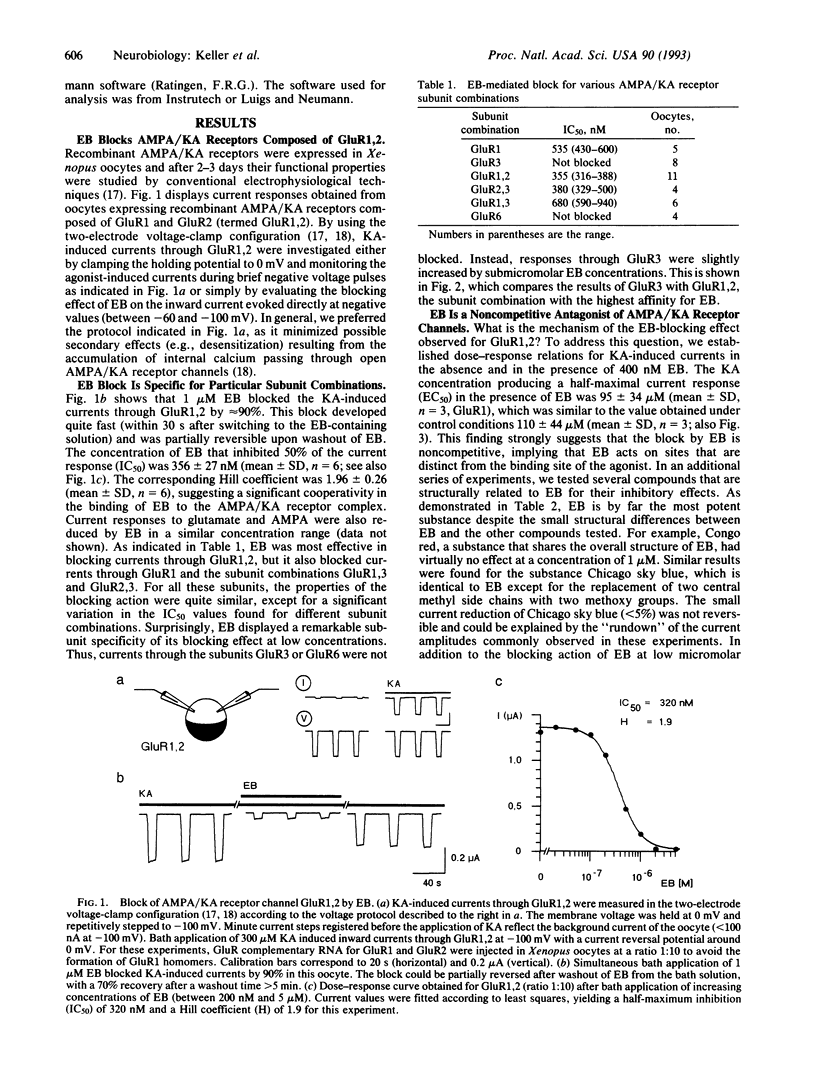
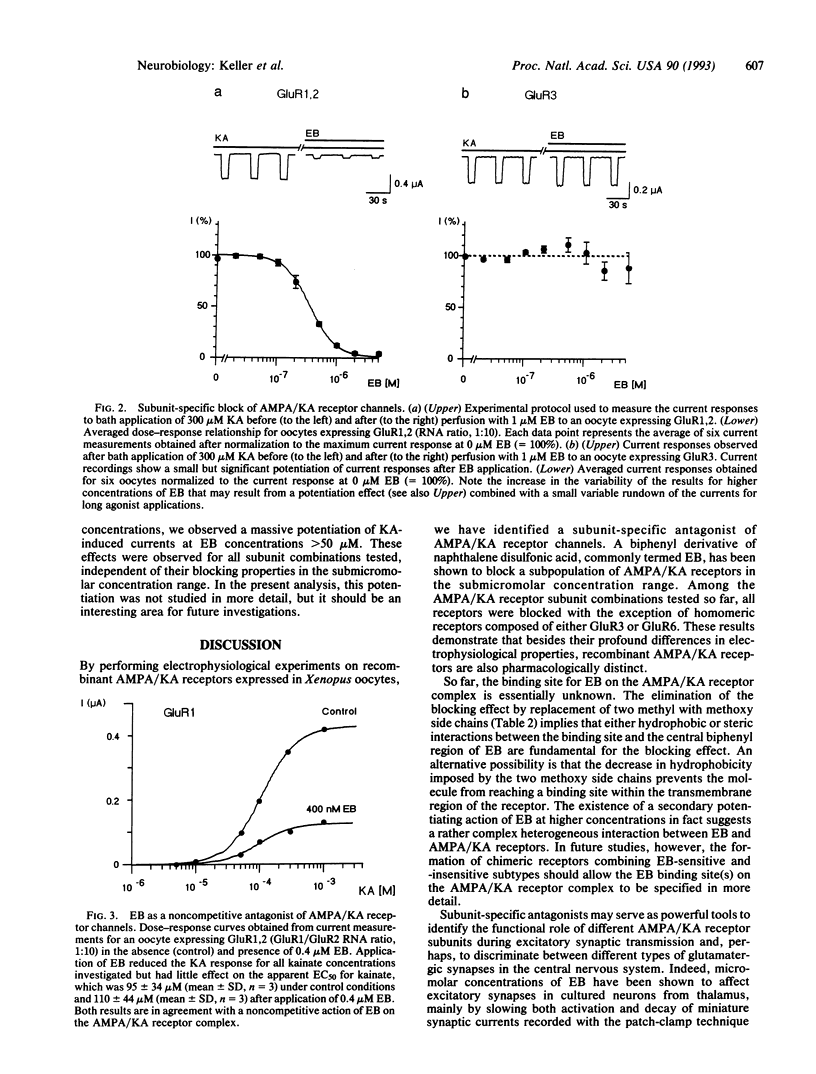
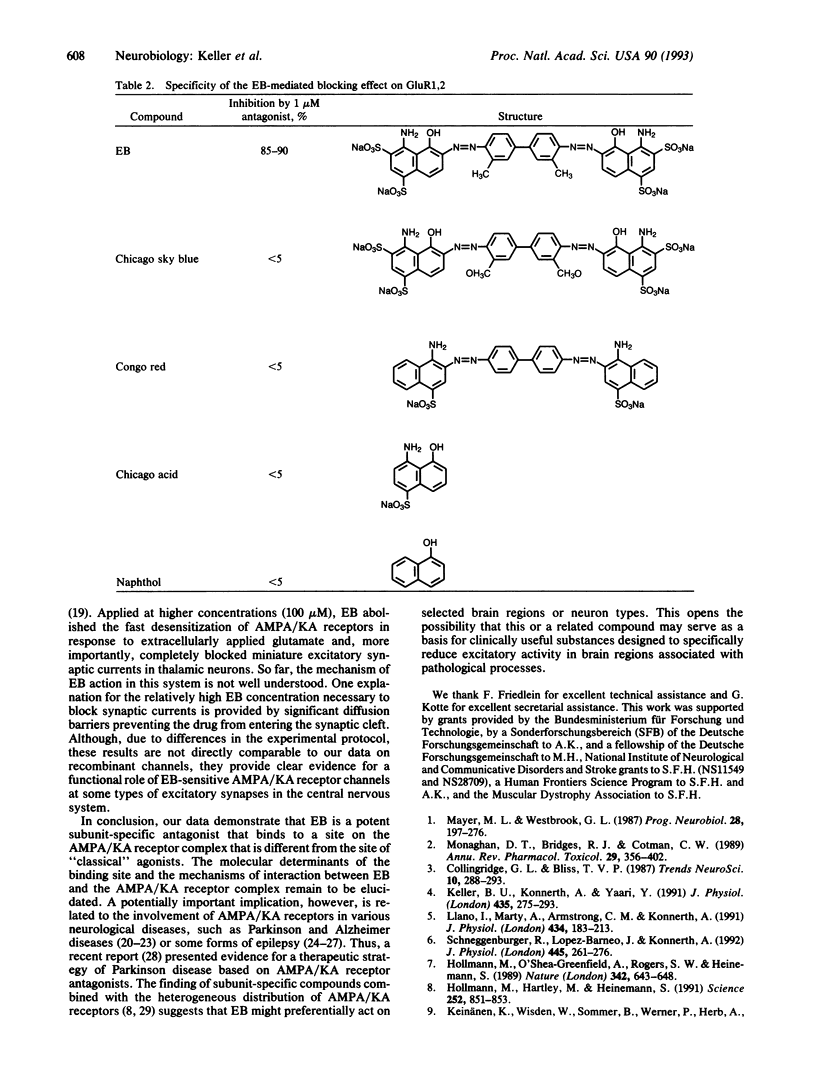
Abstract
Excitatory synaptic transmission in the mammalian central nervous system is mediated predominantly by glutamate receptor (GluR) channels of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate/kainate (AMPA/KA) receptor type. A major improvement in our understanding of glutamatergic synaptic transmission has been achieved after the identification of quinoxalinediones (e.g., 6-cyano-7-nitroquinoxaline-2,3-dione) as specific antagonists of AMPA/KA receptors. In addition to their effects on neurons, quinoxalinediones were also shown to block glutamate-induced responses mediated by recombinant AMPA/KA receptor channels expressed in heterologous systems, irrespective of their particular subunit composition. Here we report the identification of an AMPA/KA receptor antagonist that selectively blocks a subset of AMPA/KA receptors. We found that Evans blue, a biphenyl derivative of naphthalene disulfonic acid, blocks at low concentrations (IC50 = 355 nM for the subunit combination GluR1,2) KA-mediated responses of the subunits GluR1, GluR1,2, GluR1,3, and GluR2,3 expressed in Xenopus oocytes but not responses of GluR3 or GluR6. The blocking action of Evans blue was partially reversible and did not compete with KA for the agonist binding site. These findings suggest not only that Evans blue is a potent tool for elucidating the functional role of specific AMPA/KA receptor subtypes for excitatory synaptic transmission but also that it may also represent a powerful starting point for clinically useful drugs that are able to reduce the excitatory drive in specific neuronal populations of the central nervous system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulter J., Hollmann M., O'Shea-Greenfield A., Hartley M., Deneris E., Maron C., Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990 Aug 31;249(4972):1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992 Jan;8(1):189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Dewar D., Chalmers D. T., Shand A., Graham D. I., McCulloch J. Selective reduction of quisqualate (AMPA) receptors in Alzheimer cerebellum. Ann Neurol. 1990 Dec;28(6):805–810. doi: 10.1002/ana.410280612. [DOI] [PubMed] [Google Scholar]
- Difazio M. C., Hollingsworth Z., Young A. B., Penney J. B., Jr Glutamate receptors in the substantia nigra of Parkinson's disease brains. Neurology. 1992 Feb;42(2):402–406. doi: 10.1212/wnl.42.2.402. [DOI] [PubMed] [Google Scholar]
- Dingledine R., McBain C. J., McNamara J. O. Excitatory amino acid receptors in epilepsy. Trends Pharmacol Sci. 1990 Aug;11(8):334–338. doi: 10.1016/0165-6147(90)90238-4. [DOI] [PubMed] [Google Scholar]
- Egebjerg J., Bettler B., Hermans-Borgmeyer I., Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991 Jun 27;351(6329):745–748. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- Harrison P. J., Barton A. J., Najlerahim A., Pearson R. C. Distribution of a kainate/AMPA receptor mRNA in normal and Alzheimer brain. Neuroreport. 1990 Oct;1(2):149–152. doi: 10.1097/00001756-199010000-00017. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Hartley M., Heinemann S. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991 May 10;252(5007):851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Hollmann M., O'Shea-Greenfield A., Rogers S. W., Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989 Dec 7;342(6250):643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Hosford D. A., Crain B. J., Cao Z., Bonhaus D. W., Friedman A. H., Okazaki M. M., Nadler J. V., McNamara J. O. Increased AMPA-sensitive quisqualate receptor binding and reduced NMDA receptor binding in epileptic human hippocampus. J Neurosci. 1991 Feb;11(2):428–434. doi: 10.1523/JNEUROSCI.11-02-00428.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume R. I., Dingledine R., Heinemann S. F. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991 Aug 30;253(5023):1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Keller B. U., Hollmann M., Heinemann S., Konnerth A. Calcium influx through subunits GluR1/GluR3 of kainate/AMPA receptor channels is regulated by cAMP dependent protein kinase. EMBO J. 1992 Mar;11(3):891–896. doi: 10.1002/j.1460-2075.1992.tb05127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller B. U., Konnerth A., Yaari Y. Patch clamp analysis of excitatory synaptic currents in granule cells of rat hippocampus. J Physiol. 1991 Apr;435:275–293. doi: 10.1113/jphysiol.1991.sp018510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockgether T., Turski L., Honoré T., Zhang Z. M., Gash D. M., Kurlan R., Greenamyre J. T. The AMPA receptor antagonist NBQX has antiparkinsonian effects in monoamine-depleted rats and MPTP-treated monkeys. Ann Neurol. 1991 Nov;30(5):717–723. doi: 10.1002/ana.410300513. [DOI] [PubMed] [Google Scholar]
- Lessmann V., Gottmann K., Lux H. D. Evans blue reduces macroscopic desensitization of non-NMDA receptor mediated currents and prolongs excitatory postsynaptic currents in cultured rat thalamic neurons. Neurosci Lett. 1992 Oct 26;146(1):13–16. doi: 10.1016/0304-3940(92)90160-9. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Armstrong C. M., Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991 Mar;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löschmann P. A., Lange K. W., Kunow M., Rettig K. J., Jähnig P., Honoré T., Turski L., Wachtel H., Jenner P., Marsden C. D. Synergism of the AMPA-antagonist NBQX and the NMDA-antagonist CPP with L-dopa in models of Parkinson's disease. J Neural Transm Park Dis Dement Sect. 1991;3(3):203–213. doi: 10.1007/BF02259538. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- McDonald J. W., Garofalo E. A., Hood T., Sackellares J. C., Gilman S., McKeever P. E., Troncoso J. C., Johnston M. V. Altered excitatory and inhibitory amino acid receptor binding in hippocampus of patients with temporal lobe epilepsy. Ann Neurol. 1991 May;29(5):529–541. doi: 10.1002/ana.410290513. [DOI] [PubMed] [Google Scholar]
- Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986 Dec;407(6):577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Shneider N. A., Axel R. A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron. 1990 Nov;5(5):569–581. doi: 10.1016/0896-6273(90)90212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedder S. C., Wilcox R. I., Tuchek J. M., Johnson D. D., Crawford R. D. Quisqualate receptors in epileptic fowl: the absence of coupling between quisqualate and N-methyl-D-aspartate receptors. Eur J Pharmacol. 1990 Jan 3;175(1):85–91. doi: 10.1016/0014-2999(90)90156-z. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro D. E., Bennett M. V., Zukin R. S. Differential expression of three glutamate receptor genes in developing rat brain: an in situ hybridization study. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4157–4161. doi: 10.1073/pnas.88.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R., López-Barneo J., Konnerth A. Excitatory and inhibitory synaptic currents and receptors in rat medial septal neurones. J Physiol. 1992 Jan;445:261–276. doi: 10.1113/jphysiol.1992.sp018923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn T. A., Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991 Jun 21;252(5013):1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]



