Abstract
The effects of a clarifying process using pectinases and chitosan on the physicochemical characteristics, antioxidant capacity and quality attributes of açaí fruit (Euterpe oleracea Mart.) juice were evaluated. Clarification of acaí pulp resulted (P ≤ 0.05) in a 50 % loss of total anthocyanin (4.2730 mg/100 mL) and 29 % reduction in antioxidant capacity (33.60 μM FeSO4/g). A high association (P ≤ 0.05) was found between the decrease of antioxidant capacity and total anthocyanin loss. The use of pectinases associated with chitosan as an aid for clarification of açaí juice proved to be highly effective and resulted in a clear juice with a brighter purple to red color that was free of lipids, insoluble solids, and others substances that cause hazes. The obtained clarified açaí juice is a genuinely high-value anthocyanin-rich product that could be used as colorant and functional ingredient to fruit juices and soft drinks.
Keywords: Açaí, Euterpe oleracea, Pectinases, Clarification, Tropical juice, Chitosan
Introduction
In recent years, the increase in consumption of fruits and vegetables has been associated with the prevention of modern life-style related degenerative disease (Pacheco-Palencia and Talcott 2010). More attention has been given to the presence of dietary polyphenols in fruits and vegetables once they contribute towards maintaining a good health (Netzel et al. 2007). Acaí (Euterpe oleracea Mart.) is a slender, multi-stemmed, monoecious palm, widely distributed in the Amazon floodplains (Muniz-Miret et al. 1996). Moreover, acaí is a highly perishable fruit with short shelf life (Tonon et al. 2008). Beyond being a highly energetic fruit, acaí has been recognized for its functional properties for use in food and nutraceutical products, due to its high antioxidant activity, which is related to its high anthocyanin and phenolic content (Coisson et al. 2005; Schauss et al. 2006). Del Pozo-Insfran et al. (2006) reported that acaí was found to have higher antioxidant capacity than other anthocyanin-rich fruits, such as highbush blueberries, blackberries, cranberries and others, and verified that the predominant anthocyanin present in acaí pulp was cyanidin-3-glucoside (1040 mg/L pulp).
Few studies have reported the potential health benefits of açaí (Del Pozo-Insfran et al. 2006; Lichtenthaler et al. 2003) and the stability of the phytochemical compounds upon processing and storage. Due to its highly perishable nature, consumption and commercialization of açaí was mainly restricted to the regional level in Brazil. However, an increased interest from international markets has made açaí pulp and its derived products widely available to the general public. Commercialization of açaí pulp imposes new challenges since various processing steps such as pasteurization, freezing, dilution or dehydration are often employed during manufacture of retail products. Clarification of açaí juice improves aesthetic properties and market acceptability while removing lipids and insoluble solids. More recently, the consumer market has been equally receptive to clear fruit juice (Carvalho et al. 2008). Clarification is an important step in the processing of fruit juice and is most often achieved through microfiltration, enzymatic treatment or by using common clarifying aids like gelatin, bentonite, silica sol, polyvinyl pyrrolidone or a combination of these compounds. Nowadays, a great variety of new clarified fruit juices is offered in international and local markets; however, there is no clarified exotic tropical fruit juices. Therefore, clarified açaí juice with its unique exotic color and novelty flavor may be able to diversify the market, thus providing a totally new experience to consumers. For clarified products, clarity and homogeneity are two important characteristics, which are achieved by the complete removal of all suspended solids (Pacheco-Palencia et al. 2007). The edible pulp of acaí is commonly macerated with water to produce a thick, purple beverage of creamy texture, oily appearance and characteristic flavor which requires the use of enzymes in order to obtain a pulpy juice with lower viscosity due to a much smaller amount of pectin and starch which is advantageous for the filtration process. Chitosan (poly-β(1–4) N-acetyl-glucosamine) has been reported to have a number of potential industrial uses such as an adhesive, a paper-sizing agent, a chelating agent for metal ions and as fruit-juice clarifying aids (Knorr 1984). Chitosan being nontoxic and biodegradable may be used as an alternative agent for refining of fruit juices. Moreover, acid soluble crab shell chitosan and water soluble chitosan salt proved equally effective as fining agent for apple and carrot juices (Imeri and Knorr 1988; Soto-Peralta et al. 1989). The use of chitosan in this respect is hindered due to its solubility in organic acids
The use of one compound only as compared to the silica sol/gelatin/bentonite treatment and the ease of handling acid soluble chitosan should make it an attractive alternative to conventional juice fining procedures (Chatterjee et al. 2004; Rungsardthong et al. 2006). The use of pectolytic enzymes in association with clarifying agents in fruit processing is essential to get better juice yields, improve filtration rate and produce clear juices of higher quality for the concentration process. Even though there are a vast number of studies describing chemical properties of açaí and açaí products, there is lack of information on the literature regarding processing technologies of clarified açaí juice using enzymes and chitosan as clarifying agents. Therefore, the aim of this work was to evaluate the effects of a clarifying process using pectinases and chitosan on the physicochemical characteristics, antioxidant capacity and quality attributes of açaí juice. Results from this investigation will assist on assessing factors that influence the quality and physicochemical stability of açaí-based food.
Material and methods
Fruit material
Pasteurized, frozen acaí pulp (4°Brix) was obtained from local market (Fortaleza, CE, Brazil). The pulp was divided into suitable small portions and kept frozen (−18 °C) until use.
Chemicals and enzymes
Citrozym-Ultra L from Novozymes Latin America Ltda (Araucária, Brazil) was used for enzymatic treatment for açaí pulp and stored at 4 °C. Citrozym-Ultra L is a commercial enzyme preparation from Aspergillus aculeatus and Aspergillus niger, used in the food industry for fruit juice processing to reduce viscosity. It contains different food grade (generally recognized as safe – GRAS) pectinolytic and cellulolytic enzymes [endo-polygalacturonase (EC 3.2.1.15; C.A.S. No. 9032-75-1), endopectinylase (EC 4.2.2.10; C.A.S. No. 9033-35-6) and pectin esterase (EC 3.1.1.11; C.A.S. No. 9025-98-3)]. The declared activity of Citrozym-Ultra L is 4500 PECTU units/mL (pectin unit per mL). The optimum enzyme reaction conditions are at pH 3.5–6.0 and temperature range below 50 °C (Kashyap et al. 2001). Commercial chitosan from Polymar Ciência e Nutrição S/A (Fortaleza, Brazil) was used in the clarification of açaí juice. Chitosan powder was dissolved in (1 % w/v) acetic acid solution to yield a 4 % (w/v) solution.
Enzymatic treatment
The enzymatic hydrolysis of the pulp as a pretreatment for clarification was carried out on a laboratory scale. Samples of açaí pulp were gently thawed overnight under refrigeration (5 °C) the day prior to use on the clarifying experiments. The range of the variables for enzymatic treatment conditions were based on preliminary experiments conducted earlier and included incubation time of 15, 30, 45, and 60 min and enzyme concentration of 0.01–0.2 % (v/v). The fresh pulp (FP) was divided into six equal portions of 100 mL and initially heated in a water-bath at 45 °C followed by the addition of different Citrozym-Ultra L concentrations (0.01, 0.05, 0.10, 0.15 and 0.20 % (v/v)) under constant agitation at 100 rpm (±5), in which one portion remained unprocessed (control). The pH of the pulp was kept constant at its natural value of 4.0. During the mash treatment 10 mL of each treated pulp were drawn out every 15 min to accomplish the enzyme inactivation by heating the pulp to 90 °C for 5 min in a water bath. After that, in order to evaluate the optimal conditions for pulp hydrolysis, the treated samples were filtered through a Whatman No.1 filter paper and the filtrate was submitted to acidified alcohol test (IAL 2005) and standard iodine test (AOAC 1995) to detect pectin and insoluble starch, respectively.
Clarifying assays
According to preliminary assays based on an enzymatic treatment optimization (data not showed) 0.1 % Citrozym-Ultra L at 45 °C for 60 min was selected to attain optimum enzymatic hydrolysis conditions since it showed negative results for starch and pectin presence. A negative test by iodine indicates that all of the starch has been reduced to a chain length of less than nine to twelve glucose units, a size sufficiently reduced to not produce post-bottling hazes (Abdullah et al. 2007). According to Grassin and Fauquembergue (1996) the acidified alcohol test is positive when pectin reacts with ethanol acidified with chlorhydric acid forming a viscous gel.
After the enzymatic treatment the hydrolyzed pulp (HP) was suspended with distilled water to obtain 3 L of açaí cloudy juice (CJ) (30 % (w/v) pulp). The CJ juice was submitted to a filtration through cheesecloth and pasteurized in a water bath (90 °C/5 min) for clarifying experiments. As pretreatment to define the optimum clarification conditions, the CJ was divided into six equal portions of 50 mL and transferred to 250 mL Erlenmeyer flasks. Volumes ranging from 2.5 to 22.5 mL of 4 % chitosan solution in water (100 to 900 mg of soluble chitosan) were added to each flask. The control flask received only 2.5 mL of distilled water instead of chitosan solution. Flasks were then left at room temperature (28 °C) and gently agitated for 120 min and finally the juice was filtered through Whatman No. 2 filter paper in order to obtain a brighter purple to red colored juice free of visual sediments or cloudiness (pulpless juice), 5 mL aliquots of açaí juice were taken from each flask at intervals of 30 min to determine the clarity and color. Clarity of the clarified açaí juice was determined by measuring the absorbance at 660 nm according to Abdullah et al. (2007) using a UV–Vis spectrophotometer (Model B582, Micronal, Brazil) after filtering through cheesecloth and diluting with water (all treatments have the same dilution (w/v)). The analysis of color was accomplished by transmittance in the Hunter system using a Chroma Meter (Minolta Co., CR10, Japan) and was expressed with the values of Hunter L* (lightness), a* (redness-greenness), b* (yellowness-blueness). The chroma meter was calibrated using the standard white and black plates. The Commision Internationale de L’Eclairage (CIE) system reference measures the lightness (L* value) on a numerical scale, where white = 100 and black = 0 (Saxena et al. 2012). The a* and b* have no specific numerical limits (positive a* (red), negative a* (green), positive b* (yellow), negative b* (blue)). The criteria applied for clarifying optimization were as follows: maximum clarity, a* and L* values, since they are important physical indexes for quality attributes of the clarified açaí juice.
Juice clarifying process
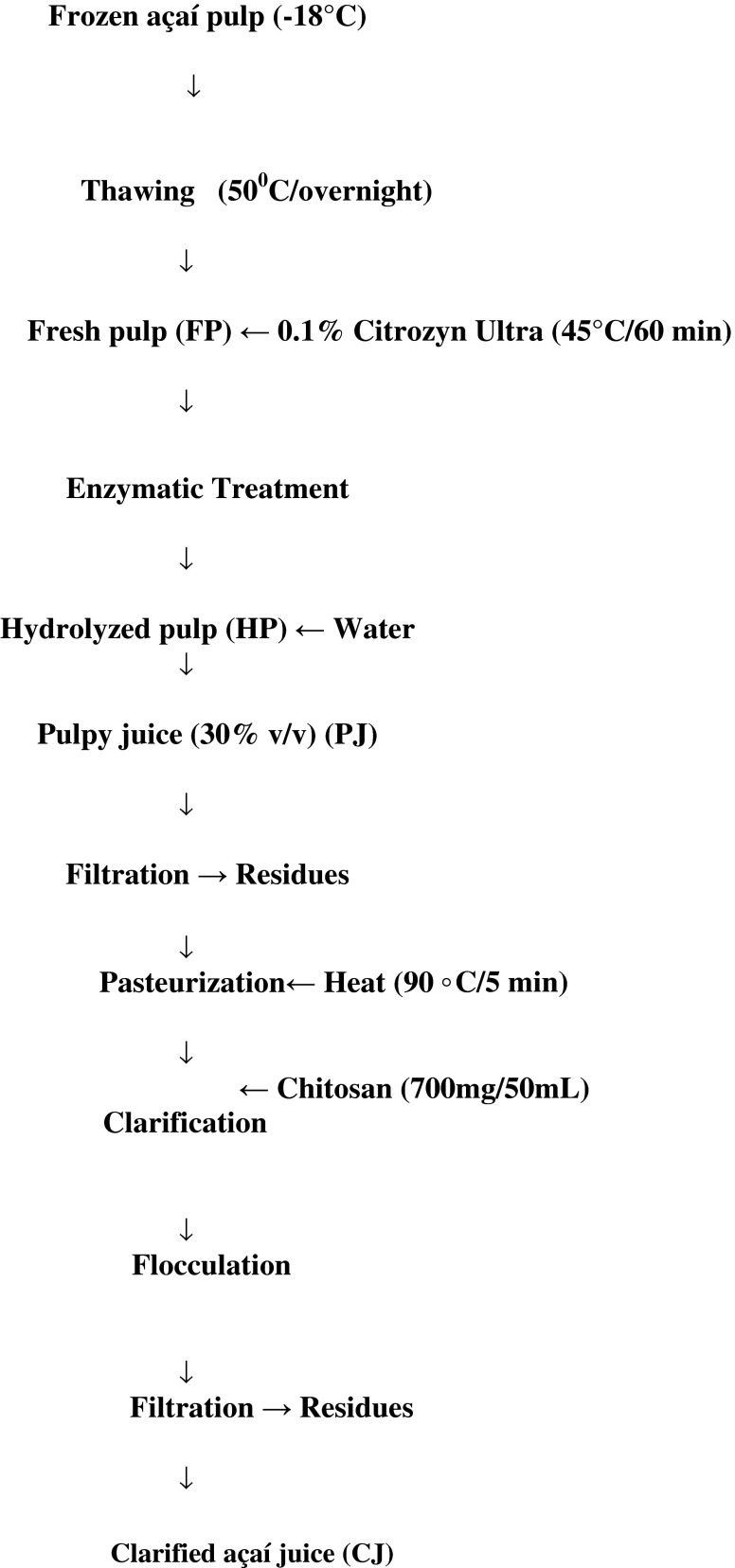
After analyzing statistically the results from the experimental clarifying assay (data not showed) the optimum conditions were 700 mg of soluble chitosan/50 mL of treated açaí juice during 90 min for flocculation. Approximately 10 kg of 0.1 % Citrozym-Ultra L treated pulp were used for further laboratory pilot-scale experiment. The treated pulp was diluted in spring water 1:3 (v/v) to produce 30 L of pulpy juice (PJ) (33 % v/v, 1.26°Brix). The juice was filtered through cheesecloth to reduce fat content and insoluble solids. The filtered moderately cloudy juice obtained was then pasteurized at 90 °C for 5 min before proceeding with the clarifying process. According to previous clarifying assay data for 30 L of filtered juice, was added 3 L of 4 % chitosan solution (700 mg of soluble chitosan/50 mL fruit juice) and held at room temperature under gentle agitation for 90 min until flocculation was complete. Following, the juice was immediately filtered using cheesecloth to obtain a clear juice (CJ). Schematic diagram of the clarifying process is showed in Fig. 1.
Fig. 1.
General processing diagram for obtaining clarified açaí juice by pectinase and chitosan
Physical and physicochemical analysis
Samples of the products obtained throughout the clarifying process were analyzed (Fig. 1): Fresh pulp (FP), hydrolyzed pulp (HP), pulpy juice (PJ) and clear juice (CL). The physicochemical analyses were: clarity (Abdullah et al. 2007); color (Brasil et al. 2012); pH was measured using a glass electrode potentiometer according to AOAC method 981.12 (1995); titratable acidity was determined by titration with 0.1 N NaOH to a pH 8.1 and expressed as % citric acid using AOAC method 932.14 (AOAC 1995); total soluble solids was measured refractometrically by use of a digital refractometer type RX 5000 (Atago, Tokio, Japan) using AOAC method 932.14 (1995); reducing and total sugar was estimated by DNSA (dinitrosalicylic acid) reagent (Miller 1959) and Anthrone reagent methods (Carvalho et al. 2008); ascorbic acid was measured following AOAC method 985.33 (2,6-dichloroindophenol titrimetric method, (AOAC 1995)); total anthocyanin content was evaluated using the method described by Giusti and Wrolstad (2003)); starch was determined using the method described by AOAC method 996.11 (AOAC 1995); and total lipid was analyzed using Soxhlet method (IAL 2005).
Determination of antioxidant capacity (FRAP Method)
Changes in antioxidant capacity during processing (FP, HP, PJ, and CJ) were determined by the ferric reducing antioxidant power (FRAP) assay method (Benzie and Strain 1996) with minor modifications. This method consists in estimating the antioxidant capacity of the products from their ability to reduce Fe(III)-2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ) complex to Fe(II)-TPTZ, the resulting intense blue color is linearly related to the amount of reductant (antioxidant) present. The absorbance at 593 nm was measured 4 min after 1 mL of a ten-fold dilution of the samples was added to 3 mL of Fe(III)-TPTZ and ferric reducing antioxidant potential (FRAP value) was interpolated from a standard curve prepared from a stock solution (Benzie and Strain 1996). The results are expressed as micromoles of ferrous equivalents per gram of fresh weight.
Statistical analysis and experimental design
The experimental design and statistical analysis were performed using Statistical Analyses System software Version 9.1 (SAS 2006). For all analyses, determinations were made in triplicate as independent experiments The components of variance for balanced split-plot experiments were determined, considering a complete factorial design with first factor distributed in the whole plots (four incubation times: 15, 30, 45 and 60 min), and a second factor in the subplots (six chitosan solution concentrations: 0, 100, 300, 500, 700 and 900 mg/50 mL of fruit juice). The dependent variables measured were clarity and color values. Analysis of variance (ANOVA) and regression were assessed to define the optimum chitosan concentration and flocculation time. Differences between the physical, physicochemical, and antioxidant activity data for each processing phase (FP, HP, PJ and CL) were tested for significance (α = 5 %) by one-way ANOVA. Significant different means (P ≤ 0.05) were separated by Tukey’s HSD test.
Results and discussion
Physicochemical characteristics, antioxidant capacity, and quality attributes
Physical and physicochemical characteristics, and antioxidant capacity from samples of each product (FP, HP, PJ, and CJ) obtained during the clarifying process are reported on Table 1. The results indicate a significant effect (P ≤ 0.05) in all studied parameters among the products obtained during the clarification process. Although the pH and titratable acidity were significantly affected by processing, the typical sour characteristic of açaí juice was kept during the clarifying process. This indicates the concentrations of organic acids in the clarified juice remained unaltered as a constant net balance. The total soluble solids (TSS) content decreased (P ≤ 0.05) during the clarification of açaí juice, a typical behaviour for clarifying processes. The TSS content found in the açaí fresh pulp is lower (P ≤ 0.05) than other tropical fruits such as guava (Brasil et al. 1995), acerola (Brasil et al. 2007), mango (Gupta and Jain 2012), and cashew apple (Maia et al. 2004). This is probably related to the presence of high-suspended solids content in the pulp such as starch that can interfere with the measurement of the refractive index (Carvalho et al. 2008). On the contrary, the increase of TSS in treated pulp is associated to the increase in reducing sugar content due to the action of pectinases (polygalacturonases and pectin lyases) on polygalacturonic chains as well as hydrolysis of non reducing sugars by weak acids at higher temperatures (pulp treatment) (Hernandez and Villegas 1987). Brasil et al. (1995) found an increase of 275 % in the reducing sugar content of guava pulp treated with 600 ppm of Clarex-L at 45 °C for 120 min. Floribeth and Lastreto (1981) detected an increase of 20 % in the reducing sugar content (galactose, arabinose and xylose) using a combination of pectic enzymes and cellulases to clarify apple juice. Carvalho et al. (2008) reported an increase of sucrose, glucose, and fructose in hydrolyzed pineapple juice using commercial pectinase (Ultrazym 100 G). Cheirsilp and Umsakul (2008) found a 15 and 39 % increase in total soluble sugars and reducing sugars in banana pulp after incubating with 0.05 % (w/w) of pectinase at 40 °C for 2 h. The decrease in TSS and reducing and total sugars in pulpy juice and clear juice is related to the pulp dilution to produce pulpy juice (33 % v/v) and the pulpy juice filtration, respectively. According to Youn et al. (2004), polysaccharides, proteins and colloidal materials are present as solid materials in juices forming gels and accumulating on the filter surface forming a secondary membrane as filtration goes on.
Table 1.
Physical and physicochemical characteristics, and antioxidant capacity of clarified açaí juice at different phases of the clarification process
| Parameters* | Fresh Pulp (FJ) | Hydrolysed Pulp (HP) | Pulpy Juice (PJ) | Clear Juice (CJ) |
|---|---|---|---|---|
| pH | 4.6908a ± 1.24 | 4.7233a ± 0.34 | 4.5733b ± 0.99 | 3.4233c ± 1.98 |
| Titratable acidity (% citric acid) | 0.0410b ± 1.23 | 0.0490b ± 0.998 | 0.0086b ± 1.12 | 0.1756a ± 0.98 |
| Total Soluble Solids (°Brix) | 3.98476a ± 1.02 | 4.8333b ± 1.98 | 1.2667c ± 301 | 1.0333c ± 2.98 |
| Reducing Sugars (% glucose) | 1.1667a ± 1.23 | 2.3646b ± 0.65 | 0.3157c ± 1.29 | 0.2996c ± 2.56 |
| Total sugars (%) | 1.5587a ± 1.02 | 1.7852a ± 2.01 | 0.4212b ± 9.12 | 0.3595c ± 2.65 |
| Ascorbic Acid (mg/100 mL) | 15.3181a ± 0.76 | 15.3067a ± 0.99 | 4.7367b ± 11.98 | 4.3600b ± 0.76 |
| Total Lipids (%) | 6.7198a ± 0.76 | 6.7094a ± 0.70 | 0.5163b ± 1.231.36 | 0.0190c ± 2.34 |
| Starch (%) | 1.1345a ± 0.99 | 0.7670b ± 0.96 | 0.4467b ± 1.13 | nd |
| Total Anthocyanins (mg/100 mL) | 53.9789a ± 0.87 | 53.9000a ± 0.97 | 8.0730b ± 1.02 | 4.2730c ± 1.12 |
| Lightness (L*) | 21.79a ± 0.87 | 20.10a ± 1.98 | 19.76b ± 1.34 | 34.34c ± 1.98 |
| Color a* | 4.01a ± 0.56 | 2.95a ± 0.52 | 33.89b ± 0.90 | 22.51c ± 0.23 |
| Color b* | 0.88a ± 1.76 | 0.66a ± 0.871.12 | −0.29b ± 0.99 | 10.87c ± 0.760 |
| Clarity (ABS. at 660 nm) | nr | nr | 0.016a | 0.007b |
| FRAP (μM FeSO4/g fresh weight) | 54.02a ± 0.98 | 53.77a ± 1.28 | 47.54b ± 0.98 | 33.60c ± 1.98 |
FRAP ferric reducing antioxidant power; Each data is a mean ± SD of three replicate experiments (n-3)*values followed by different letters in the same line are significantly different (Tukey HSD test, P ≤ 0.05), nr not realized, nd not detected.
Açaí fruit is a tropical fruit that contains considerable amounts of starch, as much as 10 % (Bobbio et al. 2002; Coisson et al. 2005; Del Pozo-Insfran et al. 2006; Lichtenthaler et al. 2003). However, the starch content found in non treated pulp (Table 1) is very low compared to the literature. According to Carvalho et al. (2008) some molecules may interfere with the starch determination by increasing or decreasing its value. Starch is estimated as glucose units or reducing sugars and if the starch granules have not been broken down completely, short-chained dextrins are left leading to retrogradation, when the short-chained dextrin recrystallize into a form that is no longer susceptible to hydrolysis, regardless of heating. Moreover, there was a significant decrease of starch content in treated pulp. This is supported by the hydrolysis of pectin present in the pulp at 45 °C. According to Carrin et al. (2004) when an aqueous suspension of starch is heated, the hydrogen bonds weaken, water is absorbed, the granules swell, rupture, and finally gelatinize causing a practically complete breaking down of the starch granule. The quantitative starch assays in CJ demonstrated that Citrozyn Ultra L (0.1 %, 45 °C for 60 min) was highly effective, even in doses lower than those recommended by the manufacturer, resulting in a complete break down of the gelatinized starch. Floribeth and Lastreto (1981) reported that up to 1 % starch may be present in clarified juice after milling and pressing causing post-process cloudiness and hindering filtration. According to Carrin et al. (2004) in the presence of starch, the following problems may occur: (i) slow filtration, (ii) membrane fouling, (iii) gelling after concentration, and (iv) post-concentration haze.
In addition, the chitosan (700 mg/50 mL for 90 min) was a highly effective clarifying agent by removing starch cloudiness, since there were significant differences in clarity and L* values for clarified açaí juice, lowest absorbance and highest values, respectively. Chitosan as a clarifying agent complexes with protein, polyphenols, and others insoluble solids inducing flocculation and sedimentation thus resulting in removal of these potential haze precursors (Soto-Peralta et al. 1989). Indeed, the total lipids of pulpy juice were significantly reduced (90 %). This result could be associated to the polycationic nature of chitosan acting as an effective clarifying agent in the separation of lipid particles from açaí pulpy juice, therefore improving aesthetic properties and further market acceptability.
Ascorbic acid content suffered a significant decrease (71.5 %) throughout the clarifying process with values ranging from 15.3 mg/100 mL (fresh pulp) to 4.3 mg/100 mL (clear juice). The vitamin C content in clarified citrus juices has been reported by other studies from no measurable loss up to 33 % compared to fruit pulp, depending on the types of clarifying aids and processing conditions (Youn et al. 2004). The low value obtained in the final product could be related to the processing phases such as pulp dilution, heat treatment, fining and due to oxygen exposure. An important criterion for the final product quality after heat treatment is the ascorbic acid retention. Yuyama et al. (1999) compared vitamin C content of açaí fruit to different exotic fruits from Amazon region such as bacuri (1.3 mg/100 g), maracujá-do-mato (6.7 mg/100 g) and murici (8.1 mg/100 g) and detected that açaí had the highest vitamin C content; however, when compared to cupuaçu (38.3 mg/100 g) and ituá (62.9 mg/100 g) both from the Amazon region, açaí fruit value was lower approaching barely to taperebá (17.2 mg/100 g). Vitamin C is highly bio-available and is consequently one of the most important water-soluble antioxidants in cells, efficient in scavenging reactive oxygen species such as O2*, OH*, peroxyl radicals and singlet oxygen (Halliwell 1996). Consequently, when considering the antioxidant activities of fruit juices to disease risk and health, it is important to account the contribution of vitamin C in addition to phenolic compounds with antioxidant activity in chemical systems (Williams 1995). According to Ozkan (2002) foods naturally low in ascorbic acid, such as acaí juice are particularly good candidates for fortification.
The clarifying process induced a significant decrease (50 %) in total anthocyanins content between pulpy juice and clear juice. Pacheco-Palencia et al. (2007) reported a 27 % loss in total polyphenolics (197 ± 6.9 mg gallic acid/100 mL) and a 20 % reduction in total anthocyanins (729 ± 3.4 mg/L) during clarification of acaí pulp. Anthocyanins are labile compounds, subject to numerous detrimental reactions during processing and storage (Wrolstad et al. 2005), among which the transformation of monomeric forms into oligomeric or polymeric pigments gives rise to important color changes toward brownish–red hues, that are generally more stable (Monagas et al. 2006). This decrease is probably related to the polycationic chitosan that bound to anthocyanin-based polymers dissolved in pulpy juice. Pacheco-Palencia et al. (2007) reported an initial detrimental effect on anthocyanins, non-anthocyanin polyphenolics, and antioxidant capacity during the clarification process of açaí pulp using diatomaceous earth.
The negative effect of chitosan on anthocyanins and non-anthocyanin polyphenolics reduction in contrast to the benefic adsorption to lipids and others insoluble solids during the clarifying process resulted in an increase (P ≤ 0.05) in lightness (L*) and decrease (P ≤ 0.05) in red color (a*). Aside from the loss of anthocyanins during clarification, there was a significant increase in red intensity (a*) after pulp dilution (pulpy juice), and this could be explained by co-pigmentation of anthocyanins. Co-pigmentation reactions involve the formation of weak linkages between anthocyanin glycosides and other non-colored components such as phenolic acids, flavonoids, and flavonol derivatives (Kirca et al. 2006). Furthermore, Boulton (2001) reported that such reactions cause anthocyanins to exhibit far stronger colors than would be expected from their concentration resulting in a hyperchromic response that may result in an over-estimation of total pigments in spectrophotometric assays. The b* values followed the a* values trend and pulpy juice samples showed a decrease (P ≤ 0.05) of the yellow intensity, with negative results. Additionally, for clear juice samples the b* values showed a significant increase that could be related to interactions between polyphenolics and carbohydrate/ascorbic acid degradation products, such as furfurals and other aldehydes, that will influence the formation of brown pigments during storage of fruit-based foods (Brasil et al. 2012). Aldehydes generally promote anthocyanin polymerization with flavonols, flavan-3-ols and their derivatives resulting in the formation of both colorless and yellow-colored compounds that contribute to browning reactions and decreased color stability of anthocyanins (Maia et al. 2004). Kirca et al. (2006) found that polyphenolic, antioxidant, and color stabilities of açaí juice are dependent on interactions among its matrix components and are influenced by processing, storage, temperature and chemical composition. Greater anthocyanin stability in açaí pulp and semi-clarified juice may be linked to several factors including the stabilizing effect of non-anthocyanin polyphenolics (Garzon and Wrolstad 2002; Skrede et al. 1992); differences in polymeric anthocyanin concentrations, or the presence of residual lipids or insoluble solids that altered oxidative reaction rates (Pacheco-Palencia et al. 2007). Color deterioration of anthocyanin-containing products during storage not only results from anthocyanin degradation but also from the transformation of monomeric anthocyanins into higher molecular weight polymeric forms (Baranac et al. 1996; Francia-Aricha et al. 1997; Johnston and Morris 1997).
There was a significant difference in antioxidant capacity between pulpy juice and clear juice and this result indicated that clarifying treatment affects the functional properties of clarified açaí juice. Clarification of açaí juice resulted in a 29 % loss in antioxidant capacity (33.60 μM FeSO4/g) and a high correlation was found between the decrease of antioxidant capacity and total anthocyanins losses (50 %). This result is comparable to previously reported values for açaí juice (Pacheco-Palencia et al. 2007) and açaí pulp (Lichtenthaler et al. 2003). Even though, there was a decrease in anthocyanin content and antioxidant capacity of the clarified açaí juice due to the clarifying process when compared to other anthocyanin-rich fruits such as highbush blueberries, blackberries, and cranberries, considered novel sources of antioxidants for food (Del Pozo-Insfran et al. 2006), its antioxidant capacity is still significantly higher.
Conclusions
The use of pectinolytic enzymes associated to chitosan as a clarifying aid for açaí juice proved to be highly effective once it resulted in a clear juice with a brighter purple to red color that was free of lipids, insoluble solids, and others substances that cause hazes. Even though, the clarifying process showed a significant decrease on total anthocyanin content and antioxidant capacity of the clarified açaí juice, its antioxidant capacity is still higher when compared to other fruits recognized as rich sources of antioxidants. The obtained clarified açaí juice is a genuinely high-value anthocyanin-rich product that could be used as colorant and functional ingredient to fruit juices and soft drinks as an interesting alternative to the growing market of food products associated to health and well-being.
Acknowledgements
The authors wish to thank the Coordenaçao de Aperfeiçoamento de Pessoal de Nivel Superior (CAPES) and the Fundaçao Cearense de Amparo a Pesquisa (FUNCAP) for the financial support.
Footnotes
Dr. Isabella Brasil is the responsible person for this project
Contributor Information
Leiliane Teles Cesar, Email: leilianetelescesar@yahoo.com.br.
Marília de Freitas Cabral, Email: marilia_defreitas@hotmail.com.
Geraldo Arraes Maia, Email: gmaia@pq.cnpq.br, Email: gamaia@netbandalarga.com.
Raimundo Wilane de Figueiredo, Email: figueira@ufc.br.
Paulo Henrique Machado de Sousa, Email: phenriquemachado@gmail.com.
Isabella Montenegro Brasil, Phone: +55-85-33669740, FAX: +55-3366-9752, Email: isabella@ufc.br.
Carmen Luiza Gomes, Email: carmen@tamu.edu.
References
- Abdullah AGL, Sulaiman NM, Aroua MK, Noor MM. Response surface optimization of conditions for clarification of carambola fruit juice using a commercial enzyme. J Food Eng. 2007;81:65–71. doi: 10.1016/j.jfoodeng.2006.10.013. [DOI] [Google Scholar]
- AOAC . Official methods of analysis of the assocation of official analytical chemistry, vol 1. 16. DC: Washington; 1995. [Google Scholar]
- Baranac JM, Petranovic NA, Dimitric-Markovic JM. Spectrophotometric study of anthocyanin copigmentation reactions. Journal of Agriculture and Food Chemistry. 1996;44(5):1333–1336. doi: 10.1021/jf950420l. [DOI] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;39:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bobbio FO, Bobbio PA, Oliveira P, Fadelli S. Stability and stabilization of the anthocyanins from Euterpe oleracea Mart. Acta Alimentarius. 2002;31:371–377. doi: 10.1556/AAlim.31.2002.4.6. [DOI] [Google Scholar]
- Boulton R. The copigmentation of anthocyanins and its role in the color of red wine: a critical review. Am J Enol Vitic. 2001;52:67–86. [Google Scholar]
- Brasil IM, Maia GA, Figueiredo RW. Physical-chemical changes during extraction and clarification of guava juice. Food Chem. 1995;54:383–386. doi: 10.1016/0308-8146(95)00066-R. [DOI] [Google Scholar]
- Brasil IM, Herculano KT, Oliveira GSF, Maia GA, Figueiredo RW. Physicochemical changes during extraction and concentration of acerola juice using pectinases and clarifying agents. Brazilian Journal of Food Technology. 2007;10:269–273. [Google Scholar]
- Brasil I, Gomes C, Puerta-Gomez A, Castell-Perez ME, Moreira RG. Freshness retention of minimally processed fruits using multilayered edible coating containing microencapsulated essential oil. LWT-Food Science Technology. 2012;47(1):39–45. doi: 10.1016/j.lwt.2012.01.005. [DOI] [Google Scholar]
- Carrin ME, Ceci LN, Lozano JE. Characterization of starch in apple juice and its degradation by amylases. Food Chem. 2004;87(2):173–178. doi: 10.1016/j.foodchem.2003.10.032. [DOI] [Google Scholar]
- Carvalho LMJ, Castro IM, Silva CAB. A study of retention of sugars in process of clarification pineapple juice (Ananas comosus, L. Meril) by micro- and ultra-filtration. J Food Eng. 2008;87:447–454. doi: 10.1016/j.jfoodeng.2007.12.015. [DOI] [Google Scholar]
- Chatterjee S, Chatterjee S, Chatterjee BP, Guha AK. Clarification of fruit juice with chitosan. Process Biochem. 2004;39:2229–2232. doi: 10.1016/j.procbio.2003.11.024. [DOI] [Google Scholar]
- Cheirsilp B, Umsakul L. Processing of banana-based wine product using pectinase and α-amylase. Journal Food Process Engineering. 2008;31:78–90. doi: 10.1111/j.1745-4530.2007.00152.x. [DOI] [Google Scholar]
- Coisson J, Travaglia F, Piana G, Capasso M, Arlorio M. Euterpe oleracea juice as a functional pigment for yogurt. Food Res Int. 2005;38(8–9):893–897. doi: 10.1016/j.foodres.2005.03.009. [DOI] [Google Scholar]
- Del Pozo-Insfran D, Percival SS, Talcott ST. Acai (Euterpe oleracea Mart.) polyphenolics in their glycoside and aglycone forms induce apoptosis of HL-60 leukemia cells. Journal of Agriculture and Food Chemistry. 2006;54:1222–1229. doi: 10.1021/jf052132n. [DOI] [PubMed] [Google Scholar]
- Floribeth V, Lastreto C. A study of the production of clarified banana juice using pectinolytic enzymes. Journal of Food Technology. 1981;16:115–125. [Google Scholar]
- Francia-Aricha EM, Guerra MT, Rivas-Gonzalo JC, Santos-Buelga C. New anthocyanin pigments formed alter condensation with flavanols. Journal of Agriculture and Food Chemistry. 1997;45:2262–2266. doi: 10.1021/jf9609587. [DOI] [Google Scholar]
- Garzon GA, Wrolstad RE. Comparison of the stability of pelargonidin-based anthocyanins in strawberry juice and concentrate. J Food Sci. 2002;67:1288–1299. doi: 10.1111/j.1365-2621.2002.tb10277.x. [DOI] [Google Scholar]
- Giusti MM, Wrolstad RE (2001) Anthocyanins. Characterization and Measurement with UV-Visible Spectroscopy. In: Wrolstad, RE. (Ed.). Current Protocols in Food Analytical Chemistry. New York: John Wiley & Sons Unit. F1.2.1-13
- Grassin C, Fauquembergue P. Application of pectinases in beverages: pectins and pectinases. Boca Raton: Elsevier Science B V; 1996. [Google Scholar]
- Gupta N, Jain SK (2012) Storage behavior of mango as affected by postharvest application of plant extracts and 315 storage conditions. Journal of Food Science and Technology. doi:10.1007/s13197-012-0774-0 [DOI] [PMC free article] [PubMed]
- Halliwell B. Vitamin C: antioxidant or pro-oxidant in vivo? Free Radic Res. 1996;25(5):439–454. doi: 10.3109/10715769609149066. [DOI] [PubMed] [Google Scholar]
- Hernandez TM, Villegas MJ. Effect of freezing storage on the quality of some tropical fruit pulps: preliminary study. Technology Chemistry. 1987;7:33–37. [Google Scholar]
- IAL . Instituo Adolfo Lutz. Physico-chemical methods for food analysis. Brasilia: ANVISA; 2005. [Google Scholar]
- Imeri AG, Knorr D. Effects of chitosan on yield and compositional data of carrot and apple juice. J Food Sci. 1988;55:1707–1709. doi: 10.1111/j.1365-2621.1988.tb07821.x. [DOI] [Google Scholar]
- Johnston TV, Morris JR. HPLC analysis of cabernet savignon and noble wine pigment fractions. J Food Sci. 1997;62:684–687. doi: 10.1111/j.1365-2621.1997.tb15435.x. [DOI] [Google Scholar]
- Kashyap DR, Vohra PK, Chopra S, Tewari R (2001) Applications of pectinases in the commercial sector: a review. Bioresour Technol 77(215–227) [DOI] [PubMed]
- Kirca A, Ozkan M, Cemeroglu B. Stability of black carrot anthocyanins in various fruit juices and nectars. Food Chem. 2006;97:598–605. doi: 10.1016/j.foodchem.2005.05.036. [DOI] [Google Scholar]
- Knorr D. Use of chitinuous polymers in foods. Food Technology. 1984;38:85–97. [Google Scholar]
- Lichtenthaler R, Marx F, Kind OM. Determination of antioxidative capacitites using an enhanced oxidant scavenging capacity (TOSC) assay. Europe Food Research and Technology. 2003;216:166–173. [Google Scholar]
- Maia GA, Souza Filho MSM, Figueiredo RW, Brasil IM. Caracterizacao quimica de pedunculos de diferentes clones de cajueiro anao precoce (Anacardium occidentale, L.) Revista Ciencia Agronomica. 2004;35:272–278. [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Monagas M, Gomez-Cordoves C, Bartolome B. Evolution of the phenolic content of red wines from Vitis vinifera L. during ageing in bottle. Food Chem. 2006;95:405–412. doi: 10.1016/j.foodchem.2005.01.004. [DOI] [Google Scholar]
- Muniz-Miret N, Vamos R, Hiraoka M, Montagnini F, Mendelsohn R. The economic value of managing the acai palm (Euterpe oleracea Mart.) in floodplains of the Amazon estuary, Para Brazil. Forest Ecol Manage. 1996;87:63–173. doi: 10.1016/S0378-1127(96)03825-X. [DOI] [Google Scholar]
- Netzel M, Netzel G, Tian Q, Shwartz S, Konczak I. Native Australian fruits: a novel source of antioxidants for food. Innovative Food Science and Emerging Technologies. 2007;8:339–346. doi: 10.1016/j.ifset.2007.03.007. [DOI] [Google Scholar]
- Ozkan M. Degradation of anthocyanins in sour cherry and pomegranate juices by hydrogen peroxide in the presence of added ascorbic acid. Food Chem. 2002;78:499–504. doi: 10.1016/S0308-8146(02)00165-6. [DOI] [Google Scholar]
- Pacheco-Palencia L, Talcott ST. Chemical stability of acai fruit (Euterpe oleracea Mart.) anthocyanins as influenced by naturally occuringand externally added polyphenolic cofactors in model systems. Food Chem. 2010;118:17–25. doi: 10.1016/j.foodchem.2009.02.032. [DOI] [Google Scholar]
- Pacheco-Palencia L, Hawken P, Talcott ST. Phytochemical, antioxidant and pigment stability of acai (Euterpe oleracea Mart.) as affected by clarification, ascorbic acid fortification and storage. Food Res Int. 2007;40:620–628. doi: 10.1016/j.foodres.2006.11.006. [DOI] [Google Scholar]
- Rungsardthong V, Wongvuttanakul N, Kongpien N, Chotiwaronon P. Application of fungal chitosan for clarification of apple juice. Process Biochem. 2006;41:589–593. doi: 10.1016/j.procbio.2005.08.003. [DOI] [Google Scholar]
- Statistical Analisys System Institute (2006) SAS System for Windows. versão 9.1. Cary: SAS Institute
- Saxena D, Sabikhi L, Chakraborty SK, Signgh D (2012) Process optimization for enzyme aided clarification of 344 watermelon juice. Journal of Food Science and Technology 49. doi:10.1007/s13197-345 012-0720-1 [DOI] [PMC free article] [PubMed]
- Schauss AG, Wu X, Prior RL, Ou B, Huang D, Owens J, Agarwal A, Jensen GS, Hart AN, Shanbrom E. Antioxidant capacity and other bioactivities of the freeze dried amazonian palm berry, Euterpe oleracea Mart (Acai) J Agric Food Chem. 2006;54:8604–8610. doi: 10.1021/jf0609779. [DOI] [PubMed] [Google Scholar]
- Skrede G, Wrolstad RE, Lea P, Enersen G. Color stability of strawberry and black currant syrups. J Food Sci. 1992;57:172–177. doi: 10.1111/j.1365-2621.1992.tb05449.x. [DOI] [Google Scholar]
- Soto-Peralta NV, Muller H, Knorr D. Effects of chitosan treatment on the clarity and color of apple juice. J Food Sci. 1989;54:495–496. doi: 10.1111/j.1365-2621.1989.tb03119.x. [DOI] [Google Scholar]
- Tonon RV, Brabet C, Hubinger MD. Influence of process conditions on the physicochemical properties of acai (Euterpe oleraceae Mart.) powder produced by spray drying. J Food Eng. 2008;94:215–221. doi: 10.1016/j.jfoodeng.2009.03.009. [DOI] [Google Scholar]
- Williams C. Healthy eating: clarifying advice about fruit and vegetables. British Medical Journal. 1995;310:453–455. doi: 10.1136/bmj.310.6992.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrolstad RE, Durst RW, Lee J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci Technol. 2005;16:423–428. doi: 10.1016/j.tifs.2005.03.019. [DOI] [Google Scholar]
- Youn KS, Hong JH, Bae DH, Kim SJ, Kim SD. Effective clarifying process of reconstituted apple juice using membrane filtration with filter-aid pretreatment. J Membr Sci. 2004;228:179–186. doi: 10.1016/j.memsci.2003.10.006. [DOI] [Google Scholar]
- Yuyama LKO, Vasquez ALV, Aguiar JPL, Macedo SHM, Yonekura L, Nagahama D, Fonseca CW. Composicao quimica e adequacao da alimentacao oferecidaaos pre-escolares de uma instituicao beneficiente de Manaus, Amazonas, Brasil. Acta Amazonica. 1999;29:549–554. [Google Scholar]