Abstract
Due to the aging and increasingly complex nature of our patients, frailty has become a high-priority theme in cardiovascular medicine. Despite the recognition of frailty as a pivotal element in the evaluation of older adults with cardiovascular disease (CVD), there has yet to be a road map to facilitate its adoption in routine clinical practice. Thus, we sought to synthesize the existing body of evidence and offer a perspective on how to integrate frailty into clinical practice. Frailty is a biological syndrome that reflects a state of decreased physiological reserve and vulnerability to stressors. Upward of 20 frailty assessment tools have been developed, with most tools revolving around the core phenotypic domains of frailty—slow walking speed, weakness, inactivity, exhaustion, and shrinking—as measured by physical performance tests and questionnaires. The prevalence of frailty ranges from 10% to 60%, depending on the CVD burden, as well as the tool and cutoff chosen to define frailty. Epidemiological studies have consistently demonstrated that frailty carries a relative risk of >2 for mortality and morbidity across a spectrum of stable CVD, acute coronary syndromes, heart failure, and surgical and transcatheter interventions. Frailty contributes valuable prognostic insights incremental to existing risk models and assists clinicians in defining optimal care pathways for their patients. Interventions designed to improve outcomes in frail elders with CVD such as multidisciplinary cardiac rehabilitation are being actively tested. Ultimately, frailty should not be viewed as a reason to withhold care but rather as a means of delivering it in a more patient-centered fashion.
Keywords: cardiovascular disease, elderly, frailty
Frailty, from the French frêle meaning of little resistance, is a biological syndrome that reflects a state of decreased physiological reserve and vulnerability to stressors (1). Stressors are broadly classified as acute or chronic illness (e.g., myocardial infarction) or iatrogenic (e.g., cardiac surgery). When exposed to such stressors, frail patients are at risk for marked and often disproportionate decompensation, adverse events, procedural complications, prolonged recovery, functional decline, disability, and mortality (2).
Frailty has become a high-priority theme in cardiovascular medicine due to the aging and increasingly complex nature of our patients (3). Evolving technical innovations have enabled clinicians to treat a wider array of patients with devices and procedures, many of whom were previously regarded as “ineligible” (4,5). Uncertainty regarding individual benefit from such treatments has been coupled with growing economic constraints on healthcare systems, such that the issue of appropriate patient selection has intensified. There is an unmet need to optimize resource allocation to prevent patients from receiving costly but futile interventions.
Assessment of frailty is instrumental to refine estimates of risk and guide patients toward personalized treatment plans that will maximize their likelihood of a positive outcome. For example, given 2 heart failure patients with similar chronological age and comorbidities, the presence of objectively-measured frailty alerts the clinician that 1 of the 2 patients has a substantially higher risk of mortality and major morbidity. Furthermore, the frail patient faces a higher risk from invasive procedures but also a potential benefit from interventions such as cardiac rehabilitation to counteract the physical weakness characteristic of frailty. A critical mass of clinicians, researchers, and policy makers have embraced the concept of frailty, yet the lack of a scientific road map to integrate frailty into practice has been a limiting factor.
The objectives of this state-of-the-art paper are to: 1) summarize the existing body of evidence for frailty in patients with cardiovascular disease (CVD); 2) offer a perspective on integrating frailty into current clinical practice; and 3) point out the knowledge gaps for future research.
Pathobiology of Frailty
Frailty biology is a field of ongoing research and debate (6). Putative mechanisms revolve around dysregulation of the immune, hormonal, and endocrine systems (7)—notably, up-regulation of inflammatory cytokines (8–10), decreased testosterone levels (11,12), and insulin resistance (13). This leads to a catabolic milieu, in which muscle breakdown exceeds muscle building, leading to a progressive decline in muscle mass and strength (sarcopenia) (9,14). Under stressed conditions, subclinical impairments are unmasked, and a vicious cycle ensues with physical inactivity and malnutrition leading to further decline (15,16) (Fig. 1).
Figure 1. Two of the Pathways Leading Toward the Phenotype of Frailty.
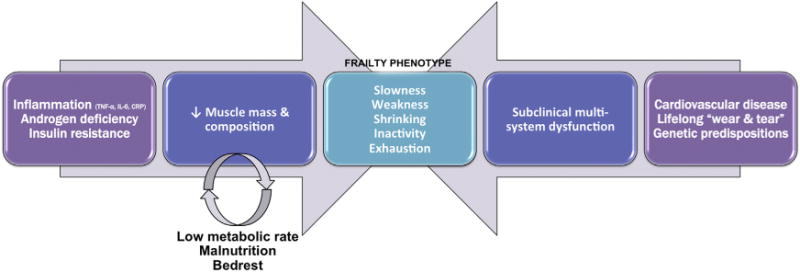
(Left) The age-associated activation of inflammatory cells and decline in androgen hormones upset the balance between catabolic and anabolic stimuli, respectively, leading to a decline in muscle mass and composition known as sarcopenia. This detrimental response is aggravated in patients with insulin resistance and metabolic syndrome. Addition of bed rest and malnutrition initiates a vicious cycle of further decline in muscle mass, limiting the necessary mobilization of amino acids in times of stress. (Right) The accumulation of subclinical impairments in multiple organ systems resulting from cardiovascular disease, lifelong “wear and tear,” and/or genetic predispositions lead to decreased homeostatic reserve and resiliency to stressors. Other pathophysiological pathways have been proposed. Biological pathways may manifest clinically as slow walking speed, weakness, weight loss, physical inactivity, and exhaustion—termed the phenotype of frailty. CRP = C-reactive protein; IL = interleukin; TNF = tumor necrosis factor.
The pathobiology of frailty and CVD shares several commonalities, particularly a consistent correlation with the inflammatory biomarkers interleukin 6 and C-reactive protein. Just as immune cells and cytokines exert nefarious effects on the arterial wall to promote atherosclerosis, so too do they impact cellular senescence and body composition to promote frailty. Moreover, by causing impairments in multiple organ systems, subclinical CVD is one of the important contributors to frailty (17). This biological link frames the epidemiological data, showing that frailty and CVD coexist in a large number of individuals (18).
Frailty Assessment Tools
Upward of 20 frailty tools have been developed to measure frailty (19); owing to a lack of consensus agreement, there is variability among studies and confusion on which tool to use. Most tools focus on 1 or more of the 5 core domains that define the frailty phenotype: slowness, weakness, low physical activity, exhaustion, and shrinking. Slowness is measured by a comfortable-pace gait speed test, weakness by a maximal handgrip strength test (using a dynamometer), and other domains by questionnaire or more specialized instruments. These domains may be considered individually or combined into a variety of scales (Table 1).
Table 1.
Recommended Frailty Assessment Tools
| Domain | Tool(s) | Operational Definition | Common Cutoffs for Frailty |
|---|---|---|---|
| Slowness | 5-m gait speed test | Patient is positioned behind start line and asked to walk at a comfortable pace past 5-m finish line; cue to trigger stopwatch is first footfall after start line and first footfall after finish line; repeated 3 times and averaged | Extremely slow: <0.50 m/s (>10 s) Very slow: <0.65 m/s (>7.7 s) Slow: <0.83 m/s (>6s) |
| Weakness | Handgrip strength test | Patient is asked to squeeze a handgrip dynamometer as hard as possible; repeated 3 times (once with each hand and then with strongest hand); maximum value is recorded | Men: <30 kg Women: <20 kg |
| Knee extensor strength test | Patient is seated on the dynamometer machine and asked to extend his/her knee against resistance; maximum isotonic force is recorded | Frailty cutoffs not yet established | |
| Low physical activity | Physical activity questionnaire | Many questionnaires have been validated; those that provide a measure of activity in kcal/week are recommended (e.g., Minnesota Leisure Time Activity, PASE, Paffenbarger Physical Activity Questionnaire) | Men: <383 kcal/week Women: <270 kcal/week |
| Portable accelerometer | Patient is asked to wear a portable accelerometer for a period of 1 to 7 days; total kcal expenditure is recorded | Frailty cutoffs not yet established | |
| Exhaustion | CES-D questionnaire | Patient is asked 2 questions: How often in the past week did you feel like everything you did was an effort?/like you could not get going? (often [i.e., ≥3 days] or not often [i.e., 0–2 days]) | Positive if often is the answer to either question |
| Anergia questionnaire | Patient is asked 7 questions pertaining to lack of energy over the past month | Positive if major criterion “sits around a lot for lack of energy” + any 2 of 6 minor criteria | |
| Shrinking | Weight loss | Self-reported or measured unintentional weight change not due to dieting or exercise | ≥10 lbs in past year |
| Appendicular muscle mass | Measured muscle mass in arms and legs using a dual-energy x-ray absorptiometry scan | Frailty cutoffs not yet established; general cutoffs >2 SD from controls Men: ≤7.23 kg/m2 Women: ≤5.67 kg/m2 |
|
| Serum albumin | Measured serum albumin | ≤3.3 g/dl |
| Scale | Components | Operational Definition | Scoring |
|---|---|---|---|
| Short Physical Performance Battery | Balance test | Patient is asked to stand in semitandem position for 10 s; if patient is able, then he/she is asked to stand in full tandem position for 10 s; if patient is not able, then he/she is asked to stand in side-by-side position for 10 s | 0 = side by side 0–9 s or unable 1=side by side 10 s 2 = full tandem 0–2 s 3 = full tandem 3–9 s 4 = full tandem 10 s |
| Chair rise test | Patient is seated on a straight-backed chair and asked to stand up 5 times as quickly as possible with arms folded across his/her chest; time to complete 5 chair rises is recorded (cue to stop stopwatch is when patient is standing after fifth chair rise) | 0 = unable 1 = ≥16.7 s 2 = 13.7–16.6 s 3 = 11.2–13.6 s 4 = ≤11.1 s |
|
| 5-m gait speed test | As described above | 0 = unable to walk 5 m 1 = ≥11.6 s (≤0.43 m/s) 2 = 8.3–11.5 s (0.44–0.60 m/s) 3 = 6.5–8.2 s (0.61–0.77 m/s) 4 = ≤6.4 s (≥0.78 m/s) Each item is scored 0–4 Frail if Composite score ≤5/12 |
|
| Fried scale | 5-m gait speed test Handgrip strength test Physical activity questionnaire CES-D questionnaire Weight loss |
As described above | Each item is scored 0–1 Frail if Composite score ≥3/5 |
CES-D = Center for Epidemiologic Studies Depression Scale; PASE = Physical Activity Scale for the Elderly.
The Fried scale (20) encompasses slowness, weakness, low physical activity, exhaustion, and shrinking (unintentional weight loss), with ≥3 of 5 criteria required for a diagnosis of frailty. This is the most frequently cited frailty scale and has been demonstrated to predict mortality and disability in large cohorts of community-dwelling elders and patients with CVD. Whether cognition and mood should be considered as the sixth and seventh domains of frailty or as modulating factors (i.e., catalyzing the transition from frailty to overt disability) remains an area of discussion (1,21).
The Short Physical Performance Battery (SPPB) (22,23) encompasses slowness, weakness, and balance. This is measured by a series of 3 timed physical performance tests (gait speed, chair rises, and tandem balance), each is scored 1 to 4 and a total score ≤5 of 12 is required for a diagnosis of frailty.
In contrast to these multi-item frailty scales, 5-m gait speed, and to a lesser extent handgrip strength, has been advocated as a single-item measure of frailty (24–26) that often outperforms more elaborate and time-consuming scales. The gait speed test has been shown to have excellent inter-rater reliability (intraclass coefficient 0.88 to 0.96) and test-retest reliability (intraclass coefficient 0.86 to 0.91) (27). It is responsive to change, with meaningful improvements in gait speed (estimated at 0.05 to 0.2 m/s [28,29]) predicting positive outcomes on a population level (30) but not necessarily an individual patient level (31). The walking distance has varied between 3 and 10 m, although the distance has little effect on measured speed (32). The 5-m distance has been adopted by large registries and is a good balance between allowing patients to achieve a steady walking speed without eliciting cardiopulmonary symptoms. The short distance and comfortable pace are well below cardiopulmonary limitations, making the focus of this test different than a typical stress test or 6-min walk test.
The aforementioned tools reflect the clinical phenotype of frailty; another school of thought reflects the accumulation of deficits (33). Deficits encompass an assortment of up to 70 symptoms, signs, comorbidities, disabilities, and frailty traits, which are counted and summed. A simplified bedside version has been developed (34). The International Academy on Nutrition and Aging Frailty Task Force (35) favored the clinical phenotype approach, stating that comorbidities and disabilities should be disentangled from frailty.
Disabilities, broadly defined as difficulty or dependency in carrying out activities of daily living (ADL) or instrumental ADL, are erroneously interchanged with “frailty” in many instances. However, disability is more correctly conceptualized as an adverse outcome associated with frailty (e.g., a frail patient becomes disabled after a myocardial infarction) or as a separate entity altogether (e.g., a nonfrail patient becomes disabled after a motor vehicle accident).
Patient heterogeneity precludes the use of a “one size fits all” scale and cutoff for frailty. There is a ceiling effect when physical performance scales such as the SPPB are administered to healthier individuals (more challenging versions are available) (36), and conversely there is a floor effect when the scales are administered to debilitated hospitalized patients (up to 30% have a score of 0). Certain scales may be effective to screen for frailty, whereas others may be required to focus on specific and potentially treatable domains. There is justifiable reason to consider various scales, more/less challenging variants of such scales, or different cutoffs to define frailty depending on the population being studied.
Frailty in CVD: Current Body of Evidence
The prevalence of frailty in community-dwelling older adults is estimated to be 10% (37), and depending on the population studied and the frailty assessment tool used, rises to 10% to 60% in older adults with CVD (18). In CVD, frailty confers a 2-fold increase in mortality, an effect that persists even after adjustment for age and comorbidities. The relevance and impact of frailty has been demonstrated across a broad spectrum, including: 1) stable CVD; 2) subclinical CVD; 3) heart failure; 4) coronary syndromes; 5) cardiac surgery; and 6) transcatheter aortic valve replacement (TAVR). These studies are outlined in Table 2 and are discussed in the following text.
Table 2.
Systematic Review of Frailty in Cardiovascular Disease
| Study | N | Design | Frailty Tool | % Frail | Main Outcome(s) for Frail vs. Nonfrail |
|---|---|---|---|---|---|
| Community dwelling | |||||
| Studenski, 2011 (42) | 34,485 | Meta-analysis of elderly in the community | Gait speed (2.4–6 m) | 32% | 12-year mortality: HR: 0.90 (95% CI: 0.89–0.91) per 0.1-m/s increase in gait speed |
| Dumurgier, 2009 (25) (Three-City Study) | 3,208 | Prospective cohort of elderly in the community | Fast-pace gait speed (6 m) | Lowest third | 5.1-yearmortality: 19% vs.10%;HR: 1.4 (95% CI: 1.0–2.0) *HR: 2.92 for cardiovascular mortality vs. HR: 1.03 for cancer mortality |
| Corti, 1996 (102) | 4,116 | Prospective, multicenter cohort of elderly in the community | Inability to walk 0.5 miles or 1 flight of stairs | 25% | 4-year CAD mortality: Men 3.5%/yr vs. 1.3%/yr; RR: 1.8 (95% CI: 1.1–3.0) Women 1.9%/yr vs. 0.6%/yr; RR: 2.2 (95% CI: 1.5–3.5) 4-year incident CAD: Men 5.8% vs. 4.5% per yr; RR: 1.2 (95% CI: 0.7–2.1) Women 5.1% vs. 2.5% per yr; RR: 1.6 (95% CI: 1.3–2.1) |
| Chin A Paw, 1999 (103) | 450 | Prospective, multicenter cohort of elderly men in the community | Chin A Paw scale | 13% | Prevalent CVD: 62% vs. 28% 3-year mortality: 50% vs. 18%; OR: 4.1 (95% CI: 1.8–9.4) |
| Klein, 2005 (104) | 2,515 | Prospective, multicenter cohort of elderly and nonelderly in the community | Klein scale (level 1–4) | 53–64 yrs: 0.7% 65–74 yrs: 5%(br/75–84 yrs: 22% ≥85 yrs: 53% |
Prevalent CVD: Men: OR: 1.33 (95% CI: 1.06–1.67) per level Women: OR: 1.43 (95% CI: 1.13–1.82) per level 4-year mortality: HR: 1.56 (95% CI: 1.27–1.92) per level |
| Woods, 2005 (38) (Women’s Health Initiative Observational Study) | 40,657 | Prospective, multicenter cohort of elderly women in the community | Modified Fried scale ≥3 | 16% | Prevalent frailty with vs. without CAD: 17% vs. 7% Incident frailty with vs. without CAD: 12% vs. 5%; OR: 1.40 (95% CI: 1.11–1.76) 5.9-year mortality: OR: 1.71 (95% CI: 1.48–1.97) |
| Chaves, 2005 (105) (Women’s Health and Aging Studies I & II) | 670 | Prospective, multicenter cohort of elderly women in the community | Fried scale ≥3 | 14% | Prevalent CVD: 41% vs. 21% |
| Bandeen-Roche, 2006 (106) (Women’s Health and Aging Studies I & II) | 786 | Prospective, multicenter cohort of elderly women in the community | Fried scale ≥3 | 11% | 3-year mortality: HR: 6.0 (95% CI: 3.0–12.1) 3-year severe ADL disability: HR: 15.8 (95% CI: 5.8–42.8) 3-year nursing home placement: HR: 24.0 (95% CI: 4.5–129.2) |
| Newman, 2006 (39) (Health ABC Study) | 3,075 | Prospective, multicenter cohort of elderly in the community | Gait speed (400 m) | N/A | Incident CVD: slowest Q 3.6%/yr vs. fastest Q 2.8%/yr; HR: 1.61 (95% CI: 1.05–2.45) 5-year mortality: slowest Q 4.0%/yr vs. fastest Q 1.4%/yr; HR: 3.23 (95% CI: 2.11–4.94) |
| Subclinical CVD | |||||
| Newman, 2001 (43) (Cardiovascular Health Study) | 4,735 | Cross-sectional study of elderly in the community | Fried scale ≥3 | 6% | Prevalent clinical CVD: 38% vs. 17%; OR: 2.79 (95% CI: 2.12–3.67) Prevalent subclinical CVD: RWMA, LVH, pre-HTN, low ABI, carotid stenosis, silent CVA |
| Elbaz, 2005 (44) | 2,572 | Prospective, multicenter cohort of elderly and nonelderly in the community | Fast pace gait speed (6 m) | Lowest third | CIMT >0.785 mm: mean gait speed 1.47 m/s (vs. 1.61 m/s in CIMT ≤0.6 mm); OR: 1.9 (95% CI: 1.4–2.8) Carotid plaques: mean gait speed 1.50 m/s (vs. 1.57 m/s in no plaque group); OR: 1.3 (95% CI: 1.0–1.7) |
| Singh, 2012 (40) (NHANES) | 3,571 | Prospective, multicenter cohort of elderly in the community, focus on those with PAD | Modified Fried scale ≥3 | 6.4% all 17.5% ABI <0.9 (PAD) |
Prevalent frailty with vs. without PAD: 18% vs. 5%; OR: 2.31 (95% CI: 1.08–4.94) 4.9-year mortality in PAD patients: 52% vs. 21%; HR: 2.88 (95% CI: 1.40–5.96) 4.9-year CVD mortality in PAD patients: 29% vs. 6%; HR: 11.02 (95% CI: 3.41–35.60) |
| McDermott, 2008 (41) (Walking and Leg Circulation Study) | 444 | Prospective multicenter cohort of patients with PAD (ABI <0.9) | Gait speed <0.76 m/s (4 m); SPPB | Lowest quartile | 4.8-year mortality: HR: 1.87 (95% CI: 1.06–3.30) 4.8-year CVD mortality: HR: 2.59 (95% CI: 1.04–6.44) |
| Cardiac surgery | |||||
| Afilalo, 2010 (61) (Frailty ABCs Study) | 131 | Prospective, multicenter cohort of elderly patients undergoing cardiac surgery | Gait speed <0.83 m/s (5 m) (i.e., >6 s to walk 5 m) | 46% | In-hospital mortality/morbidity: 35% vs. 13%; OR: 3.05 (95% CI: 1.23–7.54) Discharge to facility: 46% vs. 20%; OR: 3.19 (95% CI: 1.40–8.41) |
| Afilalo, 2012 (65) (Frailty ABCs Study) | 152 | Prospective, multicenter cohort of elderly patients undergoing cardiac surgery | Gait speed <0.83 m/s (5 m) Fried scale ≥3 Expanded Fried ≥3 MSSA subdimensions |
46% 20% |
In-hospital mortality/morbidity: Gait speed: AUC 0.68 Fried: AUC 0.60 Expanded Fried: AUC 0.58 MSSA subdimensions: AUC 0.56 |
| Lee, 2010 (62) | 3,826 | Retrospective cohort of elderly and nonelderly patients undergoing cardiac surgery | Ambulation dependence, ADL disability, or diagnosis of dementia | 4% | In-hospital mortality: 15% vs. 5%; OR: 1.8 (95% CI:1.1–3.0) 2-year mortality: 30% vs. 11%; OR: 1.5 (95% CI: 1.1–2.2) Discharge to facility: 49% vs. 9%; OR: 6.3 (95% CI: 4.2–9.4) |
| Sündermann, 2011 (63) | 400 | Prospective cohort of elderly patients undergoing cardiac surgery | CAF score ≥11 | 50% (45% moderate, 9% severe) | 30-day mortality: 10% vs. 4%; AUC 0.71 |
| Sündermann, 2011 (64) | 213 | Prospective cohort of elderly patients undergoing cardiac surgery | CAF score ≥11 | 54% (43% moderate, 8% severe) | 1-year mortality: OR: 1.11 (95% CI: 1.04–1.16) per point |
| Robinson, 2011 (74) | 223 | Prospective cohort of elderly patients undergoing major surgery (34% cardiac surgery) | Timed up-and-go ≥15 s | 30% | Discharge to facility: 67% vs. 8%; OR: 13.0 (95% CI: 5.1–33.0) |
| Lee, 2011 (107) | 262 | Prospective cohort of elderly patients undergoing abdominal aortic aneurysm surgery | Cross-sectional area of psoas muscles at L4 by computed tomography | N/A | 90-day mortality: HR: 0.33 (95% CI: 0.16–0.68) per 1,000-mm2 increase in muscle area 1-year mortality: 9% tertile 1 vs. 5% tertile 3 3-year mortality: 21% tertile 1 vs. 13% tertile 3 |
| TAVI | |||||
| Rodés-Cabau, 2010 (68) | 345 | Retrospective, multicenter cohort of patients undergoing TAVI | Subjective judgment of treating physician | 25% | Procedural complications: no differences except need for dialysis 7% vs. 1% (p = 0.009) 30-day mortality: 8% vs. 11% (p = 0.54) 8-month mortality: 22% vs. 22% (p = 1.00) |
| Ewe, 2011 (67) | 147 | Prospective, multicenter cohort of patients undergoing TAVI | Fried scale ≥3 | 33% | 9-month mortality/morbidity: HR: 4.2 (95% CI: 2.0–8.8) |
| Green, 2012 (69) | 102 | Cross-sectional study of TAVI (83%), high-risk AVR (11%), and medically managed AS (5%) | Gait speed <0.5 m/s (4.6 m) | 63% | Prevalent ADL disability: OR: 1.52 (95% CI: 1.21–1.91) per 0.1 m/s; AUC 0.81 |
| Green, 2012 (70) | 159 | Prospective cohort of patients undergoing TAVI | Modified Fried scale >median | 50% | 30-day mortality/morbidity: nonsignificant 1-year mortality: 17% vs. 7%; HR: 3.51 (95% CI: 1.43–8.62) |
| Schoenenberger, 2012 (71) | 119 | Prospective cohort of patients undergoing TAVI | In-house scale ≥3/7 | 50% | 6-month ADL change ≥1: 31.3% vs. 12.1% (OR: 3.34 for functional decline; OR: 4.21 for functional decline or death, adjusted for STS) 6-month mortality: 18.6% vs. 3.3% |
| Stortecky, 2012 (72) | 100 | Prospective cohort of patients undergoing TAVI (same cohort as Schoenenberger) | In-house scale ≥3/7 | 49% | 1-year mortality: OR: 2.93 (95% CI: 0.93–9.24) 1-year major cardiovascular and cerebral events: OR: 4.89 (95% CI: 1.64–14.60); both adjusted for STS |
| Coronary disease | |||||
| Purser, 2006 (55) | 309 | Prospective cohort of elderly patients with severe CAD admitted to cardiac unit | Fried scale ≥3 Rockwood scale ≥1 Gait speed <0.65 m/s Grip strength <25 kg Chair rise <7/30 s |
27% 63% 50% 50% 56% |
6-month mortality: Fried: 12% vs. 8%; OR: 1.9 (95% CI: 0.6–6.0) Rockwood: 11% vs. 5%; OR: 1.4 (95% CI: 0.3–5.6) Gait speed: 14% vs. 4%; OR: 4.0 (95% CI: 1.1–13.8) Grip strength: 13% vs. 5%; OR: 2.7 (95% CI: 0.7–10.0) Chair rise: 12% vs. 5%; OR: 1.5 (95% CI: 0.4–5.0) |
| Ekerstad, 2011 (59) | 307 | Prospective, multicenter cohort of elderly patients with NSTEMI admitted to cardiac or medical unit | CSHA Clinical Frailty Scale ≥5 | 49% | 30-day mortality/morbidity: 46% vs. 27%; OR: 2.17 (95% C1: 28–3.67) 30-day mortality: 15% vs. 3%; OR: 4.7 (95% CI: 1.7–13.0) |
| Singh, 2011 (56) | 629 | Prospective, multicenter cohort of elderly patients post-PCI | Fried scale ≥3 | 21% | 3-year mortality: 28% vs. 6%; HR: 2.74 (95% CI: 1.12–6.71) |
| Gharacholou, 2012 (58) | 629 | Cross-sectional analysis of elderly patients post-PCI (same cohort as Singh) | Fried scale ≥3 | 21% | SAQ: more physical limitation and lower QOL (despite same angina frequency) SF-36: lower PCS and MCS scores |
| McNulty, 2011 (57) | 101 | Retrospective, multicenter cohort of elderly and nonelderly patients post–left main PCI | Subjective judgment of treating physician (“cachexia/frailty”) |
7% | 1.5-year mortality: unadjusted HR: 14.0 (95% CI: 5.4–36.0) |
| Heart failure | |||||
| Cacciatore, 2005 (51) | 120 | Secondary analysis of cohort study of elderly patients with chronic heart failure | Lachs frailty staging system | 15% | 12-year mortality: 94% vs. 69%; HR: 1.62 (95% CI: 1.08–2.45) |
| Altimir, 2005 (108) | 360 | Cross-sectional study of elderly patients with chronic heart failure referred to HF clinic | Altimir scale | 42% | Prevalent frailty: 42% |
| Lupón, 2008 (47) | 622 | Prospective cohort of elderly patients with chronic heart failure referred to HF clinic | Altimir scale | 40% | MLWHFQ: 39 vs. 19 (p < 0.001) HF hospitalization: 21% vs. 13% (p = 0.01) 1-year mortality: 17% vs. 5%; HR: 2.09 (95% CI: 1.11–3.92) |
| Volpato, 2008 (52) | 92 | Prospective cohort of acute patients admitted to hospital (64% decompensated heart failure) | SPPB admission, discharge | N/A | Length of stay: +2.5–4 days for SPPB 0–4 on admission (+0.5 day for every SPPB point) |
| Volpato, 2011 (53) | 87 | Prospective cohort of acute patients admitted to hospital (64% decompensated heart failure) | SPPB admission, discharge, 1 month | N/A | Incident disability: +0.24 ADL limitations for SPPB 0–4 at discharge or SPPB decline at follow-up 1-year mortality or hospitalization: 75% vs. 57%; OR: 5.38 (95% CI: 1.82–15.9) for SPPB 0–4 vs. 5–12; HR: 3.59 (95% CI: 1.20–10.0) for SPPB decline at follow-up |
| Chiarantini, 2010 (54) | 157 | Prospective, multicenter cohort of patients with decompensated heart failure discharged from cardiac unit | SPPB | 51% | 15-month mortality: SPPB 0: 62 per 100 PY; HR: 6.06 (95% CI: 2.19–16.76) SPPB 1–4: 29 per 100 PY; HR: 4.78 (95% CI: 1.63–14.02) SPPB 5–8: 17 per 100 PY; HR: 1.95 (95% CI: 0.67–5.70) SPPB 9–12: 9 per 100 PY; HR: 1 (referent) |
| Tjam, 2012 (109) | 149 | Secondary analysis of cohort study of elderly patients with chronic heart failure living in long-term care | RAI 2.0 scale | N/A | 6-month mortality: AUC 0.87 |
| Khan, 2013 (47) (Health ABC Study) | 2,825 | Prospective, multicenter cohort of elderly in the community without baseline heart failure | Modified SPPB ≤2 | 31% | 11-year incident HF: HR: 1.30 (95% CI: 1.10–1.55) *Overall incidence 15.9% or 1.8 per 100 PY |
| Chaudhry, 2013 (110) (Cardiovascular Health Study) | 758 | Prospective, multicenter cohort of elderly in the community with newly diagnosed heart failure | Gait speed <0.8 m/s (4.6 m) Grip strength: men: <28.5 kg; women: <18.5 kg |
42% | 3.4-year hospitalization: Gait speed: adjusted HR: 1.28 (95% CI: 1.06–1.55) Grip strength: adjusted HR: 1.19 (95% CI: 1.00–1.42) |
| Rozzini, 2003 (111) | 995 | Prospective cohort of acute patients admitted to cardiac unit | Barthel ADL <90 MMSE <18 |
20% | 6-month mortality: 28% vs. 12% vs. 4% if both, either, or neither criteria present |
ABI = ankle-brachial index; ADL = activities of daily living; AS = aortic stenosis; AUC = area under curve; AVR = aortic valve replacement; CAD = coronary artery disease; CAF = Comprehensive Assessment of Frailty; CIMT = carotid intima media thickness; CSHA = Canadian Study of Health and Aging; CVA = cerebrovascular accident; CVD = cardiovascular disease; Health ABC = Health, Aging, and Body Composition; HF = heart failure; HR = hazard ratio; HTN = hypertension; LVH = left ventricular hypertrophy; MCS = mental component summary; MLWHFQ = Minnesota Living With Heart Failure Questionnaire; MMSE = Mini-Mental Status Examination; MSSA = MacArthur Study of Successful Aging; NHANES = National Health and Nutrition Examination Survey; NSTEMI = non–ST-segment elevation myocardial infarction; OR = odds ratio; PAD = peripheral artery disease; PCI = percutaneous coronary intervention; PCS = physical component summary; PY = person-years; QOL = quality of life; RAI = Resident Assessment Instrument; RR = relative risk; RWMA = regional wall motion abnormality; SAQ = Seattle Angina Questionnaire; SF-36 = Short-Form 36; SPPB = Short Physical Performance Battery; STS = Society of Thoracic Surgeons; TAVR = transcatheter aortic valve replacement.
Stable CVD in the community
Beyond the cross-sectional association between frailty and CVD, the Women’s Health Initiative Study revealed that women with coronary artery disease (CAD) were more likely to develop de novo frailty over 6 years (12% vs. 5%) (38), and the Health ABC (Health, Aging, and Body Composition) study showed that older adults with objectively-measured frailty were more likely to develop CAD events (3.6% vs. 2.8% per year) (39). Furthermore, the 3C (Three-City) Study showed that slow gait speed was highly predictive of cardiovascular mortality (hazard ratio [HR]: 2.9) but not mortality from cancer or other causes (HR: 1.0) (25). The EPESE (Established Populations for Epidemiologic Studies of the Elderly) Study similarly showed that impaired mobility was predictive of CAD-related mortality (relative risk [RR]: 1.8 to 2.2), with the RR increase being equivalent in magnitude to diabetes. In 2 studies focusing on peripheral arterial disease, frailty predicted cardiovascular mortality (HR: 2.6 to 11.0) more so than all-cause mortality (HR: 1.9 to 2.9) (40,41).
Studenski et al. (42) performed a patient-level metaanalysis of 9 large prospective studies and found that for every 0.1 m/s increase in gait speed, there was a 10% improvement in survival. Short-distance gait speed was a robust yet simple “indicator of vitality that integrates known and unrecognized disturbances in multiple organ systems many of which affect survival.” Those who walked at a speed of 0.8 m/s were predicted to reach an average life expectancy, whereas those who walked >1.0 m/s exceeded the average life expectancy (traffic signals at crosswalks are typically set at a pedestrian walking speed of 1.2 m/s, reflecting the expected lower limit for ambulatory citizens).
Subclinical CVD
Before frail patients manifest clinical CVD, they tend to exhibit subclinical cardiovascular derangements. A seminal substudy from the Cardiovascular Health Study screened for subclinical CVD in 4,735 older adults and found that those who were frail had an increased prevalence of undiagnosed/subclinical lesions: myocardial injury on echocardiography, brain infarcts on magnetic resonance imaging, abnormal ankle-brachial index, carotid stenosis, pre-hypertension, and left ventricular hypertrophy (43). A subanalysis from the 3C Study showed that those who had slow gait speed were more likely to have carotid intimal-medial thickening and silent carotid plaques (44). Subclinical CVD predisposes to “unsuccessful aging” (45), often defined as impaired physical or cognitive functioning and development of clinically manifest disease (46).
Heart failure
Frailty is pertinent to the development, manifestations, and prognosis of heart failure. Frailty may be apparent at the myocardial organ level by predisposing patients to a greater extent of myocardial injury and, thus, clinical heart failure in response to stressors such as coronary ischemia or pressure or volume overload. Alternatively, frailty may be apparent at the global multisystem level by predisposing patients with heart failure to decompensate at a lower threshold and require more frequent hospitalizations. The person-years accrued for studies of frailty in the heart failure setting are greater than those for other cardiac conditions, involving approximately 2,300 patients with heart failure and up to 12 years of follow-up.
The Health ABC Study followed 2,825 older patients free of baseline heart failure over a period of 11 years and found that frailty (as measured by a modified SPPB) conferred a 30% higher risk of developing new heart failure (47). Excluding heart failure events in the first year did not alter the results, implying that frailty was not merely capturing undiagnosed/imminent cardiac dysfunction.
Although traditionally considered a geriatric condition, frailty was found by Lupón et al. (48) in one-third of younger patients with heart failure. Because chronic heart failure is known to perturb skeletal muscle and body composition (49,50) (giving rise to the phenotype of “cardiac cachexia” in extreme cases), it is not surprising to observe a large proportion of younger and older patients with heart failure exhibiting frailty traits.
Patients with chronic heart failure who were frail had a higher risk of mortality at 1 year (17% vs. 5%), heart failure hospitalizations (21% vs. 13%), and impaired quality of life (48). Chaudhry et al. (51) showed that slow gait speed was the most powerful predictor of hospitalizations, conferring a 30% increase; weak grip strength was also predictive, conferring a 16% increase. In a long-term study by Cacciatore (52), patients with chronic heart failure who were frail had a substantially lower probability of surviving >10 years (6% vs. 31%).
Frailty is also relevant in acute decompensated heart failure. Volpato et al. (53,54) succeeded in administering the SPPB to patients with recently decompensated heart failure at different time points (shortly after admission, at discharge, and 1 month after discharge). A low SPPB score on admission was associated with prolonged length of stay, whereas a low SPPB score at discharge was associated with a higher risk of ADL disability, mortality, or readmission (odds ratio [OR]: 5.4). In a similar study by Chiarantini et al. (55), the yearly mortality rates were 62%, 45%, 17%, and 9% for SPPB scores of 0, 1 to 4, 5 to 8, and 9 to 12, respectively. The SPPB was responsive to change, with 63% improving versus 20% worsening from admission to discharge and 50% improving versus 18% worsening from discharge to 1 month.
Acute coronary syndromes and percutaneous coronary interventions
In a seminal study of 309 elderly patients admitted to a coronary care unit and found to have multivessel CAD, Purser et al. (56) found that the prevalence of frailty varied considerably depending on the tool used: 27% with the Fried scale, 50% with gait speed <0.65 m/s, and 63% with the Rockwood scale. Each tool was associated with a trend toward increased 6-month mortality, yet only gait speed was statistically significant (OR: 4.0).
In a study of 629 elderly patients who underwent percutaneous coronary intervention at the Mayo Clinic, the prevalence of frailty was 21% with the Fried scale administered before discharge, conferring a significant increase in 3-year mortality (28% vs. 6%; OR: 2.74) (57). Similarly, “cachexia/frailty” was the most powerful predictor of 18-month mortality (HR: 14.0) (58) in a study of 111 patients undergoing percutaneous coronary intervention for unprotected left main disease in the Kaiser Permanente database.
Gharacholou et al. (59) further showed that, despite a similar severity of angina between frail and nonfrail patients, those who were frail had lower physical functioning and quality of life. Frailty exerted a greater impact on quality of life than comorbidities. Ekerstad et al. (60) explored the relationship between frailty and comorbidities in patients with non–ST-segment elevation myocardial infarction and showed that 79% of frail patients had at least 1 severe comorbidity. The OR for frailty to predict mortality was exponentially higher when the comorbidity burden was moderate to severe.
The studies of Ekerstad, Purser, and Lupón all showed that frail patients were less aggressively managed compared with their nonfrail counterparts; whether this is for better or for worse remains unclear. They were less likely to receive angiotensin-converting enzyme inhibitors (71% vs. 81%) and beta-blockers (63% vs. 80%), less likely to be admitted to a coronary care unit (35% vs. 54%), and less likely to be referred for cardiac catheterization (15% vs. 46%) or coronary artery bypass surgery (9% vs. 16%).
Cardiac surgery
Cardiac surgery is an inherently relevant setting for frailty because surgery represents an iatrogenic physiological stressor to which the patient’s resiliency will determine their post-operative course. Surgeons have been performing de facto clinical frailty assessments termed the “eyeball test” or the “end of the bed-o-gram” for quite some time. More recently, investigators have examined the role of objective frailty tools to predict post-operative outcomes, and even the lay media has been attracted by this prospect (61). The utility of frailty to prospectively guide surgical decisions and improve outcomes has yet to be explicitly tested.
The Frailty ABCs (Frailty Assessment Before Cardiac Surgery) prospective study showed that slow 5-m gait speed was associated with a 3-fold increase in post-operative mortality or major morbidity (OR: 3.1) (62). A walking time of 6 s or longer (<0.83 m/s) was selected as the optimal cutoff based on receiver-operating characteristic analysis. Importantly, gait speed contributed incremental value above the Society for Thoracic Surgeons risk score (area under the curve 0.70 for risk score alone vs. area under the curve 0.74 for risk score plus gait speed). Patients with slow gait speed and a high risk score had a 43% incidence of mortality/morbidity, whereas those with normal gait speed and a low to intermediate risk score had a 6% incidence. There was a trend toward interaction for female patients and those undergoing aortic valve replacement (AVR), both of which had a markedly greater RR when frailty was present.
Studies by Lee et al. (63) and Sündermann et al. (64,65) showed that pre-operative frailty was associated with postoperative mortality at 30 days and 1 to 2 years. These 2 studies differed in the frailty scales used, and as a result, in the reported prevalence of frailty. Lee et al. (63) retrospectively reviewed data from the Maritime Heart Center Cardiac Surgery Registry and defined frailty as ambulation dependence, ADL disability, or diagnosed dementia. This definition represented disability more than frailty and yielded a low 4% prevalence of frailty (mixed elderly and nonelderly cohort). Sündermann et al. (64,65) defined frailty as an aggregate of 35 criteria, which yielded a 50% prevalence of frailty. The data from Afilalo et al. (66) showed a 46% prevalence of frailty using gait speed versus 20% using the Fried scale and a low 5% prevalence of ADL disability; the single measure of gait speed outperformed other scales in predicting outcomes.
The presence of frank disability is infrequent in the general cardiac surgery population, in part because disabled patients are less likely to be referred for such a surgery. Therefore, disability scales for basic ADL are insensitive to screen elderly patients in this context. Higher-level disability scales such as the Nagi scale are more sensitive and better predict outcomes. An interaction between frailty and disability has been reported, with the prognostic effect of frailty diminishing in patients who have progressed to the more advanced stage of disability (66).
In addition to predicting post-operative mortality and morbidity, 3 studies showed that frail patients were less likely to be discharged home and were more likely to require rehabilitation and/or institutionalization after cardiac surgery (OR: 3.2 to 13.0).
Thus, it is evident that frail patients who undergo cardiac surgery have higher rates of post-operative mortality, morbidity, prolonged length of stay, and need for discharge to facilities. It is not evident whether frail patients who undergo less invasive intervention (or no intervention) have improved outcomes, although this is at times extrapolated. For the time being, a more prudent extrapolation may be that the risks and benefits of cardiac surgery should be carefully weighed in frail patients, ideally with a multidisciplinary heart team, and if indicated, should proceed with thorough pre-operative optimization and heightened post-operative surveillance.
Transcatheter aortic valve implantation
TAVI was initially developed for patients with severe aortic stenosis (AS) who were considered “too frail for surgery;” thus, the concept of frailty has been intimately linked to TAVI. Patients referred for TAVI typically have advanced age, multiple comorbidities, and a prevalence of frailty as high as 63%. Frailty is 1 of the “missing parameters” not captured by traditional risk scores (67) that are relied upon by clinicians as gatekeepers to TAVI. Few studies have been published in the past 2 years, limited to approximately 100 to 150 patients each, and larger studies are underway.
Although this was not the primary aim of their study, Ewe et al. (68) found that one-third of patients undergoing TAVI were frail according to the Fried scale and that frailty was among the most powerful predictors of death, myocardial infarction, stroke, or heart failure at 9 months (HR: 4.2). Frailty was not a significant predictor when defined according to the physician’s subjective judgment in the earlier study by Rodés-Cabau et al. (69).
Green et al. (70,71) presented the experience at Columbia University and surprisingly showed that frailty was predictive of 1-year mortality (17% if frail vs. 7% if not frail; HR: 3.5) but not the composite of 30-day mortality or morbidity. The lack of 30-day event prediction was attributed to “adequacy of the standard selection process,” although it should be noted that there was no systematic frailty assessment on patients who had been screened out to substantiate the adequacy of the selection process and the absolute number of events was low. Furthermore, the trends toward greater risk in frail patients (especially for major bleeding, major vascular complications, and length of stay) were concerning. The increase in C-statistic from 0.73 to 0.77 and the net reclassification index of 0.24 were in the clinically meaningful range, yet confidence intervals were wide.
Between 19% and 35% of patients were unable to complete the short-distance gait speed test. This is a sizeable proportion of nonwalkers, larger than the <10% generally reported for other cardiac cohorts, which may reflect the heavy burden of comorbidity and disability in patients undergoing TAVI. Not being able to complete the gait speed test is an indicator of advanced frailty or perhaps even disability because nonwalkers have weaker grip strength, lower albumin levels, and more ADL disabilities. Low albumin levels and ADL disabilities were the strongest predictors in their TAVR cohort. A gait speed of 0.50 m/s was selected as the optimal cutoff based on receiver-operating characteristic analysis, slower than the 0.65 to 0.85 m/s cutoffs reported for other cardiac cohorts. The authors commented that >80% of their patients would have been considered frail if these traditional cutoffs had been used, supporting the notion that adapted cutoffs are required to achieve reasonable discrimination.
This is in slight contrast to the TAVI experience at Bern University (72,73), in which the vast majority of patients were able to complete the timed-up-and-go test (which requires standing up from a chair, walking 3 m, and turning around) and 61% of patients were able to do so faster than the usual cutoff of 20 s. Their frailty scale consisted of timed-up-and-go, mobility limitation, basic ADL disability, instrumental ADL disability, mini-mental status examination, and mini-nutritional assessment. Frailty was predictive of a 3- to 4-fold increase in functional decline at 6 months (measured by basic ADL disabilities) and major cardiac and cerebral adverse events at 1 year. There was a trend for frailty and all-cause mortality, which was stronger at 30 days compared with 1 year, although the number of events was small.
Synthesizing the evidence surrounding frailty in TAVI, 2 critical questions arise: first, are the standard frailty assessment tools (gait speed, even with adapted cutoffs, grip strength, and Fried scale) valid in this severely ill and often debilitated population or are these traits too ubiquitous, such that we should be relying on markers of more advanced frailty and frank disability (inability to walk, low albumin, ADL disability) to better discriminate risk? Second, does frailty increase the risk of short-term morbidity after TAVI (as it does in cardiac surgery) or does the less invasive nature of the transcatheter procedure mitigate this risk? In both cardiac surgery and TAVI, the rate of technical success remains high and the risk of intraprocedural mortality low in frail patients.
The Use of Frailty in Clinical Practice
There are many scenarios in day-to-day clinical practice in which frailty assessment can contribute valuable prognostic information and assist the clinicians in defining optimal care pathways for their patients. Ideally, frailty is not a reason to withhold care but rather a means of structuring care in a more patient-centered fashion.
A guiding principle is that frailty, disability, and comorbidity are inter-related but distinct entities (74). A second principle is that there is no definitive gold standard test for frailty, but rather an assortment of tools that reflect 1 or more domains of frailty. Multidomain tools do not necessarily provide incremental value above single-domain tools, and ease of implementation may be an important factor for adoption. A third principle is that frailty is a continuous spectrum, and specific cutoffs used to dichotomize frailty status in 1 group of patients may not be applicable in another group.
The tools recommended in Table 1 provide a (nonrestrictive) framework to improve consistency and comparability among studies. For investigators seeking to test new or modified tools, they are encouraged to also use 1 of the recommended tools as a comparator and to confirm the findings in a validation cohort before reporting.
High-yield clinical scenarios for assessment of frailty in cardiovascular medicine
CONSIDERATION FOR CARDIAC SURGERY
Frailty assessment tools should be employed in the pre-operative period; at a minimum, 5-m gait speed is a simple and powerful measure of frailty supported by prognostic data. However, it is premature to assume that frailty should determine eligibility for surgery at the individual patient level. Until data are available to prove a direct role for frailty in determining treatment, it is recommended to integrate frailty with other proven risk factors and risk models for decision making.
The timing of frailty assessment may be in the inpatient setting just before the surgery or in the outpatient setting, providing there is no intercurrent change or prolonged delay (arbitrarily >1 month) between the assessment and surgery. The choice of when to assess frailty tends to be logistically driven depending on feasibility and work flow at the given center.
Pre-operative optimization via a multidisciplinary approach is key to counteract the multiple physiological impairments (e.g., cardiac, neurological, muscular, respiratory, renal) that lead to the decreased physiological reserve characteristic of frailty (75). Establishing a heart team and involving the appropriate consultants are instrumental in this regard. Prompt recognition and treatment of complications are primordial; deconditioning and delirium are 2 complications that merit special attention because of their insidious and devastating course. Cardiac rehabilitation may potentially improve frailty, and although this has yet to be proven, may ultimately serve to facilitate surgical recovery for frail elderly patients. Patients may benefit, for example, if cardiac rehabilitation is initiated before a planned procedure and then continued afterward, with aerobic and strength training alongside nutritional and educational components.
CONSIDERATION FOR TAVI
Because patient selection continues to be a central and often challenging issue, there is hope that frailty can be used to pre-select high-risk patients with AS who are best served by TAVI rather than surgical AVR. Proving this hypothesis has not been straightforward, particularly because the majority of patients referred for TAVI are frail and the usefulness of frailty (or any other risk factor) becomes limited when it is endemic. Moreover, because the TAVR procedure induces less physiologic stress compared with surgery, it is unclear whether frailty will predict post-procedural outcomes similarly in TAVI and surgery.
The role of frailty assessment in TAVI programs may ultimately prove to be in identifying who is not frail and thus appropriate for conventional AVR. At the other end of the spectrum, the role of frailty assessment may be in identifying who is extremely frail and/or disabled and thus appropriate for medical management without intervention. The latter patient typically exhibits 1 or more features of cachexia, severe weakness, inability to ambulate, dementia, and ADL dependencies. Anecdotally, balloon aortic valvuloplasty has been used to allow for rehabilitation and improvements in heart failure as a bridge to TAVI.
STABLE OR RECENTLY STABILIZED HEART FAILURE OR CAD
Once identified in the inpatient or outpatient setting, frail patients may be excellent candidates for cardiac rehabilitation (targeting frail patients may be 1 strategy to overcome the underuse of cardiac rehabilitation in general), longitudinal heart function clinics, and comprehensive geriatric assessment (76). The latter may include evaluation by experts in nutrition, physical function, cognition, psychogeriatrics, and social support; each of which represents an area of potential vulnerability for frail patients and a blind spot for most cardiovascular practitioners who are not accustomed to dealing with these issues. This blind spot is increasingly being addressed at the educational level within cardiology curricula and continuing medical education programs.
Controversies and future research questions
Defining the optimal tool set to measure frailty is a high priority. We must first determine whether there is incremental value in using multi-item scales such as Fried as opposed to single-item measures such as 5-m gait speed (56,66,77,78). We must also determine the appropriate cutoff for each tool and patient group, particularly for gait speed (>10 cutoffs have been proposed ranging from 0.5 to 1.0 m/s). This underscores the need to validate frailty tools and cutoffs in the population of interest rather than extrapolating results from other studies.
The ongoing FRAILTY-AVR multicenter study (http://clinicaltrials.gov/ct2/show/NCT01845207?term=NCT01845207&rank=1) is comparing different frailty tools to determine which is most predictive in high-risk patients with AS undergoing AVR and TAVI. The Society for Thoracic Surgeons Adult Cardiac Surgery Database is collecting 5-meter gait speed data to define its value across a broad sample of patients undergoing cardiac surgery. The CoreValve U.S. pivotal trial and PARTNER II (Placement of Aortic Transcatheter Valves) trial have integrated frailty assessment in all eligible patients. The Silver-AMI Trial (http://clinicaltrials.gov/ct2/show/NCT01755052?term=NCT01755052&rank=1) is evaluating the impact of frailty alongside other risk factors in older adults hospitalized with acute myocardial infarction. Many other CVD trials have begun considering frailty.
There is an impetus to develop more robust frailty tools. Existing tools are limited in the measurement of physical activity and energy expenditure (79,80); portable pedometers and actigraphy-based tools are being investigated for this purpose (81). Whereas most tools capture muscle strength, muscle mass is only indirectly measured by weight loss. Weight loss is a flawed measure of muscle mass because excess adiposity may mask low muscle mass—termed “sarcopenic obesity” (82,83). In a study of elderly patients with cancer, 7.5% of patients were found to be underweight, whereas 46.8% were sarcopenic (84). Muscle mass is a predictor of frailty and functional decline (85,86) and can be reliably measured by computed tomography, magnetic resonance, or dual-isotope x-ray absorptiometry (87).
Exciting translational research is seeking to gain mechanistic insights into the pathobiology of frailty and, in doing so, is fueling the development of frailty therapeutics and elusive frailty biomarkers. Biomarkers of senescence such as telomere length are not correlated with frailty (88). Other biomarkers have been correlated with frailty but remain nonspecific: C-reactive protein, interleukin 6, tumor necrosis factor alpha, neutrophil count, D-dimer, plasminogen activator inhibitor 1, testosterone, insulin-like growth factor 1, albumin, vitamin D, lymphocyte count, and memory/naive CD8 T-cell ratio. Thus, efforts to develop a specific biomarker or panel of biomarkers for frailty have been unsuccessful to date (89).
With the accrual of diagnostic and prognostic data in CVD cohorts, we are now on the horizon of therapeutic trials to define how to best care for our frail cardiac patients (90,91). Interventions may be divided into those that: 1) direct frail patients toward less invasive therapeutic pathways; 2) monitor frail patients more closely to promptly detect and avert adverse events; 3) treat frail patients with therapies to improve their clinical or subclinical comorbidities; or 4) treat frail patients with therapies to reverse or reduce their intrinsic frailty.
A controversial question is to what extent a patient’s frailty is intrinsic or related to a specific comorbidity that can be treated (so-called “reversible” comorbidity-related frailty) (92). Some suggest that when the degree of frailty is out of proportion to the burden of comorbidity, it is intrinsic and less likely to improve after removal of the comorbidity. This suggestion is an oversimplification because the manifestations of frailty are not only influenced by comorbidity but also by a host of other modulating factors (e.g., cognition, mood, compliance, and social support).
The most widely studied interventions to improve frailty are exercise training, nutritional supplementation, testosterone replacement, and comprehensive geriatric assessment/management (93–98). Testosterone levels are associated with frailty (99), and the benefits of testosterone replacement appear to be consistent across sexes (95). Other interventions are aimed at improving the delivery and coordination of care for frail elders (100). Ideally, frailty should be identified before a cardiac intervention is imminent. Regardless of the intervention, the treatment of frail patients should emphasize patient-centered outcomes such as functional status and quality of life (101).
Conclusions
There is a substantial body of evidence to support the utility of frailty assessment in patients with diverse forms of CVD. The value of frailty as a prognostic marker is well demonstrated (with risk ratios that often exceed 2 and dwarf juxtaposed predictors in multivariable models). The value of frailty in guiding cardiovascular care and as a therapeutic target is beginning to emerge and should be expanded in future applications to improve patient outcomes. The frailty assessment tools outlined should facilitate this task by promoting a validated tool set that will allow us to compare and synthesize the results of different studies and provide a frame of reference when evaluating novel frailty markers.
Acknowledgments
Dr. Popma has received research grants from Medtronic, Boston Scientific, and Abbott; and has served on advisory boards for Boston Scientific. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations and Acronyms
- ADL
activities of daily living
- AS
aortic stenosis
- AVR
aortic valve replacement
- CAD
coronary artery disease
- CVD
cardiovascular disease
- SPPB
Short Physical Performance Battery
- TAVR
transcatheter aortic valve replacement
References
- 1.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–7. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shamliyan T, Talley KMC, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2012;12:719–36. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statisticsd2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagé M, Doucet M, Eisenberg MJ, Behlouli H, Pilote L. Temporal trends in revascularization and outcomes after acute myocardial infarction among the very elderly. CMAJ. 2010;182:1415–20. doi: 10.1503/cmaj.092053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodson JA, Maurer MS. Changing nature of cardiac interventions in older adults. Aging Health. 2011;7:283–95. doi: 10.2217/ahe.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fedarko NS. The biology of aging and frailty. Clin Geriatr Med. 2011;27:27–37. doi: 10.1016/j.cger.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulop T, Larbi A, Witkowski JM, et al. Aging, frailty and age-related diseases. Biogerontology. 2010;11:547–63. doi: 10.1007/s10522-010-9287-2. [DOI] [PubMed] [Google Scholar]
- 8.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–41. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 9.Cesari M, Leeuwenburgh C, Lauretani F, et al. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr. 2006;83:1142–8. doi: 10.1093/ajcn/83.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaap LA, Pluijm SMF, Deeg DJH, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–9. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Travison TG, Nguyen A-H, Naganathan V, et al. Changes in reproductive hormone concentrations predict the prevalence and progression of the frailty syndrome in older men: the Concord Health and Ageing in Men Project. J Clin Endocrinol Metab. 2011;96:2464–74. doi: 10.1210/jc.2011-0143. [DOI] [PubMed] [Google Scholar]
- 12.Schaap LA, Pluijm SMF, Deeg DJH, et al. Low testosterone levels and decline in physical performance and muscle strength in older men: findings from two prospective cohort studies. Clin Endocrinol. 2008;68:42–50. doi: 10.1111/j.1365-2265.2007.02997.x. [DOI] [PubMed] [Google Scholar]
- 13.Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med. 2007;167:635–41. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 14.Boirie Y. Physiopathological mechanism of sarcopenia. J Nutr Health Aging. 2009;13:717–23. doi: 10.1007/s12603-009-0203-x. [DOI] [PubMed] [Google Scholar]
- 15.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297:1772–4. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 16.Ferrucci L, Maggio M, Ceda GP, Beghi C, Valenti G, De Cicco G. Acute postoperative frailty. J Am Coll Surg. 2006;203:134–5. doi: 10.1016/j.jamcollsurg.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss CO, Hoenig HH, Varadhan R, Simonsick EM, Fried LP. Relationships of cardiac, pulmonary, and muscle reserves and frailty to exercise capacity in older women. J Gerontol A Biol Sci Med Sci. 2010;65:287–94. doi: 10.1093/gerona/glp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103:1616–21. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 19.de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JSM, Olde Rikkert MGM, Nijhuis-van der Sanden MWG. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10:104–14. doi: 10.1016/j.arr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 21.Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the Three-City Study. J Am Geriatr Soc. 2009;57:453–61. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–9. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 25.Dumurgier J, Elbaz A, Ducimetière P, Tavernier B, Alpérovitch A, Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. BMJ. 2009;339:b4460. doi: 10.1136/bmj.b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling CHY, Taekema D, de Craen AJM, Gussekloo J, Westendorp RGJ, Maier AB. Handgrip strength and mortality in the oldest old population: the Leiden 85-Plus Study. CMAJ. 2010;182:429–35. doi: 10.1503/cmaj.091278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muñoz-Mendoza CL, Cabañero-Martínez MJ, Millán-Calenti JC, Cabrero-García J, López-Sánchez R, Maseda-Rodríguez A. Reliability of 4-m and 6-m walking speed tests in elderly people with cognitive impairment. Arch Gerontol Geriatr. 2011;52:e67–70. doi: 10.1016/j.archger.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–9. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 29.Tilson JK, Sullivan KJ, Cen SY, et al. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther. 2010;90:196–208. doi: 10.2522/ptj.20090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55:1727–34. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 31.van Iersel MB, Munneke M, Esselink RA, Benraad CEM, Olde Rikkert MGM. Gait velocity and the timed-up-and-go test were sensitive to changes in mobility in frail elderly patients. J Clin Epidemiol. 2008;61:186–91. doi: 10.1016/j.jclinepi.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Graham JE, Ostir GV, Kuo Y-F, Fisher SR, Ottenbacher KJ. Relationship between test methodology and mean velocity in timed walk tests: a review. Arch Phys Med Rehabil. 2008;89:865–72. doi: 10.1016/j.apmr.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12:29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 36.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–9. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 37.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–92. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 38.Woods NF, Lacroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–30. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 39.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–26. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 40.Singh S, Bailey KR, Noheria A, Kullo IJ. Frailty across the spectrum of ankle-brachial index. Angiology. 2012;63:229–36. doi: 10.1177/0003319711413457. [DOI] [PubMed] [Google Scholar]
- 41.McDermott MM, Guralnik JM, Tian L, et al. Baseline functional performance predicts the rate of mobility loss in persons with peripheral arterial disease. J Am Coll Cardiol. 2007;50:974–82. doi: 10.1016/j.jacc.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newman AB, Gottdiener JS, Mcburnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–66. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 44.Elbaz A, Ripert M, Tavernier B, et al. Common carotid artery intimamedia thickness, carotid plaques, and walking speed. Stroke. 2005;36:2198–202. doi: 10.1161/01.STR.0000181752.16915.5c. [DOI] [PubMed] [Google Scholar]
- 45.Newman AB, Arnold AM, Naydeck BL, et al. “Successful aging”: effect of subclinical cardiovascular disease. Arch Intern Med. 2003;163:2315–22. doi: 10.1001/archinte.163.19.2315. [DOI] [PubMed] [Google Scholar]
- 46.Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237:143–9. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- 47.Khan H, Kalogeropoulos AP, Georgiopoulou VV, et al. Frailty and risk for heart failure in older adults: The health, aging, and body composition study. Am Heart J. 2013;166:887–94. doi: 10.1016/j.ahj.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lupón J, González B, Santaeugenia S, et al. Prognostic implication of frailty and depressive symptoms in an outpatient population with heart failure. Rev Esp Cardiol. 2008;61:835–42. [PubMed] [Google Scholar]
- 49.Pittman JG, Cohen P. The pathogenesis of cardiac cachexia. N Engl J Med. 1964;271:453–60. doi: 10.1056/NEJM196408272710908. [DOI] [PubMed] [Google Scholar]
- 50.Persinger R, Janssen-Heininger Y, Wing SS, Matthews DE, Lewinter MM, Toth MJ. Effect of heart failure on the regulation of skeletal muscle protein synthesis, breakdown, and apoptosis. Am J Physiol Endocrinol Metab. 2003;284:E1001–8. doi: 10.1152/ajpendo.00517.2002. [DOI] [PubMed] [Google Scholar]
- 51.Chaudhry SI, McAvay G, Chen S, et al. Risk factors for hospital admission among older persons with newly diagnosed heart failure: findings from the Cardiovascular Health Study. J Am Coll Cardiol. 2013;61:635–42. doi: 10.1016/j.jacc.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cacciatore F, Abete P, Mazzella F, et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005;35:723–30. doi: 10.1111/j.1365-2362.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 53.Volpato S, Cavalieri M, Guerra G, et al. Performance-based functional assessment in older hospitalized patients: feasibility and clinical correlates. J Gerontol A Biol Sci Med Sci. 2008;63:1393–8. doi: 10.1093/gerona/63.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volpato S, Cavalieri M, Sioulis F, et al. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66:89–96. doi: 10.1093/gerona/glq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiarantini D, Volpato S, Sioulis F, et al. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Card Fail. 2010;16:390–5. doi: 10.1016/j.cardfail.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54:1674–81. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 57.Singh M, Rihal CS, Lennon RJ, Spertus JA, Nair KS, Roger VL. Influence of frailty and health status on outcomes in patients with coronary disease undergoing percutaneous revascularization. Circ Cardiovasc Qual Outcomes. 2011;4:496–502. doi: 10.1161/CIRCOUTCOMES.111.961375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McNulty EJ, Ng W, Spertus JA, et al. Surgical candidacy and selection biases in nonemergent left main stenting: implications for observational studies. J Am Coll Cardiol Intv. 2011;4:1020–7. doi: 10.1016/j.jcin.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Gharacholou SM, Roger VL, Lennon RJ, et al. Comparison of frail patients versus nonfrail patients ≥65 years of age undergoing percutaneous coronary intervention. Am J Cardiol. 2012;109:1569–75. doi: 10.1016/j.amjcard.2012.01.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397–404. doi: 10.1161/CIRCULATIONAHA.111.025452. [DOI] [PubMed] [Google Scholar]
- 61.Span P. Who thrives after cardiac surgery? The New York Times. 2010 Dec 28;:1–3. [Google Scholar]
- 62.Afilalo J, Eisenberg MJ, Morin J-F, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–76. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 63.Lee DH, Buth KJ, Martin B-J, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–8. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 64.Sündermann S, Dademasch A, Praetorius J, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33–7. doi: 10.1016/j.ejcts.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 65.Sündermann S, Dademasch A, Rastan A, et al. One-year follow-up of patients undergoing elective cardiac surgery assessed with the Comprehensive Assessment of Frailty test and its simplified form. Interact Cardiovasc Thorac Surg. 2011;13:119–23. doi: 10.1510/icvts.2010.251884. [DOI] [PubMed] [Google Scholar]
- 66.Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222–8. doi: 10.1161/CIRCOUTCOMES.111.963157. [DOI] [PubMed] [Google Scholar]
- 67.Chikwe J, Adams DH. Frailty: the missing element in predicting operative mortality. Semin Thorac Cardiovasc Surg. 2010;22:109–10. doi: 10.1053/j.semtcvs.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 68.Ewe SH, Ajmone Marsan N, Pepi M, et al. Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. Am Heart J. 2010;160:1113–20. doi: 10.1016/j.ahj.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 69.Rodés-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–90. doi: 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 70.Green P, Woglom AE, Genereux P, et al. Gait speed and dependence in activities of daily living in older adults with severe aortic stenosis. Clin Cardiol. 2012;35:307–14. doi: 10.1002/clc.21974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Green P, Woglom AE, Genereux P, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. J Am Coll Cardiol Intv. 2012;5:974–81. doi: 10.1016/j.jcin.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI) Eur Heart J. 2012;34:684–92. doi: 10.1093/eurheartj/ehs304. [DOI] [PubMed] [Google Scholar]
- 73.Stortecky S, Schoenenberger AW, Moser A, et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. J Am Coll Cardiol Intv. 2012;5:489–96. doi: 10.1016/j.jcin.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 74.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 75.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250:449–55. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 76.Pulignano G, Del Sindaco D, Di Lenarda A, et al. Usefulness of frailty profile for targeting older heart failure patients in disease management programs: a cost-effectiveness, pilot study. J Cardiovasc Med (Hagerstown) 2010;11:739–47. doi: 10.2459/JCM.0b013e328339d981. [DOI] [PubMed] [Google Scholar]
- 77.Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2009;57:251–9. doi: 10.1111/j.1532-5415.2008.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 79.Neilson HK, Robson PJ, Friedenreich CM, Csizmadi I. Estimating activity energy expenditure: how valid are physical activity questionnaires? Am J Clin Nutr. 2008;87:279–91. doi: 10.1093/ajcn/87.2.279. [DOI] [PubMed] [Google Scholar]
- 80.Mahabir S, Baer DJ, Giffen C, et al. Comparison of energy expenditure estimates from 4 physical activity questionnaires with doubly labeled water estimates in postmenopausal women. Am J Clin Nutr. 2006;84:230–6. doi: 10.1093/ajcn/84.1.230. [DOI] [PubMed] [Google Scholar]
- 81.Maurer MS, Cuddihy P, Weisenberg J, et al. The prevalence and impact of anergia (lack of energy) in subjects with heart failure and its associations with actigraphy. J Card Fail. 2009;15:145–51. doi: 10.1016/j.cardfail.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bouchard DR, Dionne IJ, Brochu M. Sarcopenic/obesity and physical capacity in older men and women: data from the Nutrition as a Determinant of Successful Aging (NuAge)—the Quebec longitudinal study. Obesity (Silver Spring) 2009;17:2082–8. doi: 10.1038/oby.2009.109. [DOI] [PubMed] [Google Scholar]
- 83.Gallagher D, Ruts E, Visser M, et al. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000;279:E366–75. doi: 10.1152/ajpendo.2000.279.2.E366. [DOI] [PubMed] [Google Scholar]
- 84.Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91:1133S–7S. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]
- 85.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 86.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 87.Van Kan GA, Cderbaum JM, Cesari M, et al. Sarcopenia: biomarkers and imaging (International Conference on Sarcopenia Research) J Nutr Health Aging. 2011;15:834–46. doi: 10.1007/s12603-011-0365-1. [DOI] [PubMed] [Google Scholar]
- 88.Woo J, Tang NLS, Suen E, Leung JCS, Leung PC. Telomeres and frailty. Mech Ageing Dev. 2008;129:642–8. doi: 10.1016/j.mad.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 89.Simm A, Nass N, Bartling B, Hofmann B, Silber R-E, Navarrete Santos A. Potential biomarkers of ageing. Biol Chem. 2008;389:257–65. doi: 10.1515/BC.2008.034. [DOI] [PubMed] [Google Scholar]
- 90.Drey M, Pfeifer K, Sieber CC, Bauer JM. The Fried frailty criteria as inclusion criteria for a randomized controlled trial: personal experience and literature review. Gerontology. 2011;57:11–8. doi: 10.1159/000313433. [DOI] [PubMed] [Google Scholar]
- 91.Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52:625–34. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 92.Flint KM, Matlock DD, Lindenfeld J, Allen LA. Frailty and the selection of patients for destination therapy left ventricular assist device. Circ Heart Fail. 2012;5:286–93. doi: 10.1161/CIRCHEARTFAILURE.111.963215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Latham NK, Bennett DA, Stretton CM, Anderson CS. Systematic review of progressive resistance strength training in older adults. J Gerontol A Biol Sci Med Sci. 2004;59:48–61. doi: 10.1093/gerona/59.1.m48. [DOI] [PubMed] [Google Scholar]
- 94.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347:1068–74. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- 95.Toma M, McAlister FA, Coglianese EE, et al. Testosterone supplementation in heart failure: a meta-analysis. Circ Heart Fail. 2012;5:315–21. doi: 10.1161/CIRCHEARTFAILURE.111.965632. [DOI] [PubMed] [Google Scholar]
- 96.Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, Acha AA, Ostir GV. Androgen treatment and muscle strength in elderly men: a meta-analysis. J Am Geriatr Soc. 2006;54:1666–73. doi: 10.1111/j.1532-5415.2006.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wolfe RR. Optimal nutrition, exercise, and hormonal therapy promote muscle anabolism in the elderly. J Am Coll Surg. 2006;202:176–80. doi: 10.1016/j.jamcollsurg.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 98.Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–75. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 99.Baylis D, Bartlett DB, Syddall HE, et al. Immune-endocrine biomarkers as predictors of frailty and mortality: a 10-year longitudinal study in community-dwelling older people. Age (Dordr) 2013;35:963–71. doi: 10.1007/s11357-012-9396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bakker FC, Robben SHM, Olde Rikkert MGM. Effects of hospital-wide interventions to improve care for frail older inpatients: a systematic review. BMJ Qual Saf. 2011;20:680–91. doi: 10.1136/bmjqs.2010.047183. [DOI] [PubMed] [Google Scholar]
- 101.Rumsfeld JS, Alexander KP, Goff DC, et al. Cardiovascular health: the importance of measuring patient-reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127:2233–49. doi: 10.1161/CIR.0b013e3182949a2e. [DOI] [PubMed] [Google Scholar]
- 102.Corti MC, Salive ME, Guralnik JM. Serum albumin and physical function as predictors of coronary heart disease mortality and incidence in older persons. J Clin Epidemiol. 1996;49:519–26. doi: 10.1016/0895-4356(95)00562-5. [DOI] [PubMed] [Google Scholar]
- 103.Chin A, Paw MJ, Dekker JM, Feskens EJ, Schouten EG, Kromhout D. How to select a frail elderly population? A comparison of three working definitions. J Clin Epidemiol. 1999;52:1015–21. doi: 10.1016/s0895-4356(99)00077-3. [DOI] [PubMed] [Google Scholar]
- 104.Klein BE, Klein R, Knudtson MD, Lee KE. Frailty, morbidity and survival. Arch Gerontol Geriatr. 2005;41:141–9. doi: 10.1016/j.archger.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 105.Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60:729–35. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- 106.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–6. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 107.Lee JS, He K, Harbaugh CM, et al. Michigan Analytic Morphomics Group (MAMG) Frailty, core muscle size, and mortality in patients undergoing open abdominal aortic aneurysm repair. J Vasc Surg. 2011;53:912–7. doi: 10.1016/j.jvs.2010.10.111. [DOI] [PubMed] [Google Scholar]
- 108.Altimir S, Lupón J, González B, et al. Sex and age differences in fragility in a heart failure population. Eur J Heart Fail. 2005;7:798–802. doi: 10.1016/j.ejheart.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 109.Tjam EY, Heckman GA, Smith S, et al. Predicting heart failure mortality in frail seniors: comparing the NYHA functional classification with the Resident Assessment Instrument (RAI) 2.0. Int J Cardiol. 2012;155:75–80. doi: 10.1016/j.ijcard.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 110.Chaudhry SI, McAvay G, Chen S, et al. Risk factors for hospital admission among older persons with newly diagnosed heart failure: findings from the Cardiovascular Health Study. J Am Coll Cardiol. 2013;61:635–42. doi: 10.1016/j.jacc.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rozzini R, Sabatini T, Frisoni GB, Trabucchi M. Frailty is a strong modulator of heart failure-associated mortality. Arch Intern Med. 2003;163:737–8. doi: 10.1001/archinte.163.6.737-b. [DOI] [PubMed] [Google Scholar]


