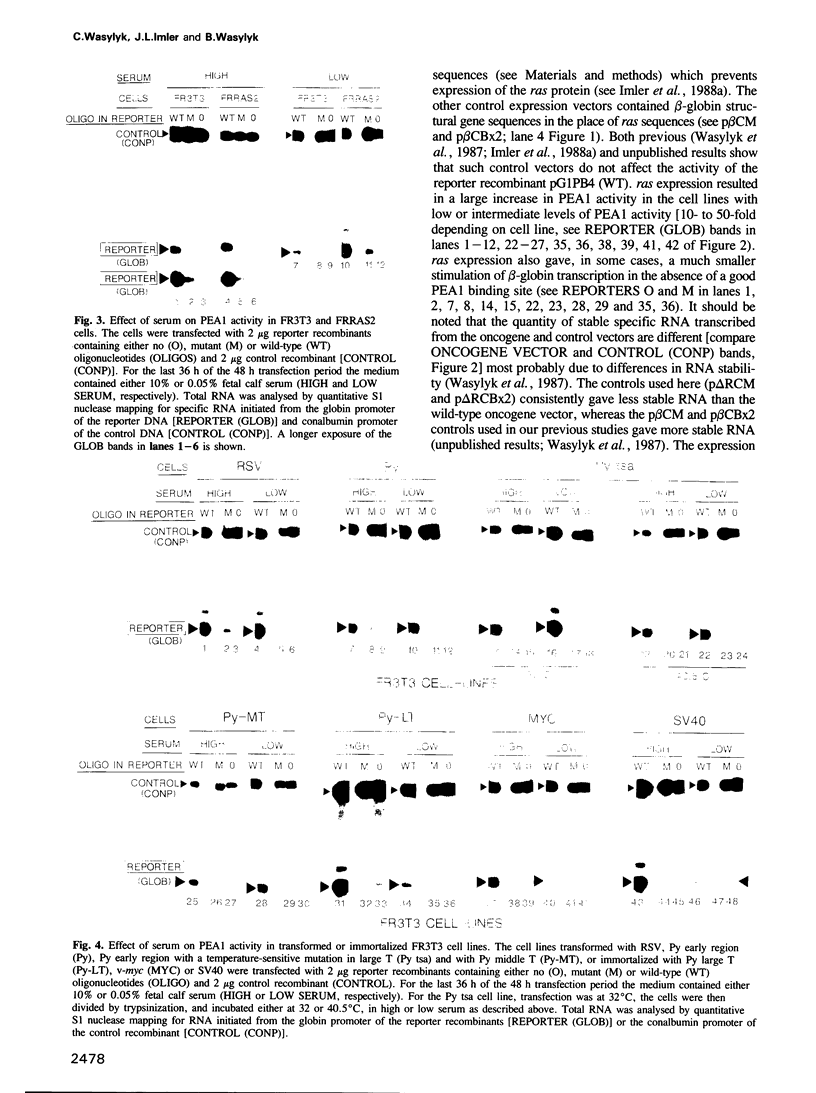
Abstract
The transcription factor PEA1 (a homologue of AP1 and c-jun) is highly active in several fibroblast cell lines, compared to its low activity in a myeloma and an embryo-carcinoma (EC) cell line. Serum components are essential to attain these high levels of PEA1 activity in fibroblasts. This serum requirement is abrogated by transformation with the oncogenes c-Ha-ras, v-src and polyoma middle T (Py-MT) but not by immortalization with polyoma large T (Py-LT), v-myc, c-myc or SV40 large T (SV40T). Expression in myeloma cells of the same transforming oncogenes, as well as v-mos and c-fos, activates PEA1, whereas expression of the same immortalizing oncogenes and EIA does not. These results suggest that a common target for transforming oncogenes is PEA1. Serum components have no effect on PEA1 activity in the myeloma and EC cell lines. In contrast, retinoic acid treatment of F9 EC cells augments PEA1 activity. These results suggest that transforming oncogene expression compensates for the absence of cell type-specific factors which are required to activate PEA1. Activation of PEA1 may lead to altered transcription of a set of transformation-related genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Allegretto E. A., Okino S. T., Hattori K., Boyle W. J., Hunter T., Karin M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature. 1988 Mar 10;332(6160):166–171. doi: 10.1038/332166a0. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M. Protein kinase C as the receptor for the phorbol ester tumor promoters: sixth Rhoads memorial award lecture. Cancer Res. 1988 Jan 1;48(1):1–8. [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Neuberg M., Burckhardt J., Almendral J., Wallich R., Müller R. Involvement of common and cell type-specific pathways in c-fos gene control: stable induction of cAMP in macrophages. Cell. 1987 Jan 30;48(2):251–260. doi: 10.1016/0092-8674(87)90428-4. [DOI] [PubMed] [Google Scholar]
- Cavalieri F., Ruscio T., Tinoco R., Benedict S., Davis C., Vogt P. K. Isolation of three new avian sarcoma viruses: ASV 9, ASV 17, and ASV 25. Virology. 1985 Jun;143(2):680–683. doi: 10.1016/0042-6822(85)90412-x. [DOI] [PubMed] [Google Scholar]
- Chiu R., Imagawa M., Imbra R. J., Bockoven J. R., Karin M. Multiple cis- and trans-acting elements mediate the transcriptional response to phorbol esters. Nature. 1987 Oct 15;329(6140):648–651. doi: 10.1038/329648a0. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Connan G., Rassoulzadegan M., Cuzin F. Focus formation in rat fibroblasts exposed to a tumour promoter after transfer of polyoma plt and myc oncogenes. Nature. 1985 Mar 21;314(6008):277–279. doi: 10.1038/314277a0. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Heber A. An 81 kd protein complexed with middle T antigen and pp60c-src: a possible phosphatidylinositol kinase. Cell. 1987 Sep 25;50(7):1031–1037. doi: 10.1016/0092-8674(87)90169-3. [DOI] [PubMed] [Google Scholar]
- Distel R. J., Ro H. S., Rosen B. S., Groves D. L., Spiegelman B. M. Nucleoprotein complexes that regulate gene expression in adipocyte differentiation: direct participation of c-fos. Cell. 1987 Jun 19;49(6):835–844. doi: 10.1016/0092-8674(87)90621-0. [DOI] [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Ohashi T., Yamanishi K., Taniguchi T. Regulation of human interleukin-2 gene: functional DNA sequences in the 5' flanking region for the gene expression in activated T lymphocytes. Cell. 1986 Aug 1;46(3):401–405. doi: 10.1016/0092-8674(86)90660-4. [DOI] [PubMed] [Google Scholar]
- Glaichenhaus N., Léopold P., Cuzin F. Increased levels of mitochondrial gene expression in rat fibroblast cells immortalized or transformed by viral and cellular oncogenes. EMBO J. 1986 Jun;5(6):1261–1265. doi: 10.1002/j.1460-2075.1986.tb04355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Siegfried Z., Ziff E. B. Mutation of the c-fos gene dyad symmetry element inhibits serum inducibility of transcription in vivo and the nuclear regulatory factor binding in vitro. Mol Cell Biol. 1987 Mar;7(3):1217–1225. doi: 10.1128/mcb.7.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griep A. E., DeLuca H. F. Decreased c-myc expression is an early event in retinoic acid-induced differentiation of F9 teratocarcinoma cells. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5539–5543. doi: 10.1073/pnas.83.15.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Imler J. L., Schatz C., Wasylyk C., Chatton B., Wasylyk B. A Harvey-ras responsive transcription element is also responsive to a tumour-promoter and to serum. Nature. 1988 Mar 17;332(6161):275–278. doi: 10.1038/332275a0. [DOI] [PubMed] [Google Scholar]
- Imler J. L., Ugarte E., Wasylyk C., Wasylyk B. v-jun is a transcriptional activator, but not in all cell-lines. Nucleic Acids Res. 1988 Apr 11;16(7):3005–3012. doi: 10.1093/nar/16.7.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Pallas D. C., White M., Cantley L., Roberts T. M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987 Sep 25;50(7):1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Raptis L., Garcea R. L., Pallas D., Roberts T. M., Cantley L. Phosphatidylinositol metabolism and polyoma-mediated transformation. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3624–3628. doi: 10.1073/pnas.83.11.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Klein E. Evolution of tumours and the impact of molecular oncology. Nature. 1985 May 16;315(6016):190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Genetics of human cancer. Annu Rev Genet. 1986;20:231–251. doi: 10.1146/annurev.ge.20.120186.001311. [DOI] [PubMed] [Google Scholar]
- Kryszke M. H., Piette J., Yaniv M. Induction of a factor that binds to the polyoma virus A enhancer on differentiation of embryonal carcinoma cells. Nature. 1987 Jul 16;328(6127):254–256. doi: 10.1038/328254a0. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lech K., Anderson K., Brent R. DNA-bound Fos proteins activate transcription in yeast. Cell. 1988 Jan 29;52(2):179–184. doi: 10.1016/0092-8674(88)90506-5. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., Glaichenhaus N., Gesnel M. C., Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 1985 Jun;4(6):1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. L., Henning-Chubb C., Huberman E., Verma I. M. c-fos expression is neither sufficient nor obligatory for differentiation of monomyelocytes to macrophages. Cell. 1986 May 23;45(4):497–504. doi: 10.1016/0092-8674(86)90281-3. [DOI] [PubMed] [Google Scholar]
- Montarras D., Pinset C. Proto-oncogenes. Biochimie. 1987 Mar;69(3):171–176. doi: 10.1016/0300-9084(87)90042-3. [DOI] [PubMed] [Google Scholar]
- Mougneau E., Lemieux L., Rassoulzadegan M., Cuzin F. Biological activities of v-myc and rearranged c-myc oncogenes in rat fibroblast cells in culture. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5758–5762. doi: 10.1073/pnas.81.18.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Selinger Z., Scolnick E. M., Bassin R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J. D., Cook F., Roberts P. C., Clewley J. P., Avery R. J. Expression of Kirsten murine sarcoma virus in transformed nonproducer and revertant NIH/3T3 cells: evidence for cell-mediated resistance to a viral oncogene in phenotypic reversion. J Virol. 1984 May;50(2):439–444. doi: 10.1128/jvi.50.2.439-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo S., Yamada E., Endo T., Itoh H., Hidaka H. Vitamin A acid-induced activation of Ca2+-activated, phospholipid-dependent protein kinase from rabbit retina. Biochem Biophys Res Commun. 1984 Jan 30;118(2):460–466. doi: 10.1016/0006-291x(84)91325-1. [DOI] [PubMed] [Google Scholar]
- Ohlsson R. I., Pfeifer-Ohlsson S. B. Cancer genes, proto-oncogenes, and development. Exp Cell Res. 1987 Nov;173(1):1–16. doi: 10.1016/0014-4827(87)90327-2. [DOI] [PubMed] [Google Scholar]
- Owen R. D., Ostrowski M. C. Rapid and selective alterations in the expression of cellular genes accompany conditional transcription of Ha-v-ras in NIH 3T3 cells. Mol Cell Biol. 1987 Jul;7(7):2512–2520. doi: 10.1128/mcb.7.7.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B., Rassoulzadegan M. Distinct transformation phenotypes induced by polyoma virus and simian virus 40 in rat fibroblasts and their control by an early viral gene function. J Virol. 1980 Feb;33(2):697–707. doi: 10.1128/jvi.33.2.697-707.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Hirai S., Yaniv M. Constitutive synthesis of activator protein 1 transcription factor after viral transformation of mouse fibroblasts. Proc Natl Acad Sci U S A. 1988 May;85(10):3401–3405. doi: 10.1073/pnas.85.10.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Yaniv M. Two different factors bind to the alpha-domain of the polyoma virus enhancer, one of which also interacts with the SV40 and c-fos enhancers. EMBO J. 1987 May;6(5):1331–1337. doi: 10.1002/j.1460-2075.1987.tb02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Prywes R., Roeder R. G. Purification of the c-fos enhancer-binding protein. Mol Cell Biol. 1987 Oct;7(10):3482–3489. doi: 10.1128/mcb.7.10.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Perbal B., Cuzin F. Growth control in simian virus 40-transformed rat cells: temperature-independent expression of the transformed phenotype in tsA transformants derived by agar selection. J Virol. 1978 Oct;28(1):1–5. doi: 10.1128/jvi.28.1.1-5.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Sambucetti L. C., Curran T., Distel R. J., Spiegelman B. M. Common DNA binding site for Fos protein complexes and transcription factor AP-1. Cell. 1988 Feb 12;52(3):471–480. doi: 10.1016/s0092-8674(88)80039-4. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Rüther U., Wagner E. F., Müller R. Analysis of the differentiation-promoting potential of inducible c-fos genes introduced into embryonal carcinoma cells. EMBO J. 1985 Jul;4(7):1775–1781. doi: 10.1002/j.1460-2075.1985.tb03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. The DNA-binding domains of the jun oncoprotein and the yeast GCN4 transcriptional activator protein are functionally homologous. Cell. 1987 Sep 11;50(6):841–846. doi: 10.1016/0092-8674(87)90511-3. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y., Whitman M., Cantley L. C., Erikson R. L. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2117–2121. doi: 10.1073/pnas.81.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Identification and purification of a polypeptide that binds to the c-fos serum response element. EMBO J. 1987 Sep;6(9):2711–2717. doi: 10.1002/j.1460-2075.1987.tb02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Bos T. J., Doolittle R. F. Homology between the DNA-binding domain of the GCN4 regulatory protein of yeast and the carboxyl-terminal region of a protein coded for by the oncogene jun. Proc Natl Acad Sci U S A. 1987 May;84(10):3316–3319. doi: 10.1073/pnas.84.10.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Imler J. L., Perez-Mutul J., Wasylyk B. The c-Ha-ras oncogene and a tumor promoter activate the polyoma virus enhancer. Cell. 1987 Feb 13;48(3):525–534. doi: 10.1016/0092-8674(87)90203-0. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Wasylyk B. The immunoglobulin heavy-chain B-lymphocyte enhancer efficiently stimulates transcription in non-lymphoid cells. EMBO J. 1986 Mar;5(3):553–560. doi: 10.1002/j.1460-2075.1986.tb04246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. The action of oncogenes in the cytoplasm and nucleus. Science. 1985 Nov 15;230(4727):770–776. doi: 10.1126/science.2997917. [DOI] [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]
- Zarbl H., Latreille J., Jolicoeur P. Revertants of v-fos-transformed fibroblasts have mutations in cellular genes essential for transformation by other oncogenes. Cell. 1987 Nov 6;51(3):357–369. doi: 10.1016/0092-8674(87)90632-5. [DOI] [PubMed] [Google Scholar]
- van der Hoorn F. A., Müller V. Differential transformation of C3H10T1/2 cells by v-mos: sequential expression of transformation parameters. Mol Cell Biol. 1985 Sep;5(9):2204–2211. doi: 10.1128/mcb.5.9.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]