Abstract
Cinnamon deodorised aqueous extract (CinDAE) was prepared and evaluated for its total phenolic (315.3 ± 35.4 mg GAE/g) and flavonoid (99.3 ± 9.6 mg RE/g) contents. Stabilizing efficiency of CinDAE, for chicken meatballs, was measured against oxidative deterioration as function of storage time under chilled conditions. For this purpose, oxidative stability [2-thiobarbituric acid reactive substances (TBARS); peroxide value (PV)], colour and sensory acceptability were measured in the control meatballs (C), and those stabilized with 200 ppm of: CinDAE (T1), ascorbic acid (T2), BHA/BHT (50/50; w/w) (T3). In comparison to “C”, induction period (IP) and redness (a* value) of the stabilized samples (T1, T2 and T3) were increased, while PV and TBARS were decreased throughout storage (8 ± 1 °C) significantly (p < 0.05). Meanwhile, CinDAE slightly decreased L* value of the meatballs as compared to other tested samples. Conclusively, CinDAE improved stability and redness of chicken meatballs without negatively affecting its sensory acceptability (Hedonic test) up to a comparable extent to that of ascorbic acid/BHA/BHT and may potentially function as a dietary antioxidant for meat products.
Keywords: Cinnamon bark, Deodorised extract, Oxidative stability, Chicken meatballs, Colour, Sensory acceptability
Introduction
The industry of meat and meat products demands eagerly for what is perceived as eating quality as well as nutritional quality. Inevitably, lipid peroxidation begins since the moment of slaughter and continues progressively during processing or storage of meat and meat products, which noticeably affects the nutritional and sensorial properties of meat products (Shahidi et al. 1992). Heating of meat, followed by low temperature storage, always results in development of warmed-over flavours (WOF) (Stodolak et al. 2007). Besides, products of lipid peroxidation may potentially contribute to various disease syndromes (Anwar et al. 2007). Hence, it is crucial to prevent or delay the process of lipid peroxidation in meat products.
Addition of antioxidants has always been an effective means to control lipid peroxidation in lipid-based foods including meat products (Shahidi and Wanasundara 1992). Synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) have been effectively being used for the stabilization of meat products against lipid peroxidation, but safety concerns associated with and their role in causing chronic diseases like cancer have restricted their use in food products (Iqbal et al. 2007). A good alternative for these synthetic antioxidants are natural antioxidants which are safer, economical and capable of preventing oxidative deterioration of food products and alleviating metabolic diseases at the same time (Ismail et al. 2010a, b; Iqbal et al. 2012a). A number of these natural antioxidants, derived mainly from routine dietary items or indigenous materials have been explored and proven effective for the stabilization of vegetable oil, cookies and meat products (Iqbal and Bhanger 2007; Bhanger et al. 2008; Chan et al. 2012a, b).
Spices have been used extensively as natural flavouring, colouring and preservative agents in food products for centuries, particularly in India, Pakistan, China and other Southeast Asian countries. Apart from being used as flavouring agents in the culinary arts, potential of spices has also been highlighted previously to improve protection of meat (Hernandez-Ochoa et al. 2011) and the oxidative stability of food products (Bukhari et al. 2008). Cinnamon (Cinnamomum sp.) is one of the very frequently used spices in many countries since ancient time. It is often added to food for better taste and aroma of food. It is even used as part of Ayurvedic treatments in India. Cinnamon bark is a common culinary spice used in Malaysian curry formulations. Its availability along the year makes it further popular. Lately, cinnamon bark has been reported to be possessing potent antioxidants (Singh et al. 2007; Chan et al. 2012a); comparable to that of synthetic antioxidants (Mathew and Abraham 2006) with promising potentials to improve oxidative stability of foods. Use of cinnamon could not gain high momentum due to pungent odour, taste of cinnamon powder, essential oils and organic solvents-extracted oleoresins for direct use in food products (Hinneburg et al. 2006). Moreover, cinnamon extracts might contain residual organic solvents and this adds to the aversion of incorporating these extracts into food products.
To overcome these concerns limiting the usage of this source of potent natural antioxidant at major scale, aqueous extraction is carried out under optimized set of conditions. As water is reported to have lower extractability of pungent flavoured compounds in comparison to organic solvents like alcohols (Anderson et al. 2003; Galedar et al. 2010), aqueous extraction is best used for the production of food ingredients with considerably good consumer acceptability. Moreover, aqueous extraction is cheaper, non-toxic and environmental friendly. No report describing the potential of cinnamon extract in stabilizing meat products is presented so far. Thus, the current study aimed to investigate the effects of adding cinnamon bark deodorized aqueous extract (CinDAE) on the oxidative stability and sensory acceptability of chicken meatballs in comparison to synthetic antioxidant (BHA/BHT) and natural antioxidant (ascorbic acid). This work, for the first time, presents an economical, safer and effective extraction protocol coupled with comprehensive instigations on oxidative stability and sensory acceptability of chicken meatballs, supplemented with CinDAE.
Materials and methods
Plant material and chemicals
Dried cinnamon barks (Cinnamomum verum) were purchased from Giant Hypermarket, Kajang, Selangor, Malaysia.
All the chemicals used in this study were of analytical grade as: L (+)-ascorbic acid, soluble starch, sodium thiosulphate (99.5 %), 2-thiobarbituric acid (TBA; ≥98 %), butylated hydroxyanisole (BHA; 90 % 3-isomer: 9 % 2-isomer), butylated hydroxytoluene (BHT; 99 %), gallic acid, rutin and aluminium trichloride (Sigma–Aldrich, Steinheim, Bayern, Germany); Folin-Ciocalteu reagent, chloroform (99.4 %) and trichloroacetic acid (TCA; 99.5 %) (Merck, Darmstadt, Hesse, Germany); sodium carbonate, methanol (99.8 %) and potassium iodide (99.5 %) (BDH VWR International, Lutterworth, Leichestershire, England); and concentrated hydrochloric acid (37 %) (R&M Chemicals, Chelmsford, Essex, United Kingdom).
Preparation of cinnamon bark deodorised aqueous extracts (CinDAE)
The cinnamon barks were cleaned and dried in an oven (Binder, Great River, New York, USA) at 50 °C until constant weight was attained; with the final moisture content being less than 5 %. The cinnamon barks were then pulverized for 3 min with a stainless steel blender (Waring Commercial, Torrington, CT, USA) followed by sieving through a sieve of mesh size 30 before being added to hot water (100 °C) at ratio of 1:20. The mixture was stirred using a magnetic stirrer for 15 min and filtered through Whatman No. 1 filter paper. Water was removed from filtrate under reduced pressure (Rotavapor R210, Buchi, Postfach, Flawil, Switzerland). Finally, the yield of CinDAE was calculated and CinDAE was kept at −18 °C prior to further analyses.
Determination of total phenolic and flavonoid contents
Total phenolic content
Determination of total phenolic content of CinDAE was carried out following a previously reported method with slight modifications (Iqbal et al. 2012b). Briefly, 0.1 ml of spice extracts was mixed with 1 ml of distilled water and resulting mixture was added to 0.5 ml of Folin-Ciocalteu reagent. The mixture was vortexed for 3 min before the addition of sodium carbonate (1.5 ml; 20 %) and distilled water (6.9 ml). Subsequently, the mixture was mixed well and incubated at 40 °C for 30 min. Finally, the absorbance of the samples was measured at 765 nm by using a spectrophotometer (Pharmaspec uv-1700, Shimadzu, Kyoto, Japan). Gallic acid was used to generate the calibration curve (linear between 60 and 2,000 ppm) and calibration equation for gallic acid was Y = 0.0013× + 0.004,1 (R2 = 0.9998). Total phenolic content was expressed as mg gallic acid equivalents (GAE)/g CinDAE.
Total flavonoid content
Total flavonoid content was determined following a previously reported procedure (Iqbal et al. 2007) with minor modifications. Equal amounts of CinDAE were reacted with 2 % (w/v) AlCl3 for 10 min. After that, the absorbance of the mixture was measured at 415 nm. Rutin was used to prepare calibration curve (Y = 0.011 × +0.0663, R = 0.9955), with linearity in the range of 25 to 500 ppm rutin. Total flavonoid contents were expressed as mg rutin equivalents (RE)/g CinDAE).
Chicken meatball processing and storage
In brief, the chicken meatball samples consisted of C (control meatballs), T1 (meatballs supplemented with 200 ppm of CinDAE), T2 (meatballs supplemented with 200 ppm of ascorbic acid) and T3 (meatballs supplemented with 200 ppm of BHA and BHT combination i.e. 50 % + 50 %). The storage quality evaluation of chicken meatballs was carried out according to Accelerated Shelf Life Testing (ASLT) protocol as described by Institute of Food Science and Technology (IFST 1993).
Chicken meatballs were prepared in the laboratory using following ingredients (w/w): 65 % minced chicken breast, 20 % Socfat, 40 palm fat (Cargill palm products Sdn. Bhd., Klang, Selangor, Malaysia), 6.5 % cold distilled water, 6.0 % potato starch (Griffith Laboratories, Phra Pradaeng, SamutPrakan, Thailand), 1.5 % sunflower seed oil (Mazola, Amornchai Co. Ltd., Bangrak, Bangkok, Thailand) and 1.0 % salt.
Firstly, minced chicken breast meat was mixed well with cold distilled water (<4 °C), potato starch and salt in a mixer (N-50, Hobart, North York, Ontario, Canada) for 1.5 min followed by addition of sunflower seed oil and Socfat 40 palm fat into the batter and mixing again for 3.5 min to form an emulsified batter. After that, 200 ppm CinDAE or BHA/BHT or ascorbic acid were mixed thoroughly for 5 min into the batter and moulded into balls with 20 ± 5 mm diameter and weighing up to 15 ± 1 g. Subsequently, the chicken meatballs were flash fried at 180 °C for 30 s in 1 kg of palm olein (Soon Hup Edible Oil Sdn. Bhd., Kepong, Kuala Lumpur, Malaysia) before cooking in the oven (Panasonic, Kadoma, Osaka, Japan) at 250 °C for 4 min. The chicken meatballs were then cooled immediately to 4 °C in a refrigerator. When the internal temperature of the chicken meatballs dropped to approximately 12 °C, they were immediately transferred to a plastic container covered with an oxygen semi-permeable polyvinyl chloride (PVC) film and stored in dark at 8 ± 1 °C for 12 days. Analyses were done regularly after 1, 3, 6, 9 and 12 days.
Oxidative stability assessment
Peroxide value (PV) determination
For determination of peroxide value (PV), oil was obtained from meatball samples according to method reported previously (Kinsella et al. 1977). In brief, meatball samples (90 g) were homogenised with methanol and chloroform at ratio of 1:2:1 (w:v:v), using Ultra-Turrax T25 basic (IKA®123-WERKE GmbH & Co. KG, Staufen, Germany) for 2 min before it was added with another 90 ml of chloroform and distilled water, respectively. Subsequently, the mixture was homogenised for another 30 s and filtered through Whatman No. 1 filter paper by separation funnel. Approximately after 1 h, chloroform layer of the filtrate was withdrawn and solvent was removed using rotary evaporator, under reduced pressure (Rotavapor R210, Buchi, Postfach, Flawil, Switzerland). Oil obtained, following the above mentioned methodology, was used for PV determination protocol (Iqbal and Bhanger 2007). Peroxide value of the tested sample was expressed in meq peroxide/kg sample.
Thiobarbituric acid reactive substances (TBARS) assay
Thiobarbituric acid reactive substances (TBARS) of the meatball samples were determined according to method described previously (Iqbal and Bhanger 2007) with slight modifications. In brief, 0.5 g of blended meatball samples were respectively added to 2.5 ml of 0.25 N HCl, 2.5 ml of TCA (15 %, w/v) and 2.5 ml of TBA (0.375 %, w/v). The mixture was then vortexed and heated in a hot water bath (100 °C) for 10 min to develop the pink chromogen. Then, the mixtures were cooled under tap water before adding chloroform (1 ml). Next, the mixtures were centrifuged at 5,500 rpm for 25 min. Finally, the absorbance of the supernatant was measured at 532 nm against a blank. TBARS value of the meatball samples was expressed in mg malonaldehyde (MDA)/kg sample.
Colour evaluation
The colour evaluation of the chicken meatball samples was conducted using Chromameter Minolta CR-100 Tristimulus Colour Analyzer, which gave CIELAB colour evaluation in the form of lightness (L*), redness (a*) and yellowness (b*). Three random measurements per sample were taken. The colourimeter was calibrated by using a standard white ceramic plate prior to colour measurement.
Sensory evaluation
A 7-scale hedonic test was performed on 70 untrained panellists in a sensory laboratory, i.e. Food Science Program, School of Chemical Sciences and Food Technology, Faculty of Science and Technology, National University of Malaysia, to determine the sensory acceptability of chicken meatballs supplemented with CinDAE. The panellists were students and staffs of National University of Malaysia. Only fresh chicken meatball samples that were kept for 1 day were used in this test and the sensory attributes evaluated were colour, aroma, taste, texture and overall acceptance.
Statistical analysis
Data obtained from the study was analysed statistically with analysis of variance (ANOVA) and Tukey’s test by using SAS Version 6.21 (1995) to identify the significant difference between samples. Each analysis was conducted thrice, results were averaged and data is reported as mean ± S.D.
Results and discussion
Total phenolic and flavonoid contents
Total phenolic content of cinnamon bark deodorised aqueous extract (CinDAE) was calculated to be 315.3 ± 35.4 mg GAE/g CinDAE using standard curve of gallic acid. This is in agreement with the findings of an earlier study (Shan et al. 2005), which showed that cinnamon contains a high amount of phenolics. Total flavonoid content was found to be 99.3 ± 9.6 mg RE/g CinDAE through the rutin standard curve. The appreciable contents of phenolics and flavonoids suggest CinDAE to be a potent antioxidant.
Oxidative stability of chicken meatballs
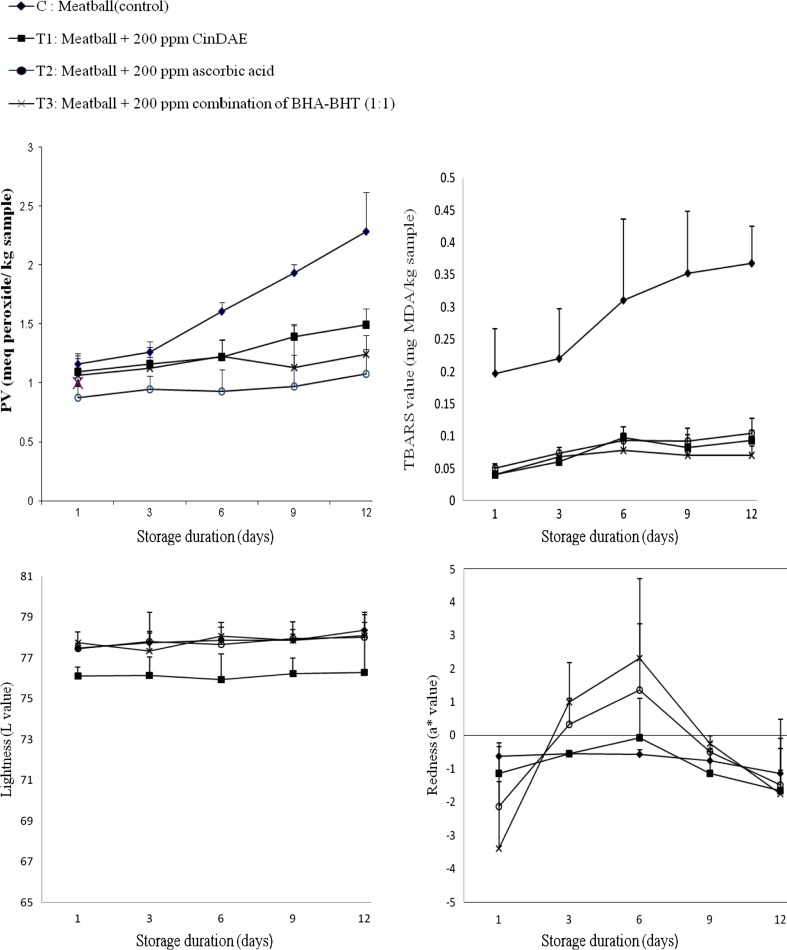
The meatball samples were subjected to peroxide value (PV) and thiobarbituric acid reactive substances (TBARS) assays to determine the primary and secondary oxidation products formed, respectively. Figure 1 represents PV and TBARS values of tested chicken meatballs samples, i.e. C, T1, T2 and T3 over the entire range of 12 days chilled storage period. The PV and TBARS values were in the range of 0.878–1.161 meq peroxide/kg sample and 0.041–0.197 mg MDA/kg sample respectively on Day 1 of chilled storage. At the end of 12-day storage duration, the range was 1.076–2.287 meq peroxide/kg sample and 0.070–0.386 mg MDA/kg sample correspondingly. The increase of both PV and TBARS values mark the commencement and progression of lipid oxidation in the meatball samples.
Fig. 1.
Changes in rancidity, Hunter colour values of chicken meatballs during storage at 8 ± 1 °C (n = 3). Abbreviations expansion: CinDAE cinnamon bark deodorised aqueous extracts; ppm part per million; BHT butylated hydroxytoluene; BHA butylated hydroxyanisole
From Fig. 1, the control sample showed minimal PV changes, which indicate the occurrence of an induction period (IP) from Day 1 to 3 of chilled storage followed by sharp rise in PV in the following days; suggesting termination of IP (p < 0.05). Meatballs supplemented with CinDAE (T1) showed the termination of IP on 9th day of storage, the difference in shelf-life is too much as compared to control; revealing appreciable stabilizing potential of CinDAE against lipid peroxidation. The induction period is a common occurrence during lipid autoxidation, in which the oxidative changes are minimal. The rate of lipid oxidation increases after the induction period, causing easier detection of rancidity or a “warmed-over” flavour (Gordon 2001; Herken and Guzel 2010). On the other hand, other meatball samples (T2 and T3) did not show the termination of IP, even till the end of storage period, implying that supplementation of 200 ppm ascorbic acid and BHA/BHT can inhibit the formation of hydroperoxide in the meatball samples, upto much longer periods of time.
Throughout the entire storage period, it was found that meatball samples T1, T2 and T3 demonstrated lower PV compared to control sample (C). At the end of storage duration, CinDAE (T1), ascorbic acid T2) and BHA/BHT (T3) were able to reduce the PV value in chicken meatball for approximately 34.8–53 %, compared to C; in the order given as: ascorbic acid ≥ BHA/BHT ≥ cinnamon bark extract (p > 0.05). The lowered PV suggests that less primary oxidative products were formed in meatball samples, likely caused by the action of chain-breaking antioxidants such as phenolics and flavonoids present in CinDAE, which inhibit further lipid peroxidation in the meatball samples.
Meanwhile, Fig. 1 shows that the control sample (C) possessed the highest TBARS value, i.e. 0.197 mg MDA/kg sample, on the first day of storage compared to all other meatball samples (p < 0.05). However, the PV for all samples was similar and in the range of 0.878–1.161 meq peroxide on Day 1 of chilled storage. This indicates that hydroperoxides in the control sample are broken down to form malondialdehyde at a higher rate than all other samples; at the beginning of storage period. The acceleration of lipid peroxidation is most likely due to cooking of the meatball samples at 250 °C before chilled storage. The heating process is one of the main prooxidative processes of meat products preparation and it might last till the early stages of refrigeration (Jo et al. 2003). However, additions of CinDAE, BHA/BHT and ascorbic acid to the meatball samples have impeded the progression of lipid oxidation in the meatballs, thus resulting in lower TBARS values compared to control sample (C).
Samples that were mixed with CinDAE, ascorbic acid and BHA/BHT, i.e. T1, T2 and T3, exhibited the ability to decrease TBARS values of tested chicken meatballs as much as 71.7–81 % in comparison to the control sample (C) in the following sequence: BHA/BHT (T3) > CinDAE (T1) > ascorbic acid (T2) (p < 0.05). Reduction of TBARS values in meatball samples supplemented with CinDAE, ascorbic acid and BHA/BHT is likely due to lower accumulation of hydroperoxides in T1, T2 and T3 as mentioned above. In a previous study, Kumar et al. (2011) reported TBARS value of 0.40–0.45 mg MDA/kg in chicken nuggets formulated with 4 % green banana and soybean hulls flour on the 15th day of refrigeration. Hence, it can be inferred that CinDAE may possess hydroperoxide inhibition capability or secondary antioxidant activity comparable or better than the synthetic antioxidants used in this study. However, the secondary antioxidant action of CinDAE is not clearly demonstrated in the present study.
Data obtained from PV and TBARS assays suggest that CinDAE is a good antioxidant with activity comparable to both the reference antioxidants, i.e. ascorbic acid and BHA/BHT, at the same concentration of 200 ppm. An earlier study (Singh et al. 2007) showed that cinnamon oils and oleoresins have comparable antioxidant activities with BHA and propyl gallate (PG), as demonstrated by TBARS, PV and linoleic acid systems. These findings are parallel to the present study, which supports that extracts from cinnamon bark possess high antioxidant activity, thus ensuring that CinDAE can effectively lower both PV and TBARS values of the tested meatball samples. In addition, our previous study (data not shown) showed that CinDAE contains large amounts of phenolic compounds, i.e. gallic acid and other flavonoids, which might be responsible for the antioxidant activity of CinDAE. As such, this suggests that the cinnamon extract obtained through hot water extraction also possessed high antioxidant activity as proven through the meatball model in the present study.
Colour evaluation for chicken meat ball
As shown in Fig. 1, all samples experienced minimal changes in the degree of lightness (L*) throughout the storage period. L* values for all samples were in the range of 76.115 ± 0.431 to 77.758 ± 0.523 on Day 1 and 76.281 ± 1.883 to 78.359 ± 0.755 at the end of the storage period. No significant differences were observed between the control sample (C) with CinDAE (T1) and the standard antioxidants (T2 and T3) (p < 0.05). However, addition of 200 ppm CinDAE decreased L* value of sample T1 more than the control sample and reference antioxidants (T2 and T3) (p < 0.05). Reduction of the degree of lightness is possibly due to the dark brownish colour of CinDAE. Previously, the incorporation of thuja cones extract (TCE) and peach seed extract (PSE) into raw chicken ground meat also observed gradual decrease in L value during refrigerated (4 ± 1 °C) storage (Yogesh and Ali 2012).
Degree of redness (a*) is generally considered as a parameter used as a colour indicator to determine the freshness of meat products. (Tang et al. 2006) Based on Fig. 1, comparison between all samples (T1, T2 and T3) with the control sample (C) did not show significant difference in a* value throughout the storage period. Generally, a* values for all samples were in the range of −1.261 to −0.517 on the first day and −1.146 to 0.16 on the final day of chilled storage.
The control sample (C) showed negative a* values throughout the storage period, indicating that only green colour was detected in the sample. This is possibly due to the occurrence of exhaustive oxidation of the red-pigmented myoglobin in the meatballs to metmyoglobin after the heating process. During this process, high temperature causes denaturation of protein globin thus accelerating the oxidation process of myoglobin to metmyoglobin, hence giving a slightly greenish colour to the chicken meatball samples (Ledward 1971). Moreover, the exhaustive oxidation of all myoglobin to metmyoglobin is possible because of the low distribution of myoglobin in chicken breast meat (Blessing and Muller 1974).
Comparison between samples show that meatballs supplemented with 200 ppm CinDAE (T1), ascorbic acid (T2) and BHA/BHT (T3) exhibited only marginal increment of a* values (without significant difference) compared to the control sample (C) in the order: ascorbic acid > BHA/BHT > CinDAE (p > 0.05). This data implies that CinDAE may serve as a suitable antioxidant with activities similar to both the reference antioxidants, which are commonly used as the agents to prevent decolourisation of meat products (USDA 1994). The gallic acid and other flavonoids in CinDAE might be the compounds, which prevent oxidation of myoglobin during cooking and storage. These figures correlate with the data obtained from PV and TBARS assays, in which the meatballs supplemented with CinDAE (T1) exhibited the highest oxidative stability.
From the data collected, the degree of yellowish (b*) for all chicken meatball samples (C, T1, T2, and T3) that were chilled (8 ± 1 °C) for 12 days showed no significant difference (p > 0.05). In general, b* values for all samples were in the range of 11.482 to 13.480 on the first day and 11.258 to 13.150 on the final day of the storage duration. However, according to (Juncher et al. 2003), the importance of yellowish tinge of meat products is unclear as the degree of redness is viewed as a more important parameter.
Sensory evaluation
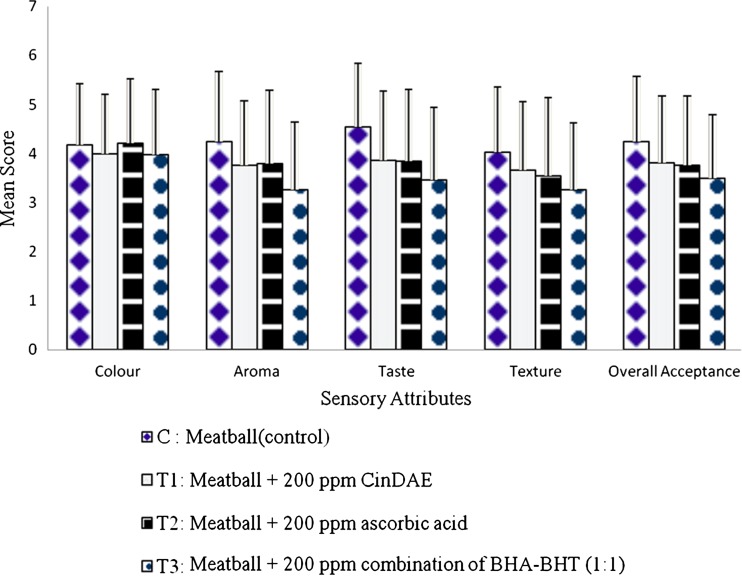
Figure 2 represents the mean score of sensory attributes for 4 chicken meatball samples (C, T1, T2 and T3). In general, mean scores for colour, aroma, taste, texture and overall acceptance are in the range of 3.971–4.214, 3.257–4.243, 3.457–4.543, 3.257–4.029 and 3.486–4.243 respectively. All the samples did not any significant difference in the mean score for all the tested attributes (p < 0.05). This indicates that the supplementation of 200 ppm CinDAE does not assert negative effects on the sensory acceptability of the meatball samples. However, it is previously reported (Dwivedi et al. 2006) that supplementation of cinnamon powder reduced the sensory acceptance of cooked ground beef in which the taste of cinnamon was too spicy to the extent where it masked the taste of the beef sample. Despite that, CinDAE seems to have low pungency as the panelists were not averse to the taste of the meatballs supplemented with CinDAE.
Fig. 2.
Mean score for sensory attributes of 4 chicken meatball samples (n = 70). Abbreviations expansion: CinDAE cinnamon bark deodorised aqueous extracts; ppm part per million; BHT butylated hydroxytoluene; BHA butylated hydroxyanisole
Despite a high TBARS value of control sample (C) on the day 1, the sensory acceptance of the meatball sample was not affected. This is possibly because of the fact that rancidity of the meatball samples is below the sensory threshold value of 0.5–2.0 MDA/kg sample (Boles 1990) and hence cannot be detected by the panelists. Furthermore, the slight decrease of L* values and the marginal increase of a* values of the meatball samples treated with the CinDAE did not affect the acceptance of the panellists thus implying that these two aspects might be perceived as less important criterions for the tested meatball samples.
Conclusion
CinDAE has the potential to replace synthetic antioxidants in meat products, as it exhibited appreciable effectiveness in controlling lipid peroxidation in chicken meatball models; without affecting sensory properties.
References
- Anderson ML, Lauridsen K, Skibsted LH. Phytochemical functional foods. Cambridge: Woodhead Publishing Ltd; 2003. Optimising the use of phenolic compounds in foods. [Google Scholar]
- Anwar F, Siddiq A, Iqbal S, Rafique Asi M. Stabilization of sunflower oil with Moringa oleifera leaves under ambient storage. J Food Lipids. 2007;14:35–49. doi: 10.1111/j.1745-4522.2006.00069.x. [DOI] [Google Scholar]
- Bhanger MI, Iqbal S, Anwar F, Imran M, Akhtar M, Zia ul Haq M. Antioxidant potential of rice bran extracts and its effects on stabilisation of cookies under ambient storage. Int J Food Sci Technol. 2008;43:779–786. doi: 10.1111/j.1365-2621.2007.01515.x. [DOI] [Google Scholar]
- Blessing M, Muller G. Myoglobin concentration in the chicken, especially in the gizzard (a biochemical, light and electron microscopic study) Comp Biochem Physiol Part A: Physiol. 1974;47(2):535–536. doi: 10.1016/0300-9629(74)90017-6. [DOI] [PubMed] [Google Scholar]
- Boles J. Sensory and Chemical characteristics of precooked mircrowave reheatable pork roasts. J Food Sci. 1990;55:618–620. doi: 10.1111/j.1365-2621.1990.tb05190.x. [DOI] [Google Scholar]
- Bukhari SB, Iqbal S, Bhanger M. Antioxidant potential of commercially available cumin (Cuminum cyminuml inn) in Pakistan. Int J Food Sci N. 2008;60:240–247. doi: 10.1080/09637480701695583. [DOI] [PubMed] [Google Scholar]
- Chan KW, Iqbal S, Khong NMH, Babji AS. Preparation of deodorized antioxidant rich extracts from 15 selected spices through optimized aqueous extraction. J Med Plants Res. 2011;5(25):6067–6075. [Google Scholar]
- Chan KW, Iqbal S, Khong NMH, Ch'ng SE, Babji AS (2012a) Preparation of clove buds deodorized aqueous extract (CDAE) and evaluation of its potential to improve oxidative stability of chicken meatballs in comparison to synthetic and natural food antioxidants. J Food Qual 35(3):190–199
- Chan KW, Khong NMH, Iqbal S, Ismail M (2012b) Simulated gastrointestinal pH condition improves antioxidant properties of wheat and rice flours. Int J Mol Sci 13:7496–7507 [DOI] [PMC free article] [PubMed]
- Dwivedi S, Vasavada MN, Cornforth D. Evaluation of antioxidant effects and sensory attributes of Chinese 5 spice ingredients in cooked ground beef. J Food Sci. 2006;71:C12–C17. doi: 10.1111/j.1365-2621.2006.tb12381.x. [DOI] [Google Scholar]
- Galedar MN, Tabatabaeefar A, Jafari A, Sharifi A, Mohtasebi S, Fadaei H. Moisture dependent geometric and mechanical properties of wild pistachio (Pistacia vera L.) nut and kernel. Int J Food Prop. 2010;13:1323–1338. doi: 10.1080/10942910903062099. [DOI] [Google Scholar]
- Gordon MH. The development of oxidative rancidity in foods. In: Pokorny J, Yanishlieva, Gordon M, editors. Antioxidants in food: practical applications. Cambridge: Woodhead Publishing Ltd; 2001. [Google Scholar]
- Herken EN, Guzel S. Total antioxidant capacity and total phenol contents of selected commercial fruit juices in Turkey. Int J Food Prop. 2010;13:1373–1379. doi: 10.1080/10942912.2010.499039. [DOI] [Google Scholar]
- Hernandez-Ochoa L, Aguirre-Prieto YB, Nevarez-Moorillon GV, Gutierrez-Mendez N, Salas-Munoz E (2011) Use of essential oils and extracts from spices in meat protection. J Food Sci T Technol. doi:10.1007/s13197-011-0598-3 [DOI] [PMC free article] [PubMed]
- Hinneburg I, Damien Dorman H, Hiltunen R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006;97:122–129. doi: 10.1016/j.foodchem.2005.03.028. [DOI] [Google Scholar]
- IFST . Shelf life of foods - Guidelines for its determination and prediction. London: Institute of Food Technology; 1993. [Google Scholar]
- Iqbal S, Bhanger M. Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chem. 2007;100:246–254. doi: 10.1016/j.foodchem.2005.09.049. [DOI] [Google Scholar]
- Iqbal S, Bhanger M, Anwar F. Antioxidant properties and components of bran extracts from selected wheat varieties commercially available in Pakistan. LWT-Food Sci Technol. 2007;40:361–367. doi: 10.1016/j.lwt.2005.10.001. [DOI] [Google Scholar]
- Iqbal S, Younas U, Chan KW, Zia-Ul-Haq M, Ismail M (2012a) Chemical composition of Artemisia annua L. leaves and antioxidant potential of extracts as a function of extraction solvents. Molecules 17:6020–6032 [DOI] [PMC free article] [PubMed]
- Iqbal S, Younas U, Sirajuddin, Chan KW, Sarfraz RA, Uddin, MK (2012b) Proximate composition and antioxidant potential of leaves from three varieties of mulberry (Morus spp.): A comparative study. Int J Mol Sci 13:6651–6664 [DOI] [PMC free article] [PubMed]
- Ismail M, Al-Naqeep G, Chan KW. Nigella sativa thymoquinone-rich fraction greatly improves plasma antioxidant capacity and expression of antioxidant genes in hypercholesterolemic rats. Free Radic Biol Med. 2010;48(5):664–672. doi: 10.1016/j.freeradbiomed.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Ismail HI, Chan KW, Mariod AA, Ismail M. Phenolic content and antioxidant activity of cantaloupe (Curcumis melo) methanolic extracts. Food Chem. 2010;119:643–647. doi: 10.1016/j.foodchem.2009.07.023. [DOI] [Google Scholar]
- Jo C, Son JH, Son CB, Byun MW. Functional properties of raw and cooked pork patties with added irradiated, freeze-dried green tea leaf extract powder during storage at 4 C. Meat Sci. 2003;64:13–17. doi: 10.1016/S0309-1740(02)00131-6. [DOI] [PubMed] [Google Scholar]
- Juncher D, Rønn B, Beck Hansen T, Henckel P, Karlsson A, Skibsted LH, Bertelsen G. Effect of pre-slaughter physiological conditions on the oxidative stability of colour and lipid during chill storage of sliced, retail packed roast ham. Meat Sci. 2003;63:151–159. doi: 10.1016/S0309-1740(02)00054-2. [DOI] [PubMed] [Google Scholar]
- Kinsella J, Shimp J, Mai J, Weihrauch J. Fatty acid content and composition of freshwater finfish. J Am Oil Chem Soc. 1977;54:424–429. doi: 10.1007/BF02671025. [DOI] [PubMed] [Google Scholar]
- Kumar V, Biswas AK, Sahoo J, Chatli MK, Sivakumar S (2011) Quality and storability of chicken nuggets formulated with green banana and soybean hulls flours. J Food Sci Technol. doi:10.1007/s13197-011-0442-9 [DOI] [PMC free article] [PubMed]
- Ledward D. On the nature of cooked meat hemoprotein. J Food Sci. 1971;36:883–888. doi: 10.1111/j.1365-2621.1971.tb15552.x. [DOI] [Google Scholar]
- Mathew S, Abraham TE. Studies on the antioxidant activities of cinnamon (Cinnamomum verum) bark extracts, through various in vitro models. Food Chem. 2006;94:520–528. doi: 10.1016/j.foodchem.2004.11.043. [DOI] [Google Scholar]
- Shahidi F, Wanasundara PK. Phenolic antioxidants. CRC Cr Rev Food Sci. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Wanasundara PKJPD, Hong C. Antioxidant activity of phenolic compound in meat model systems. In: Chi TH, Chong YL, Moh TH, editors. Phenolic compounds in food and their effects on health I. vol 1. Washington: ACS Press; 1992. [Google Scholar]
- Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agr Food Chem. 2005;53:7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- Singh G, Maurya S, Delampasona M, Catalan CAN. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem Toxicol. 2007;45:1650–1661. doi: 10.1016/j.fct.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Stodolak B, Starzynska A, Czyszczon M, Zyla K. The effect of phytic acid on oxidative stability of raw and cooked meat. Food Chem. 2007;101:1041–1045. doi: 10.1016/j.foodchem.2006.02.061. [DOI] [Google Scholar]
- Tang S, Ou S, Huang X, Li W, Kerry J, Buckley D. Effects of added tea catechins on colour stability and lipid oxidation in minced beef patties held under aerobic and modified atmospheric packaging conditions. J Food Eng. 2006;77:248–253. doi: 10.1016/j.jfoodeng.2005.06.025. [DOI] [Google Scholar]
- USDA Ascorbic acid, erythrobic acid, citric acid, sodium ascorbate and sodium citrate on beef, lamb and pork cuts. Fed Regist. 1994;59:12536–12538. [Google Scholar]
- Yogesh K, Ali J (2012) Antioxidant potential of thuja (Thuja occidentalis) cones and peach (Prunus persia) seeds in raw chicken ground meat during refrigerated (4 ± 1 °C) storage. J Food Sci Technol. doi:10.1007/s13197-012-0672-5 [DOI] [PMC free article] [PubMed]