Abstract
Shrimp waste is an important source of astaxanthin, which occur as a complex with proteins, and protein isolates as well as carotenoids are known to possess antioxidant activity. Investigations were carried out to optimize hydrolysis of shrimp waste using a bacterial protease to obtain antioxidant activity rich protein isolate. The effect of three process variables namely enzyme concentration to waste, incubation temperature and time on carotenoid recovery, protein content, trichloro acetic acid (TCA) soluble peptide content and DiPhenyl Picryl Hydrazylchloride (DPPH) scavenging activity was evaluated using a fractionally factorial design. A high correlation coefficient (>0.90) between the observed and the predicted values indicated the appropriateness of the design employed. Maximum carotenoid recovery was obtained by hydrolysing the shrimp waste with 0.3 % enzyme for 4 h. DPPH radical scavenging activity of carotenoprotein isolate was markedly affected by enzyme concentration, temperature and time of hydrolysis. The study indicated that in order to obtain the carotenoprotein from shrimp waste with higher carotenoid content hydrolysing with an enzyme concentration of 0.2–0.4 %, at lower temperature of 25–30° upto 4 h is ideal. However, in order to obtain the protein isolate with increased antioxidant activity hydrolysing at higher temperature of 50 °C, with higher enzyme concentration of 0.5 % for shorter duration is more ideal.
Keywords: Shrimp waste, Carotenoid, Carotenoprotein, Antioxidant, RSM, DPPH scavenging
Introduction
Processing of crustaceans such as shrimps generates large quantities of solid wastes accounting for approximately 35–45 % of whole shrimp weight (Sachindra et al. 2005; 2006a). These waste spoils rapidly, thus causing environmental problems. Further, as shrimp waste being a rich source of protein, chitin, carotenoid and enzymes, considerable interest has been shown recently to recover these valuable components as marketable products.
Astaxanthin is the major carotenoid present in crustacean waste, and occurs as carotenoprotein complexes, where carotenoids are bound to proteins (Ghidalia 1985; Shahidi et al. 1998). Complexing of carotenoids to protein results in display of various colors in crustaceans and provides stability to carotenoids, which are otherwise very unstable (Zagalsky 1985; Zagalsky et al. 1990). Attempts have been made to recover carotenoids from shrimp waste either as carotenoids or as carotenprotein complex. Studies have been carried out on recovery of carotenoids and carotenoproteins from crustacean waste. Carotenoids from shrimp waste has been recovered using solvent extraction and oil extraction (Sachindra and Mahendrakar 2005; Sachindra et al. 2006b) and its stability under different storage conditions has been reported (Sachindra and Mahendrakar 2010). Enzymatic hydrolysis of shrimp waste was found to enhance the oil extractability of carotenoids (Sachindra and Mahendrakar 2011). Carotenoproteins from shrimp waste can be isolated by enzymatic and fermentation techniques. Chelating agents like EDTA and the proteolytic enzyme trypsin has been used to recover carotenoprotein from shrimp waste (Simpson and Haard 1985; Cano-Lopez et al. 1987). Trypsin hydrolysis of snow crab waste followed by ammonium sulphate precipitation yielded carotenoprotein with increased carotenoid content (Manu-Tawai and Haard 1987).
Fermentation ensilaging was found to be better option for stabilizing the carotenoids in shrimp waste without affecting its recovery (Sachindra et al. 2007a) and a fermentation process has been standardized for recovery of carotenoprotein rich in essential amino acids (Bhaskar et al. 2010). Lyophilised fermentation liquor from Indian shrimp waste was found to be rich in carotenoids and exhibited strong antioxidant activity (Sachindra and Bhaskar 2008).
In most of the studies on isolation of carotenoprotein from shrimp byproducts by enzymatic technique, focus was on increased yield of protein and maximizing its recovery (Klomklao et al. 2009; Holanda and Netto 2006; Armenta and Guerrero-Legarreta 2009; Cao et al. 2008; 2009). Protein isolates as well as carotenoids are known to possess strong antioxidant activity. Hydrolyzed proteins from many animal and plant sources, individual peptides and amino acids have been found to possess antioxidant activity. Some amino acids were reported as having strong antioxidant activity in linoleic acid and methyl linoleate model systems (Marcuse 1962). Protein hydrolysate obtained during preparation of chitin from shrimp waste by Bacillus protease was found to have good antioxidant activity (Manni et al. 2010). An autolytic process to prepare antioxidant activity rich carotenoprotein from shrimp waste has been reported recently (Sowmya et al. 2011). The method for isolation of shrimp waste proteins by pH shift technique and the antioxidant activity of such protein isolate has been reported (Meenata et al. 2011). Huang et al. (2011) studied the effect of enzyme type and defatting on the antioxidant activity of shrimp byproduct hydrolysate. In the present study hydrolysis conditions were optimized using response surface methodologies (RSM) to recover, with focus on antioxidant activity of the protein hydrolysate.
Material and methods
Materials
Shrimp waste from Penaeus indicus comprising of head and carapace was collected from a local market, and transported to the laboratory under chilled condition. The material was homogenized in a table top vertical cutter (Robo-Coupe) before use. Alcalase, a bacterial protease, from M/s Genencor was used for hydrolysis.
Optimization of hydrolysis conditions
The effect of three process variables namely enzyme concentration to waste (X1), incubation temperature (X2) and time (X3) on recovery of carotenoid in the hydrolysate (Y1), protein content (Y2), TCA soluble peptide content (Y3) and DPPH scavenging activity (Y4) was evaluated using a fractionally factorial design . The homogenized shrimp waste was mixed with three different levels of enzyme (dissolved in buffer) and incubated at 3 different temperatures for 3 different periods. After specified period, the hydrolysate was recovered by centrifugation. The content of carotenoid, protein, TCA soluble peptide and DPPH scavenging activity was determined in the supernatant. The 9 combinations (Table 1) of the independent variables (X1, X2, X3) were determined with the aid of the software STATISTICA (Statsoft Inc 1999).
Table 1.
Observed and predicted dependent variables at different combinations of independent variables
| Run No. | X1 Enzyme concentration | X2 Temperature | X3 Incubation Time | Y1 Carotenoid recovery (%) | Y2 Protein recovered (mg/g waste) | Y3 TCA soluble peptide recovered (mg/g waste) | Y4 DPPH scavenging activity (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Predicted | Observed | Predicted | Observed | Predicted | Observed | Predicted | ||||
| 1 | 0.1 | 20 | 60 | 71.85 | 74.78 | 32.55 | 31.30 | 3.79 | 3.81 | 28.49 | 39.33 |
| 2 | 0.1 | 35 | 240 | 73.81 | 71.52 | 25.87 | 28.49 | 3.84 | 4.11 | 36.44 | 29.40 |
| 3 | 0.1 | 50 | 150 | 43.92 | 43.29 | 24.69 | 23.33 | 5.11 | 4.82 | 65.14 | 61.34 |
| 4 | 0.3 | 20 | 240 | 82.51 | 81.88 | 28.84 | 27.47 | 3.96 | 3.68 | 42.81 | 39.00 |
| 5 | 0.3 | 35 | 150 | 71.20 | 74.12 | 28.94 | 27.68 | 3.87 | 3.89 | 35.51 | 46.36 |
| 6 | 0.3 | 50 | 60 | 47.81 | 45.51 | 19.17 | 21.79 | 4.56 | 4.83 | 79.52 | 72.48 |
| 7 | 0.5 | 20 | 150 | 78.47 | 76.18 | 13.76 | 16.38 | 3.83 | 4.10 | 77.23 | 70.19 |
| 8 | 0.5 | 35 | 60 | 68.67 | 68.04 | 17.22 | 15.85 | 4.82 | 4.54 | 75.54 | 71.74 |
| 9 | 0.5 | 50 | 240 | 41.39 | 44.32 | 8.93 | 7.68 | 5.33 | 5.35 | 75.54 | 86.39 |
| r | 0.9890 | 0.9685 | 0.9208 | 0.9204 | |||||||
The factors, their levels and codes for the level were as follows.
| Factors | Codes | Level | ||
| −1 | 0 | +1 | ||
| Enzyme concentration (% of wet waste) | X1 | 0.1 | 0.3 | 0.5 |
| Incubation temperature (°C) | X2 | 20 | 35 | 50 |
| Incubation time (mins) | X3 | 60 | 150 | 240 |
Statistical analysis
All the statistical analyses were carried out using the software STATISTICA (Statsoft Inc 1999). The optimization data was analyzed for determination of regression coefficients to arrive at the regression equation. Regression model containing 7 coefficients including linear and quadratic effect of factors was assumed to describe relationships between response (Y) and the experimental factors (X1, X2, X3) as follows,
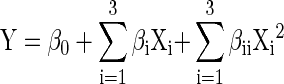 |
1 |
where β0 is the constant coefficient, βi is the linear coefficient of main factors, βii is the quadratic coefficient for main factors. The regression equation arrived was used to predict the different dependent variables in optimization and validation experiments. The 3D response graph and contour plots were plotted using the software (Statsoft Inc 1999). The regression model was further validated using different combinations of the independent variables and determining the regression coefficient between observed and predicted values of different dependent variables.
Determination of carotenoid content, protein and TCA soluble peptide content
Carotenoids in the samples (homogenized waste and the hydrolysate) were extracted by homogenizing the sample with 50 ml of acetone. The extract was filtered and the residue was repeatedly extracted with fresh solvent and filtrate collected till the filtrate is colorless. The solvent extracts were pooled together and were phase separated with equal quantity of hexane. The hexane extract was repeatedly washed with equal quantity of 0.1 % saline to remove traces of acetone if any, and dried with 25 g of sodium sulphate, filtered, flushed with nitrogen for 5 mins, and then evaporated under vacuum at 40 °C using a rotary flash evaporator. The resulting carotenoids concentrate was taken up in hexane and made up to 100 ml and the absorbance of the appropriately diluted extract was measured at 468 nm using spectrophotometer. The carotenoid content was calculated as astaxanthin (Simpson and Haard 1985) using the equation,
 |
Where, A is absorbance, V is volume of extract and 0.2 is the A468 of 1 μg/ml of standard astaxanthin and W is weight of sample in grams.
Protein content in the filtrate was determined by Lowry’s method (Lowry et al. 1951). Total protein content in hydrolysate was calculated and the total protein recovered was determined per gram of wet waste. To determine TCA soluble peptide content in the filtrate, 1 ml of the sample was mixed with 1 ml of 5 % TCA, mixed well using cyclomixer and then the contents were filtered. Protein content in the TCA filtrate was determined by Lowry’s method.
DPPH radical scavenging activity
DPPH radical scavenging was measured by the method of Duan et al. (2006). For determination of DPPH radical scavenging activity an aliquot of sample equivalent to 250 mg protein was made upto 2 ml and mixed with 2 ml of 0.16 mM DPPH in methanol and incubated at 37 °C for 30 min in dark. Sample blank was prepared by replacing the DPPH with methanol. The absorbance of the sample after incubation was measured at 517 nm and the scavenging activity was calculated.
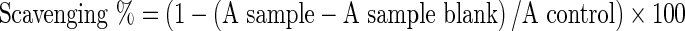 |
Results and discussion
Shrimp waste, especially the head is known to be rich in digestive enzymes such as proteases (Heu et al. 2003; Aoki et al. 2004) and autolysis of shrimp waste utilizing the insitu proteases for recovery of carotenoprotein has been reported (Cao et al. 2008, 2009; Sowmya et al. 2011). Addition of commercial proteases for recovery of shrimp waste carotenoprotein also has been attempted to enhance the yield of carotenoid and protein in hydrolysate (Klomklao et al. 2009). The antioxidant activity of protein hydrolysates is known to be influenced by degree of hydrolysis and enzyme type (Klompong et al. 2006). Further, the functional properties of protein hydrolysates are influenced by several factors such as type and concentration of enzyme, temperature, time and substrate concentration (Kristinsson and Rasco 2000). This study focussed on optimization of hydrolysis condition for recovery of carotenoprotein from shrimp waste with special reference to enhanced antioxidant activity.
Hydrolysis of homogenised shrimp waste was carried out at with different level of enzyme (Alcalase) at different temperatures for different period of time. The resultant hydrolysate was analysed for carotenoid content, protein, TCA soluble peptide and DPPH scavenging activity. The observed values were compared with the predicted value (Table 1) obtained by the regression coefficients derived using the software (Table 2). A high correlation coefficient (>0.90) between the observed and the predicted values indicate the appropriateness of the design employed.
Table 2.
Regression coefficients for predicting different dependent variables
| Carotenoid recovery | Extractable protein | TCA soluble peptide | DPPH radical scavenging | |
|---|---|---|---|---|
| Mean/Interaction (βo) | 44.972 | 21.431 | 4.6813 | 76.769 |
| X1. Enzyme (L) (βi) | 61.405 | 41.173 | −3.807 | −24.873 |
| X2. Enzyme (Q) (βii) | −103.802 | −128.631 | 8.074 | 177.923 |
| X2. Temperature (L) (βi) | 2.076 | 0.587 | −0.0404 | −3.028 |
| X2. Temperature (Q) (βii) | −0.045 | −0.012 | 0.0011 | 0.055 |
| X3. Time (L) (βi) | 0.024 | 0.004 | −0.0044 | 0.054 |
| X3. Time (Q) (βii) | −0.00002 | −0.00005 | 0.0001 | −0.00036 |
With increase in enzyme concentration the recovery yield of carotenoid in carotenoprotein isolate increased at lower temperature of hydrolysis (Fig. 1a). However, hydrolysis at higher temperature resulted in decreased carotenoid yield. An enzyme concentration of 0.2 to 0.4 % and temperature around 25 °C was found to be optimum for higher carotenoid recovery in carotenoprotein isolate. The combined effect of enzyme concentration and time of hydrolysis at a temperature of 35 °C (Fig. 1b) showed that carotenoid recovery slightly increased with increase in hydrolysis time, and lower concentration of enzyme is sufficient to recover higher carotenoids by hydrolysing for longer time. Maximum carotenoid recovery was obtained by hydrolysing the shrimp waste with 0.3 % enzyme for 4 h.
Fig. 1.
Effect of enzyme concentration and temperature on carotenoid recovery (Time = 150 mins) (a) and enzyme concentration and hydrolysis time on carotenoid recovery (Temperature = 35 °C) (b)
Hydrolysis of shrimp waste with proteases was found to enhance the carotenoid recovery (Simpson and Haard 1985; Cano-Lopez et al. 1987; Sachindra and Mahendrakar 2011). Carotenoids occur as a complex with protein in crustaceans (Shahidi et al. 1998) and proteases disrupt the protein-carotenoid bond, thus increasing the carotenoid recovery. Protease treatment for long time enhanced carotenoid recovery when the target is to recover carotenoid either by oil or solvent extraction (Sachindra and Mahendrakar 2011). However, if the target is to extract carotenoprotein, extreme hydrolysis may completely disrupt this bond resulting in lower carotenoid content in the protein isolate. Thus controlled hydrolysis of shrimp waste with proteases with lower enzyme level at lower temperature will help in obtaining the protein isolate rich in carotenoid content.
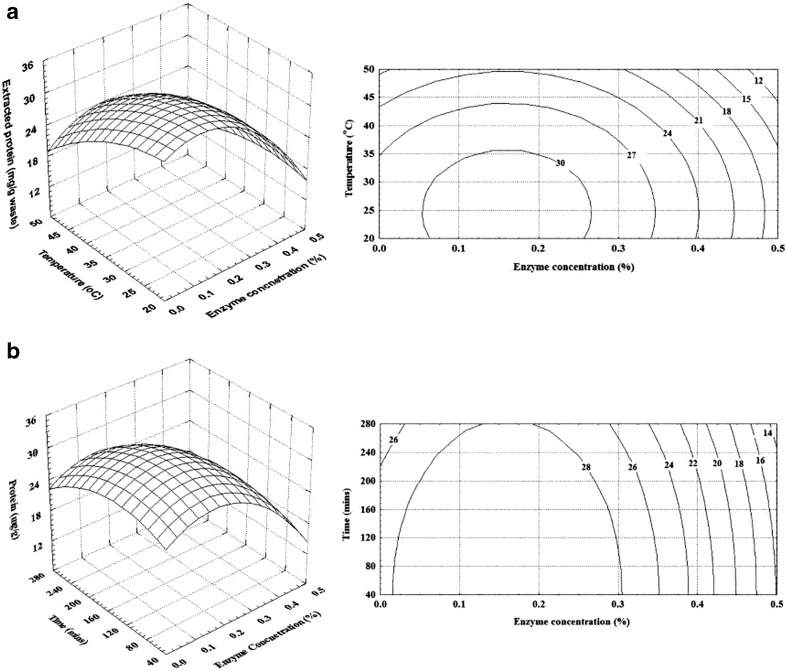
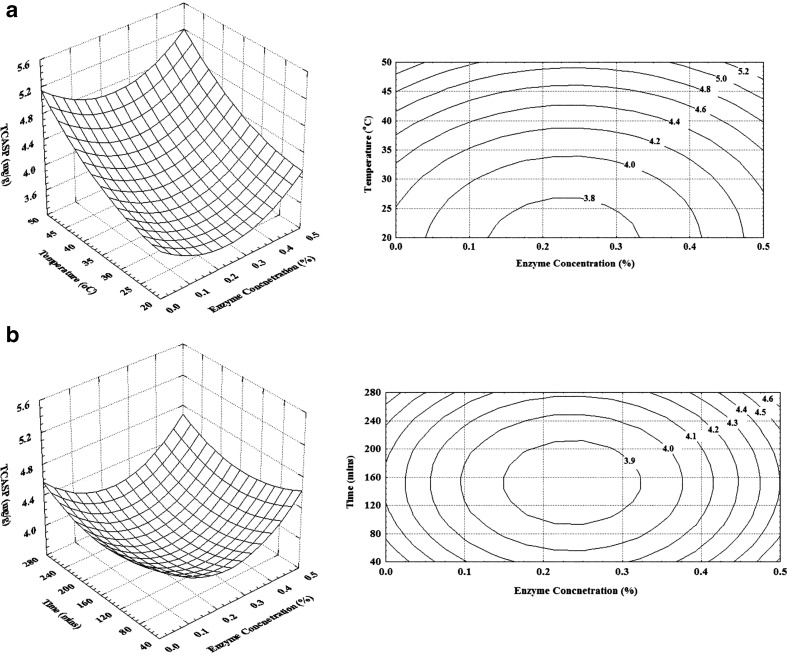
The functional properties of protein isolates obtained by hydrolysis is influenced by degree of hydrolysis (Kristinsson and Rasco 2000) and hence it is important to determine the amount of protein and TCA soluble peptide content in the protein isolates. Extractable protein content decreased with increase in temperature (Fig. 2a). At lower temperature the increase in enzyme concentration did not increase the extractable protein content. Highest extractable protein content was observed at an enzyme concentration of 0.25 % and a temperature of 35 °C. With increase in hydrolysis time not much difference was observed in extractable protein content (Fig. 2b). Enzyme concentration did not show marked effect on the TCA soluble peptide content (Fig. 3a). However, with increase in temperature the TCA soluble peptide content increased considerably, indicating higher degree of protein hydrolysis. TCA soluble peptide content also increased with increase in hydrolysis time (Fig. 3b). TCA soluble peptide content indicates the extent of hydrolysis, increasing with increase in degree of hydrolysis (Sowmya et al. 2011).
Fig. 2.
Effect of enzyme concentration and temperature on extractable protein (Time = 150 mins) (a) and enzyme concentration and hydrolysis time on extractable protein content (Temperature = 35 °C) (b)
Fig. 3.
Effect of enzyme concentration and temperature on TCA soluble peptide (TCASP) (Time = 150 mins) (a) and enzyme concentration and hydrolysis time on TCA soluble peptide (Temperature = 35 °C) (b)
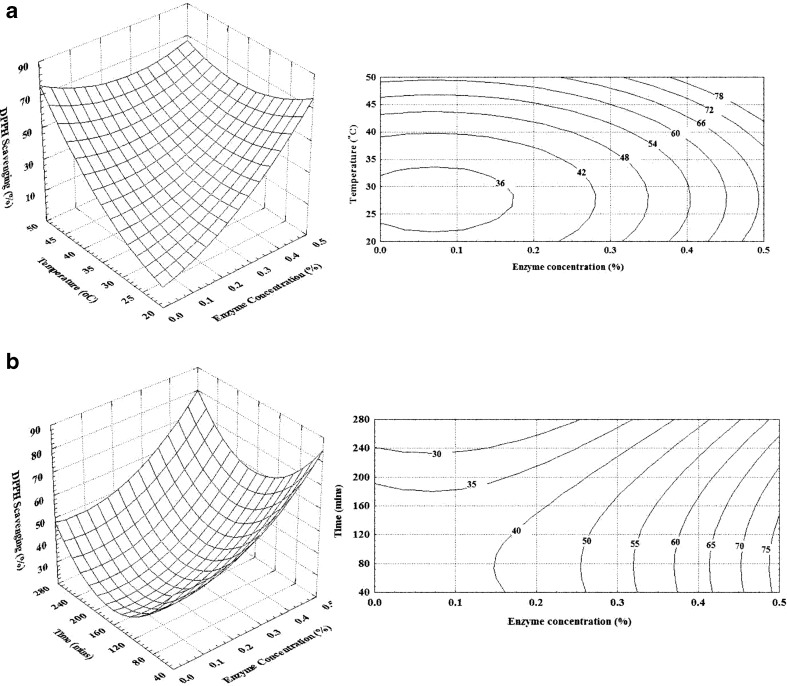
Degree of hydrolysis affects the antioxidant activity of resultant protein hydrolysate (Klompong et al. 2006) with peptides of higher molecular weight possessing higher antioxidant activity (Wu et al. 2003). DPPH scavenging activity is commonly used to evaluate the antioxidant activity. In the present study also DPPH scavenging activity of hydrolysates obtained was determined to arrive at an optimized hydrolysis condition for obtaining antioxidant activity rich protein isolate from shrimp waste. The results showed that DPPH radical scavenging activity of carotenoprotein isolate is markedly affected by enzyme concentration, temperature and time of hydrolysis (Fig. 4a and b). With increase in enzyme concentration to 0.5 % and a temperature of 50 °C more than 78 % scavenging was observed. However time of hydrolysis did not had a marked effect on DPPH scavenging activity of the protein isolate. At lower enzyme concentration, increase in hydrolysis time did not result in higher scavenging activity, but with increased enzyme concentration, shorter time was sufficient to obtain higher antioxidant activity in the hydrolysate.
Fig. 4.
Effect of enzyme concentration and temperature of hydrolysis on DPPH scavenging activity (Time = 150 mins) (a) and enzyme concentration and hydrolysis time on DPPH scavenging activity of carotenoprotein isolate (Temperature = 35 °C) (b)
Carotenoprotein from shrimp comprises of carotenoids and hydrolysed protein, and both exhibit antioxidant activity (Suetsuna 2000; Sachindra et al. 2007b). Shrimp protein hydrolysates exhibit strong antioxidant activity and the peptides present in shrimp cephalothorax were found to be responsible for antioxidant activity (Binsan et al. 2008). Shrimp waste also contains other natural antioxidants such as phenolic compounds (Seymour et al. 1996). Hence several factors influence the antioxidant activity of protein isolates from shrimp waste. In the present study the DPPH scavenging activity was determined at a protein concentration of 250 mg. When the DPPH scavenging activity was compared with the carotenoid content and TCA soluble peptide content of the sample equivalent to 250 mg protein, it was observed that the correlation coefficient was 0.65 and 0.67 respectively. This indicates that the antioxidant activity of the isolates does not depend on any one component of the hydrolysate.
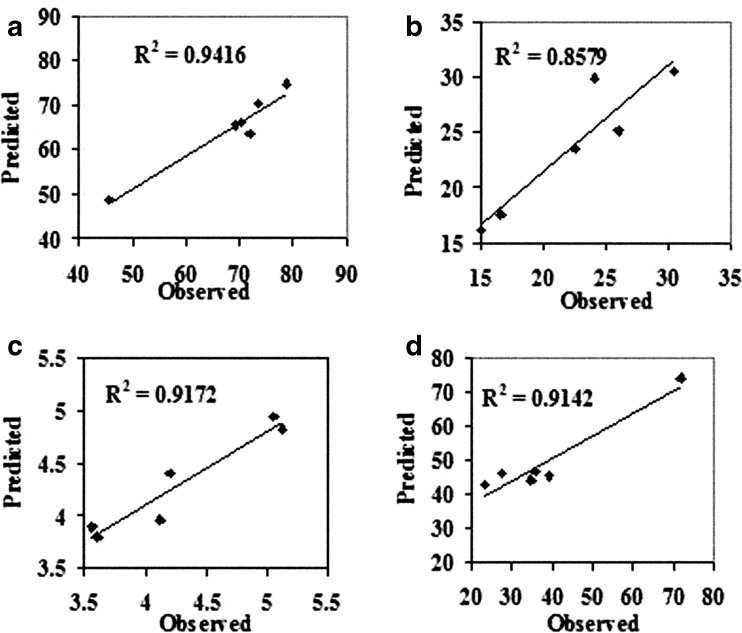
By optimization experiments regression coefficients for different independent factors were obtained (Table 2). By substituting these regression coefficients in the regression equation (Eq. 1) the values for different dependent variables can be predicted at different combinations of independent variables. For validation of the regression model 6 different combinations of independent variables (enzyme concentration, temperature and time) were used and different dependent variables (carotenoid yield, extractable protein content and DPPH scavenging activity) were determined. The observed and predicted values were plotted to obtain regression coefficient (R2) between the two (Fig. 5). It was observed that the regression coefficient was above 0.9 for all the three variables indicating the fit of the regression model for prediction.
Fig. 5.
Predicted V/s observed carotenoid yield (a), extractable protein content (b), TCA soluble peptide content (c) and DPPH scavenging activity (d) in validation experiment
Conclusion
The study indicated that in order to obtain the carotenoprotein from shrimp waste with higher carotenoid content hydrolysis using the bacterial enzyme Alcalase, an enzyme concentration of 0.2–0.4 % and hydrolysing at lower temperature of 25–30° upto 4 h is ideal. However, in order to obtain the protein isolate with increased antioxidant activity hydrolysing at higher temperature of 50 °C, with higher enzyme concentration of 0.5 % for shorter duration is more ideal. The resultant carotenoprotein isolate, due to its high antioxidant activity would find use in food and feed applications. The efficient use of the shrimp waste would benefit not only the industry but also results in reduction in the pollution potential of the waste.
Acknowledgments
Authors wish to thank Director, CFTRI for his encouragement and for the facilities provided. This study formed a part of the project funded by Department of Biotechnology, Govt. of India.
References
- Aoki H, Ahsan M, Matsuo K, Hagiwara T, Watabe S. Partial Purification of proteases that are generated by processing of the Northern shrimp Pandalus borealis and which can tenderize meat. Int J Food Sci Technol. 2004;39:471–480. doi: 10.1111/j.1365-2621.2004.00806.x. [DOI] [Google Scholar]
- Armenta RE, Guerrero-Legarreta I. Amino acid profile and enhancement of the enzymatic hydrolysis of fermented shrimp carotenoproteins. Food Chem. 2009;112:310–315. doi: 10.1016/j.foodchem.2008.05.075. [DOI] [Google Scholar]
- Bhaskar N, Suresh PV, Sakhare PZ, Lalitha RG, Sachindra NM. Yield and chemical composition of fractions obtained from fermented shrimp biowaste. Waste Manag Res. 2010;28:64–70. doi: 10.1177/0734242X09337658. [DOI] [PubMed] [Google Scholar]
- Binsan W, Benjakul S, Visessanguan W, Roytrakul S, Tanaka M, Kisimura H. Antioxidative activity of mungoong an extract paste from the cephalothorax of white shrimp (Litopenaeus vannamei) Food Chem. 2008;106:185–193. doi: 10.1016/j.foodchem.2007.05.065. [DOI] [Google Scholar]
- Cano-Lopez A, Simpson BK, Haard NF. Extraction of carotenoprotein from shrimp process wastes with the aid of trypsin from Atlantic cod. J Food Sci. 1987;52:503–506. doi: 10.1111/j.1365-2621.1987.tb06656.x. [DOI] [Google Scholar]
- Cao W, Zhang C, Hong P, Ji H. Response surface methodology for autolysis parameters optimization of shrimp head and amino acids released during autolysis. Food Chem. 2008;109:176–183. doi: 10.1016/j.foodchem.2007.11.080. [DOI] [PubMed] [Google Scholar]
- Cao W, Zhang C, Hong P, Ji H, Hao J, Zhang J. Autolysis of shrimp head by gradual temperature and nutritional quality of the resulting hydrolysate. LWT Food Sci Technol. 2009;42:244–249. doi: 10.1016/j.lwt.2008.05.026. [DOI] [Google Scholar]
- Duan XJ, Zhang WW, Li XM, Wang BG. Evaluation of antioxidant property of extract and fractions obtained from red alga Polysiphonia urceolata. Food Chem. 2006;95:37–43. doi: 10.1016/j.foodchem.2004.12.015. [DOI] [Google Scholar]
- Ghidalia W. Structural and biological aspects of pigments. In: Bliss DE, Mantel LH, editors. The biology of crustacea. New York: Academic; 1985. pp. 301–394. [Google Scholar]
- Heu MS, Kim JS, Shahidi F, Jeong YH, Jeon YJ. Characteristics of protease from shrimp processing discards. J Food Biochem. 2003;27:221–236. doi: 10.1111/j.1745-4514.2003.tb00278.x. [DOI] [Google Scholar]
- Holanda HD, Netto FM. Recovery of components from shrimp (Xiphopenaeus kroyeri) processing waste by enzymatic hydrolysis. J Food Sci. 2006;71:298–303. doi: 10.1111/j.1750-3841.2006.00040.x. [DOI] [Google Scholar]
- Huang G, Zhao J, Jiang J. Effect of defatting and enzyme type on antioxidative activity of shrimp processing byproducts hydrolysate. Food Sci Biotechnol. 2011;20:651–657. doi: 10.1007/s10068-011-0092-8. [DOI] [Google Scholar]
- Klomklao S, Benjakul S, Visessanguan W, Kishimura H, Simpson BK. Extraction of carotenoprotein from black tiger shrimp shells with the aid of bluefish trypsin. J Food Biochem. 2009;33:201–217. doi: 10.1111/j.1745-4514.2009.00213.x. [DOI] [Google Scholar]
- Klompong V, Benjakul S, Kanatachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2006;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Kristinsson HG, Rasco BA. Fish protein hydrolysates: production biochemical and functional properties. Crit Rev Food Sci Nutr. 2000;40:43–81. doi: 10.1080/10408690091189266. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Fan AL, Randall RJ, Rosebrough NJ. Protein measurement with Folin Phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Manni L, Ghorbel-Bellaaj O, Jellouli K, Younes I, Nasri M. Extraction and characterization of chitin chitosan and protein hydrolysates prepared from shrimp waste by treatment with crude protease from Bacillus cereus SV1. Appl Biochem Biotechnol. 2010;162:345–357. doi: 10.1007/s12010-009-8846-y. [DOI] [PubMed] [Google Scholar]
- Manu-Tawai W, Haard NF. Recovery of carotenoprotein from the exoskeleton of snow crab Chinocetes opilio. Can Inst Food Sci Technol J. 1987;20:31–33. doi: 10.1016/S0315-5463(87)70666-X. [DOI] [Google Scholar]
- Marcuse R. The effect of some amino acids on the oxidation of linoleic acid and its methyl ester. J Am Oil Chem Soc. 1962;39:97–103. doi: 10.1007/BF02631680. [DOI] [Google Scholar]
- Meenata K, Sowmya R, Rathinaraj K, Sachindra NM. Antioxidant activity of carotenoprotein isolate from shrimp processing discards. J Aquac Food Prod Technol. 2011;20:209–221. doi: 10.1080/10498850.2011.559618. [DOI] [Google Scholar]
- Sachindra NM, Bhaskar N. In-vitro antioxidant activity of liquor from fermented shrimp biowaste. Bioresour Technol. 2008;99:9013–9016. doi: 10.1016/j.biortech.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Sachindra NM, Mahendrakar NS. Extractability of carotenoids from shrimp waste in vegetable oils and process optimization. Bioresour Technol. 2005;96:1195–1200. doi: 10.1016/j.biortech.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Sachindra NM, Mahendrakar NS. Stability of carotenoids recovered from shrimp waste and their use as colorant in fish sausage. J Food Sci Technol. 2010;47:77–83. doi: 10.1007/s13197-010-0019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachindra NM, Mahendrakar NS. Effect of protease treatment on oil extractability of carotenoids from shrimp waste. J Aquac Food Prod Technol. 2011;20:22–31. doi: 10.1080/10498850.2010.526754. [DOI] [Google Scholar]
- Sachindra NM, Bhaskar N, Mahendrakar NS. Carotenoids in different body components of Indian shrimps. J Sci Food Agric. 2005;85:167–172. doi: 10.1002/jsfa.1977. [DOI] [Google Scholar]
- Sachindra NM, Bhaskar N, Mahendrakar NS. Carotenoids in Solonocera indica and Aristeus alcocki, deep-sea shrimps from Indian waters. J Aquac Food Prod Technol. 2006;15:5–16. [Google Scholar]
- Sachindra NM, Bhaskar N, Mahendrakar NS. Recovery of carotenoids from shrimp waste in organic solvents. Waste Manage. 2006;26:1092–1098. doi: 10.1016/j.wasman.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Sachindra NM, Bhaskar N, Siddegowda GS, Sathisha AD, Suresh PV. Recovery of carotenoids from ensiled shrimp waste. Bioresour Technol. 2007;98:1642–1646. doi: 10.1016/j.biortech.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Sachindra NM, Hosokawa M, Miyashita K. Biofunctions of marine carotenoids. In: Hou CT, Shaw J, editors. Biocatalysis and biotechnology for functional foods and industrial products. NewYork: CRC Press; 2007. pp. 91–110. [Google Scholar]
- Seymour TA, Li SJ, Morrissey MT. Characterisation of a natural antioxidant from shrimp shell waste. J Agri Food Chem. 1996;44:682–685. doi: 10.1021/jf950597f. [DOI] [Google Scholar]
- Shahidi F, Metusalach, Brown JA (1998) Carotenoid pigments in seafoods and aquaculture. CRC Crit Rev Food Sci 38:1–67 [DOI] [PubMed]
- Simpson BK, Haard NF. The use of enzymes to extract carotenoprotein from shrimp waste. J Appl Biochem. 1985;7:212–222. [Google Scholar]
- Sowmya R, Rathinaraj K, Sachindra NM. An autolytic process for recovery of antioxidant activity rich carotenoprotein from shrimp heads. Mar Biotechnol. 2011;13:918–927. doi: 10.1007/s10126-010-9353-4. [DOI] [PubMed] [Google Scholar]
- Statsoft . Statistics for windows. Tulsa: Statsoft Inc; 1999. [Google Scholar]
- Suetsuna K. Antioxidant peptides from the protease digest of prawn (Penaeus japonicus) muscle. Mar Biotechnol. 2000;2:5–10. doi: 10.1007/s101269900002. [DOI] [PubMed] [Google Scholar]
- Wu HC, Chen HM, Shiau CY. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackeral (Scomber austriasicus) Food Res Int. 2003;36:949–957. doi: 10.1016/S0963-9969(03)00104-2. [DOI] [Google Scholar]
- Zagalsky PF. Invertebrate carotenoproteins. Methods Enzymol. 1985;111:216–247. doi: 10.1016/S0076-6879(85)11011-6. [DOI] [PubMed] [Google Scholar]
- Zagalsky PF, Eliopoulos EE, Findlay JBC. The architecture of invertebrate carotenoproteins. Comp Biochem Physiol. 1990;97B:1–18. doi: 10.1016/0305-0491(90)90171-o. [DOI] [PubMed] [Google Scholar]