Abstract
Enterococcus faecium MTCC 5695 possessing potential probiotic properties as well as enterocin producing ability was used as starter culture. Effect of time (12–24 h) and inoculum level (3–7 % v/v) on cell growth, bacteriocin production, antioxidant property, titrable acidity and pH of curd was studied by response surface methodology (RSM). The optimized conditions were 26.48 h and 2.17%v/v inoculum and the second order model validated. Co cultivation studies revealed that the formulated product had the ability to prevent growth of foodborne pathogens that affect keeping quality of the product during storage. The results indicated that application of E. faecium MTCC 5695 along with usage of optimized conditions attributed to the formation of highly consistent well set curd with bioactive and bioprotective properties. Formulated curd with potential probiotic attributes can be used as therapeutic agent for the treatment of foodborne diseases like Traveler’s diarrhea and gastroenteritis which thereby help in improvement of bowel health.
Keywords: Enterococcus faecium, Probiotic curd, Optimization, Co cultivation, Food borne diseases
Introduction
There has been a growing demand for fermented foods among the consumers globally. Among them, milk based products are of utmost importance as they are consumed by all age groups from infancy to geriatrics. In India, among fermented foods, curd is the most popular in comparison to cheese, kefir and yoghurt, which are more popular among the consumers in the western countries (Granato et al. 2010). Even though the traditional practice involves the application of lactic acid bacteria (LAB) as starter cultures for food fermentation, they can also be used as protective cultures against microbial pathogens and spoilage organisms in minimally processed foods, Vereecken and Impe (2002). Different antimicrobials, such as lactic acid and acetic acid, hydrogen peroxide, carbon dioxide and bacteriocins, produced by these bacteria, can inhibit pathogenic and spoilage micro-organisms, extending the shelf-life and enhancing the safety of food products Aymerich et al. (2000).
Indian fermented milk also known as ‘Dahi’/curd is a popular milk product prepared in most households which is available commercially. They are prepared and consumed in various forms such as whole milk curd, skim milk curd, sweet curd, sour curd and sweetened curd. Hence, they can be used as an efficient medium for transport of probiotic microorganisms to the consumers to impart health benefits, Vijayendra and Gupta (2011).
Bacteriocins are ribosomally synthesized antimicrobial peptides with activity that is directed usually against closely related species (Klaenhammer 1993). They can be effectively used to replace added chemical preservatives, thereby increase the shelf-life of the product by natural means (Tamime 2002). Enterococci isolated from dairy products have also been reported to produce bacteriocins (enterocins) having antimicrobial activity against a broad spectrum of spoilage and pathogenic organisms such as Listeria monocytogenes, Staphylococcus aureus, Clostridium spp. and Bacillus spp. (Sarantinopoulos et al. 2002; De Vuyst et al. 2003). Specific enterococcal strains have been used as probiotic adjunct cultures in Cheddar cheese because of their ability to improve the intestine microbial balance (Giraffa 2003).
Combinative interactions of parameters with the growth and required metabolite production by microorganisms are legion. Therefore optimum processes should be designed using effectual experimental methods. Response surface methodology (RSM) is one of the techniques which include statistical and mathematical methods that can be used to design experiments, build models, investigate the effect of factors on one or more dependent variables, and search optimum conditions of factors for desirable responses Koh et al. (2010). It uses quantitative data to simultaneously determine and solve multivariate equations, graphically represented as response surfaces Amit et al. (2010). Although numerous reports on probiotic yoghurt formulation are cited (Cruz et al. 2010; Ibarra et al. 2012), no data is available on the optimization of conditions for curd formulation using RSM.
Thus, the aim of this study was to optimize the formulation of probiotic curds with good consistency and acceptability using E. faecium MTCC 5695 as starter culture by central composite design (CCD). Modeling and evaluation of the combined effect of fermentation time and inoculum on the growth of E. faecium MTCC 5695, bacteriocin production, titrable acidity, antioxidative property and pH of curd were assessed. In addition, microbiological and sensory characteristics of the final formulated product were studied.
Materials and methods
Substrates and chemicals
All microbiological media were procured from Hi-Media (M/s Hi-Media Laboratory Ltd, Mumbai, India). 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich Chemicals (Steinheim, Germany). Taurodeoxycholic acid sodium salt (TDCA) was obtained from Sisco Research Labs, India. Ox- bile was procured from Fluka, US. Milk and curds to be used as control were obtained from a local dairy, Mysore. The pathogens used for the tests were obtained from various collection centers and maintained as BHI glycerol stocks at −20 °C and subcultured periodically. All other chemicals, solvents and reagents used in the study were of analytical grade, unless otherwise mentioned.
Bacterial strains and culture conditions
E. faecium MTCC 5695, which was examined for its antibacterial activity in our previous study (Vrinda et al. 2012) was used. E. faecium MTCC 5695 was maintained as frozen stocks on MRS soft agar overlaid with glycerol at −20 °C and sub-cultured periodically.
Evaluation of probiotic properties of E. faecium MTCC 5695
E. faecium MTCC 5695 was checked for its probiotic properties like acid and bile tolerance, and bacterial adhesion to hydrocarbon test (BATH) (Table 1). In all the probiotic assays, E. faecium MTCC 5153 was used as a positive control. The strains used for the study were also screened for their pathogenicity on blood agar plates. The strain was also tested for its virulence by checking for the presence of cyl genes associated with cytotoxin production, Badarinath and Halami (2011).
Table 1.
Comparison of probiotic properties of E. faecium strains
| Cultures | Acid tolerance | Bile tolerance | BATH assay | |||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | Delay in growth (min) | Result | A0 | A | Adherence, % | |
| E.faecium MTCC 5695 | 100 | 78 | 42 | 30 | Tolerant | 1.011 | 0.77 | 23 |
| E.faecium MTCC 5153 | 100 | 72 | 38 | 10 | Resistant | 1.1 | 0.98 | 11 |
Acid tolerance
Survival of E. faecium MTCC 5695 at low pH was analyzed by the method described by Raghavendra and Halami (2009). Briefly, MRS broth was adjusted to pH 2.5 with 1.0 M HCl and 5 ml of medium was dispensed to each culture tube and sterilized by autoclaving. Overnight grown culture of approximately 109 cfu/ml was inoculated into the MRS broth (pH 2.5) and the viability during 0, 1 and 2 h of incubation was assessed by plating appropriate dilution on MRS agar plates.
Bile tolerance
Bile tolerance of E. faecium MTCC 5695 was detected as described by Dora and Glenn (2002). E. faecium MTCC 5695 was grown for 16 h at 37 °C and centrifuged at 8000 rpm for 10 min at 4 °C. The pellet was washed twice with saline and 5 % of cell suspension was inoculated separately into the freshly prepared MRS broth (control) and MRS broth containing 0.3%w⁄v ox-bile. Samples were withdrawn at intervals of 1 h and incubated at 37 °C up to 4 h and the optical density (OD) was observed at A600 nm in UV-visible spectrophotometer.
Bacterial adhesion to hydrocarbons (BATH) assay
E. faecium MTCC 5695 was assessed for its adhesion ability by BATH test with xylene as the source of hydrocarbon as per procedure described by (Canzi et al. 2005). Overnight grown culture (1 ml) was washed with phosphate- buffered saline (PBS: 140 mM NaCl, 3 mM KCl, 8 mM Na2HPO4, 2 mM KH2PO4, pH 7.2) and resuspended in the same buffer until an absorbance of about 0.5 was reached (A600 nm). Then an equal volume of xylene was added. The two phase system was thoroughly mixed by vortexing for 3 min. The aqueous phase was removed after 1 h of incubation at room temperature and its A600 was measured. Adhesion percentage was calculated according to the formula;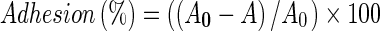 , where the A0 and A are absorbance before and after extraction with organic solvents, respectively.
, where the A0 and A are absorbance before and after extraction with organic solvents, respectively.
Bile salt hydrolase (BSH) assay
BSH activity of the culture was evaluated using the procedure described by Pereira and Gibson (2002). Overnight grown culture was streaked on MRS agar plates supplemented with 0.5 % w/v TDCA followed by incubation at 37 °C for 72 h. MRS agar without supplementation of TDCA was used as a control.
Optimization of conditions for curd formulation by RSM
Culture preparation
E. faecium MTCC 5695 was grown for 18–24 h and after three successive transfers, the activated organism was used for the production of curd. The culture was used as per the required concentration for inoculation into cow milk. Milk samples obtained from local vendors were subjected to pasteurization by heating at 95 °C for 15 min and sudden cooling at 4 °C.
Experimental design and statistical analysis
A CCD was employed for optimization of curd formulation using 2 independent variables, time (A; h) and inoculum level (B; %v/v), as per levels shown in Table 2. The design consisted of 12 runs (4 factorial runs, 4 star points, 4 central runs) as shown in Table 3. Cell growth (Y1), pH (Y2) and bacteriocin (Y3) were recorded as the main responses. Antioxidant property (Y4) and titrable acidity (Y5) were taken as the additional responses. Each run consisted of 100 ml of pasteurized milk, which was inoculated with respective levels of inoculum and incubated at 37 °C for predetermined time (Table 3). Generation of the experimental design and analysis of results obtained was accomplished by STATISTICA software (Statsoft 1999).
Table 2.
Maximum and minimum levels of variables used in Central Composite Design
| Factors | Levels | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | α | |
| A | 9.51 | 12 | 18 | 24 | 26.48 |
| B | 2.17 | 3 | 5 | 7 | 7.82 |
Table 3.
Central composite design for optimization of independent variables affecting curd formulation with the observed dependent responses
| Run | Independent variables | Responses | |||||
|---|---|---|---|---|---|---|---|
| A | B | Y1 | Y2 | Y3 | Y4 | Y5 | |
| 1 | 12 | 3 | 2.21 | 6.28 | 6400 | 94.9 | 0.54 |
| 2 | 12 | 7 | 2.384 | 6.16 | 6400 | 95.2 | 0.72 |
| 3 | 24 | 3 | 3.666 | 4.80 | 102400 | 97.1 | 0.81 |
| 4 | 24 | 7 | 3.733 | 4.66 | 102400 | 97.8 | 0.99 |
| 5 | 9.51472 | 5 | 2.42 | 6.31 | 6400 | 92.0 | 0.45 |
| 6 | 26.48528 | 5 | 3.514 | 4.45 | 204800 | 97.8 | 0.90 |
| 7 | 18 | 2.1715729 | 3.135 | 5.26 | 12800 | 98.0 | 0.63 |
| 8 | 18 | 7.8284271 | 2.844 | 4.92 | 25600 | 99.0 | 0.90 |
| 9 | 18 | 5 | 2.445 | 4.32 | 12800 | 95.6 | 0.81 |
| 10 | 18 | 5 | 2.413 | 4.38 | 12800 | 95.0 | 0.81 |
| 11 | 18 | 5 | 2.4 | 4.35 | 12800 | 95.4 | 0.81 |
| 12 | 18 | 5 | 2.435 | 4.34 | 12800 | 95.0 | 0.81 |
A incubation time (h), B inoculum level (%v/v); Y 1 cell growth (OD600), Y 2 pH, Y 3 bacteriocin activity (AU/ml), Y 4 DPPH free radical scavenging activity and Y 5 Total titrable acidity (gm/ml)
Measurement of growth
The curd samples were removed at regular intervals and diluted in the ratio 1:10 using sterile double distilled water and the optical density was measured at 600 nm using Shimadzu UV 1800. The measurements were performed in triplicate for each curd sample.
Measurement of pH
The pH of samples was determined using a pH meter (model μPHCAL5, Analab Scientific, India). The measurements were performed in triplicate for each curd sample.
Bacteriocin production
Antibacterial activity was performed for whey samples by agar well assay as described by Geis et al. (1983) using Listeria monocytogenes Scott A as indicator. Cell-free supernatants were prepared by centrifugation and subjected to two- fold serial dilution with sterile distilled water upto 1:512 dilution. 5 μl of the diluted samples was spotted on to the indicator lawn plate. After storage at 4 °C for 2 h, the plates were incubated at 37 °C for 18 h. The reciprocal of the highest dilution showing a visible zone of inhibition was defined as one arbitary unit (AU). The amount of bacteriocin produced (AU/ml) was calculated by multiplying the reciprocal of the highest active dilution by a factor of 200. Each sample was assayed in triplicates.
DPPH radical scavenging activity
The DPPH radical scavenging activity was performed as described by Ganesan et al. (2008). Curd sample (1 ml) was taken in micro centrifuge tube, centrifuged at 8000 rpm for 10 min; supernatant (whey) was collected and filtered using Whatman No: 1 filter paper. The samples were added (100 μl) and the volume was made up to 2 ml using distilled water. Then, 2 ml of 0.16 mM DPPH solution (in methanol) was added to the above sample. Blank was prepared using 2 ml of methanol and 2 ml of distilled water. The mixture was vortexed and kept in dark at room temperature for 30 min. Absorbance of all the samples at 517 nm was measured in the UV- visible spectrophotometer. The scavenging effect (%) was calculated by using the formula,
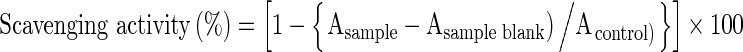 |
Measurement of titrable acidity
The titrable acidity of the curd samples was measured as per a modified method, Yoon et al. (2006) by titrating with 0.1N NaOH solution and expressed as equivalents of lactic acid (1 ml of 0.1N NaOH amounts to 0.009 g equivalents of lactic acid).
Validation of the second order model
The second order model obtained after optimization was validated with random runs within the experimental area. The observed values were compared with the values as predicted by the second order regression equation obtained from the model.
Curd formulation under optimized conditions
Pasteurized milk (1000 ml) was inoculated with optimized levels of inoculum and incubated at 37 °C for the optimized period of time. Bacteriocin production, pH, cell growth, DPPH free radical scavenging activity and titrable acidity were checked. The sensory and microbiological analysis of the formulated curd was carried out.
Co cultivation studies and viable count against food borne pathogens
Co cultivation studies were carried out as per the method described by Somkuti and Steinberg (2010). Curds were formed by using E. faecium MTCC 5695 as starter culture. Into the formulated curd 3 h grown Listeria monocytogenes Scott A (108 cfu/ml) was inoculated. The curd samples containing the pathogen as well as the starter culture were collected at regular intervals (2 h) from 0th to 8th h. The samples were then serially diluted, pour plated using the selective medium (Listeria Oxford medium agar) and incubated at 37 °C for 24 h and the viable count of L. monocytogenes Scott A was determined. Similarly, co cultivation experiments were also performed with Staphylococcus aureus FB271 and Escherichia coli MTCC 118. Baird Parker agar base and Violet Red Bile agar were used as selective media for estimating viable counts of respective cultures.
Sensory analysis
Sensory analysis was carried out for curd prepared using E. faecium MTCC 5695 and control curd obtained from Nandini as starter culture. Evaluations were conducted under white fluorescent light, with the booth area maintained at temperature 22 ± 2 °C and RH 50 ± 5 %. A suitable score card was developed using “Free-Choice Profiling” method selecting suitable terminology. Samples were presented in beakers (25 ml) coded with 3-digit random numbers, to the panelists. A glass of water was also presented to cleanse the palate in between the samples. Quantitative Descriptive Analysis (QDA) Allgeyer et al. (2010) was used to assess the quality of samples. Panelists were asked to mark on a scale of 0–15 cm to indicate the intensity of each attribute listed on the score card. The scale was anchored at 1.25 cm on either end, representing ‘Recognition Threshold’ and ‘Saturation Threshold’, respectively. The scores given for all the attributes for each sample were tabulated. Next, the mean value was calculated for each attribute of a sample, representing the panel’s judgement about the sensory quality of the product.
Results and discussion
In the present study, the potent culture E. faecium MTCC 5695 was characterized for its probiotic properties as well as bioactive and bioprotective (antibacterial and antioxidant) properties. Furthermore, it was used as a starter culture for probiotic curd formulation. The strain used showed α-hemolysis on blood agar plates implying its non pathogenicity (data not shown). The strain showed lack of amplification for cyl gene specific PCR indicating that it is devoid of cytolysin biosynthesis genes (data not shown) and can be used as a safe starter culture for curd formulation. The test strain E. faecium MTCC 5695 was selected for this study due to its high proteolytic and enterocin producing ability. The parameters for efficient curdling and formation of well set consistent curds were fixed and the experiments were designed and optimized by CCD using STATISTICA software.
Probiotic properties of E. faecium MTCC 5695
One of the important properties of probiotic cultures is the ability to survive in the gastrointestinal tract, where the pH is 2–2.5 before it reaches the colon, Badarinath and Halami (2009). E. faecium MTCC 5695 was found to show a survival index of 78 % after 1 h and 42 % after 2 h of incubation. The survival rate was more than that of the standard E. faecium MTCC 5153, which showed a survival index of 72 % and 38 % after 1 and 2 h of incubation, respectively (Table 1).
The test strain showed tolerance in presence of 0.3 % bile and the delay in growth was 30 min which was more than that of the standard E. faecium MTCC 5153 (Table 1). Since the physiological concentration of bile acids existing in the intestine ranges from 5000 to 20000 μmoles, Badarinath and Halami (2009), these conditions have been used for the assay and concentrations of 0.15–0.3 % bile salts are generally used, Fernandez et al. (2003). Time delay to reach log phase under bile conditions is a measure of tolerance or sensitivity of the strain to bile acids.
Adhesion of cultures to intestinal mucosa is one of the probiotic properties. Xylene was the hydrocarbon used for the assay. BATH test has been extensively used for measuring cell surface hydrophobicity in Lactobacillus Vinderola et al. (2004). The adherence property of MTCC 5695 was found to be 23 %, which was mor than twice the value in comparison to the standard strain used for the study E. faecium MTCC 5153 (Table 1).
BSH is one of the prerequisite for selection of a probiotic organism, Pereira and Gibson (2002), since consumption of probiotic LAB with BSH property helps in reduction in serum cholesterol by deconjugation of bile salts, Pereira and Gibson (2002). Plate assay supplemented with TDCA showed appearance of white precipitate surrounding the colonies indicating positive reaction for BSH activity (data not shown). The white precipitate formation was due to the conversion of taurocholic acid to taurine and deoxy cholic acid (an insoluble compound which precipitates out) by BSH.
Optimization of conditions for curd formulation by CCD
The effect of two parameters viz. inoculum level and incubation time on the formation of curd obtained by fermentation of milk with E. faecium MTCC 5695 was examined by CCD in levels as shown in Table 3. The observed values for the dependent variables i.e., cell growth (Y1), pH (Y2), bacteriocin production (Y3), antioxidant property (Y4) and titrable acidity (Y5) are depicted in Table 3 along with the experimental runs. The variables showing statistically significant effects were tested by Student’s t-test for ANOVA (Tables 4 and 5). Factors presenting p-values less than 0.05 were viewed to have significant effects on curd formation. The graphical representation of the significance of the responses is presented by Pareto charts (Fig. 1). Pareto chart is the representation of the size effect of each of the variables investigated upon the resolution of the main peaks of the main responses. Here, a variable is found to be significant, if the size of effect is greater than p = 0.05. It was observed that both inoculum level and incubation time had significant effect on all the responses except for bacteriocin production, where incubation time had no effect. Incubation time was more significant than inoculum level on all 5 responses i.e. (p < 0.0005). Also, the interactive effect between the two independent variables had no effect on any of the responses.
Table 4.
ANOVA table for main responses i.e. cell growth (Y1), pH (Y2) and bacteriocin activity (Y3) in curd produced by E.faecium MTCC 5695 as affected by incubation time (A) and inoculum level (B)
| Source | Df | Y1 | Y2 | Y3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | MS | F value | p value | SS | MS | F value | p value | SS | MS | F value | p value | ||
| A | 1 | 2.36765 | 2.36765 | 56.1375 | 0.00029 | 3.9346258 | 3.9346258 | 170.52091 | 1.24E-05 | 2.79E + 10 | 2.79E + 10 | 145.681 | 1.96E-05 |
| A2 | 1 | 0.49062 | 0.49062 | 11.6328 | 0.01431 | 2.1252102 | 2.1252102 | 92.10349 | 7.319E-05 | 1.26E + 10 | 1.26E + 10 | 65.8399 | 0.000188 |
| B | 1 | 0.00364 | 0.00364 | 0.08619 | 0.77896 | 0.0686041 | 0.0686041 | 2.9732019 | 0.1354179 | 40960000 | 40960000 | 0.21375 | 0.660136 |
| B2 | 1 | 0.5313 | 0.5313 | 12.5973 | 0.01208 | 1.1902498 | 1.1902498 | 51.58368 | 0.0003683 | 9216000 | 9216000 | 0.04809 | 0.833685 |
| AB | 1 | 0.00286 | 0.00286 | 0.06786 | 0.80318 | 1.00E-04 | 1.00E-04 | 0.0043339 | 0.9496503 | 0 | 0 | 0 | 1 |
| Error | 6 | 0.25306 | 0.04218 | 0.1384449 | 0.0230742 | 1.15E + 09 | 1.92E + 08 | ||||||
| Total SS | 11 | 3.47898 | 6.9326917 | 4.21E + 10 | |||||||||
| R2 | 0.92726 | 0.98003 | 0.9727 | ||||||||||
| R2(adj) | 0.86665 | 0.96339 | 0.94995 | ||||||||||
Table 5.
ANOVA table for additional responses i.e. DPPH free radical scavenging activity (Y4) and Total titrable acidity (Y5) in curd produced by E.faecium MTCC 5695 as affected by incubation time (A) and inoculum level (B)
| Source | Df | Y4 | Y5 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SS | MS | F value | p value | SS | MS | F value | p value | ||
| A | 1 | 0.002116 | 0.00212 | 59.16949 | 0.000253 | 0.172988 | 0.17299 | 196.908 | 8.2E-06 |
| A2 | 1 | 5.37E-05 | 5.4E-05 | 1.501235 | 0.266393 | 0.02025 | 0.02025 | 23.05 | 0.003 |
| B | 1 | 6.85E-05 | 6.9E-05 | 1.916002 | 0.215583 | 0.06879 | 0.06879 | 78.3023 | 0.00012 |
| B2 | 1 | 0.001481 | 0.00148 | 41.41324 | 0.000666 | 0.00081 | 0.00081 | 0.922 | 0.37403 |
| AB | 1 | 3.79E-06 | 3.8E-06 | 0.106043 | 0.755752 | 0 | 0 | 0 | 1 |
| Error | 6 | 0.000215 | 3.6E-05 | 0.005271 | 0.00088 | ||||
| Total SS | 11 | 0.00412 | 0.2673 | ||||||
| R2 | 0.94773 | 0.98028 | |||||||
| R2(adj) | 0.9045 | 0.96385 | |||||||
Fig. 1.
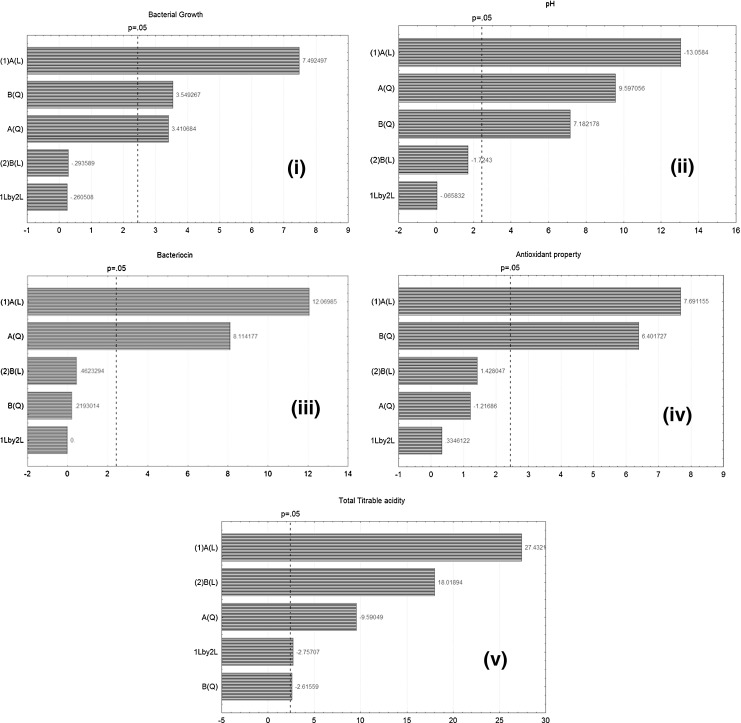
Pareto chart showing the significance of dependent parameters, their quadratic and interactive effects on cell growth (i), pH (ii), bacteriocin production (iii), DPPH free radical scavenging activity (iv) and total titrable acidity (v)
The response surface graphs (RSG) for cell growth, pH, bacteriocin production, DPPH free radical scavenging activity and total titrable acidity, as a function of incubation time and inoculum level are shown in Fig. 2. Bacterial growth showed a continuous increase with increase in time, whereas in the case of inoculum level there was a gradual decrease up to 5.5%v/v followed by an increase (Fig. 2). This may probably be due to the initial lag in doubling time of cells which was later found to enter an exponential phase of cell growth with increase in time. A decrease in pH was observed with increase in time which indicates the accumulation of lactic acid in the medium. However, it was seen that at high percentage of inoculum (above 6%v/v) there was no significant decrease in pH (Fig. 2). This may be probably due to the fact that higher inoculum favored lower cell growth at constant volume of medium. Inoculum level did not have any effect on bacteriocin production, whereas there was a continuous increase with increased incubation time (Fig. 2). RSG for antioxidant property showed a similar pattern to bacterial growth (Fig. 2). This was suggestive of a positive correlation between growth and antioxidant property. RSG for total titrable acidity revealed that there was an increase with time and decrease with increase in inoculum level (Fig. 2).
Fig. 2.
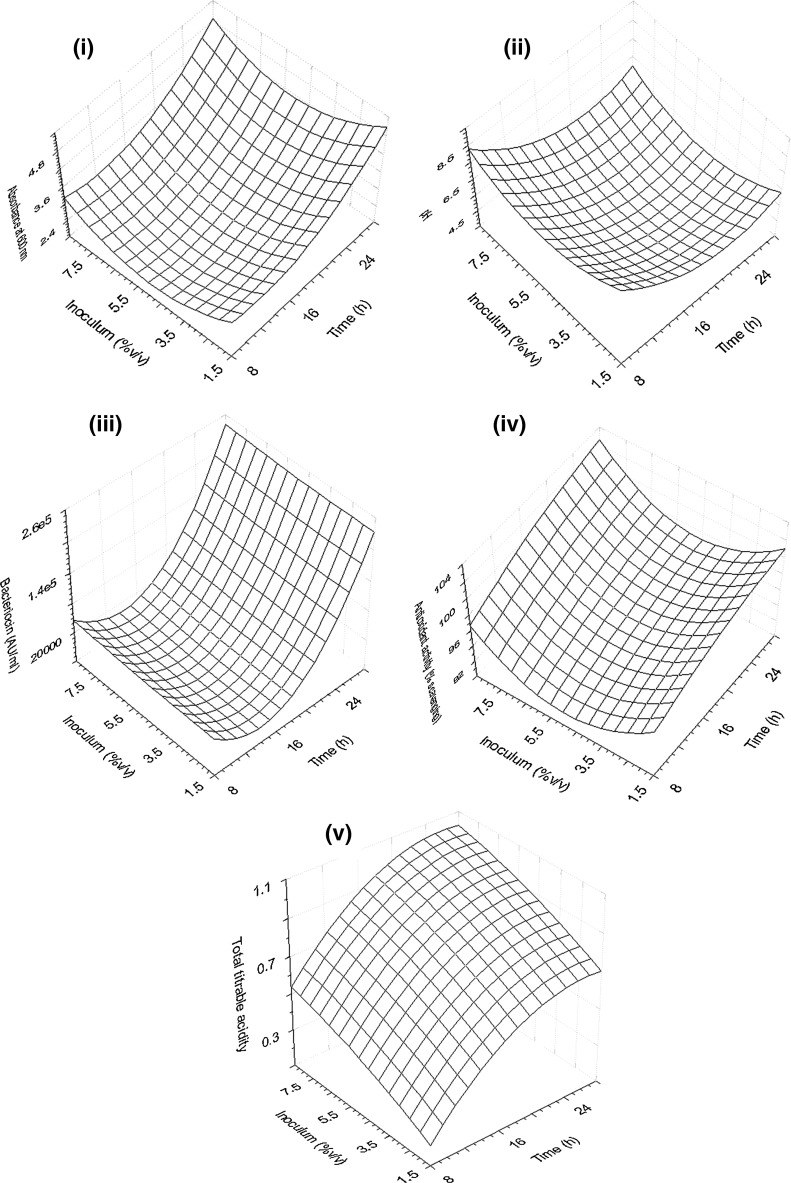
Response surface graphs showing the effect of incubation time (a) and inoculum level (b) on cell growth (i), pH (ii), bacteriocin production (iii), DPPH free radical scavenging activity (iv) and total titrable acidity (v)
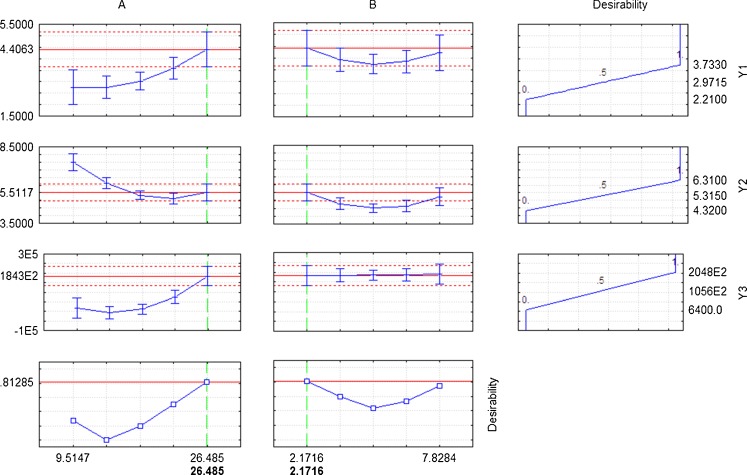
The optimized levels of variables (A and B) were determined using desirability profiles (Fig. 3) for Y1, Y2 and Y3 by assigning 0 to least observed value of response and 1 to highest observed value of response. The optimized factors for curd obtained on fermentation with E. faecium MTCC 5695 having highest cell growth and bacteriocin production were 26.48 h at 2.17 % (v/v) inoculum level. The regression analysis obtained after ANOVA resulted in the following second order equations for all the responses, as a function of independent variables (A and B) and their linear and quadratic interactions:
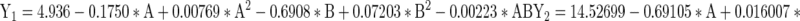 |
Fig. 3.
Desirability profiles for predicted main responses and the desirability levels for incubation time and inoculum level for optimum curd formation by Enterococcus faecium MTCC 5695
The fit of the model was estimated by the coefficient of determination (R2). The closer the R2 value is to 1.00, the stronger the model is and the better it predicts the response. An R2 value >0.75 indicates the good fitness of the model, Mandenius and Brundin (2008). The R2 values for the models predicting the main responses i.e., cell growth, pH and bacteriocin production were 0.9272, 0.98 and 0.9727, respectively, indicating the model as significant. The model was validated by conducting random experiments within the experimental area. The predictability of the model for all the responses was determined by comparing the observed values and values predicted by the model (Table 6). The validation experiments showed that RSM was reliable with a variation of 3.47 % for cell growth, 2.93 % for pH, 2.31 % for bacteriocin production, 4.97 % for DPPH radical scavenging activity and 5.28 % for total titrable acidity, from the experimental data. RSM has been applied for the optimization of tofu (soymilk curd) using solids content of soy milk, concentration of coagulant, mixing temperature and stirring time as the independent variables, (Shih et al. 1997). Also the conditions for optimization of yoghurt using combination cultures of Lactobacillus and Streptococcus species was reported, Kristo et al. (2003).
Table 6.
The observed values and values of validation runs as predicted by the second order model for the dependent responses
| A | B | Ya1 | Yb1 | Ya2 | Yb2 | Ya3 | Yb3 | Ya4 | Yb4 | Ya5 | Yb5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 | 5 | 3.24 | 3.28 | 4.22 | 4.31 | 116273 | 102400 | 96.5878 | 96.6 | 0.9008 | 0.9 |
| 18 | 7 | 2.69 | 2.60 | 4.68 | 4.78 | 16263 | 12800 | 97.0643 | 97.41 | 0.8914 | 0.9 |
| 15 | 3 | 2.51 | 2.56 | 5.36 | 5.54 | −6699 | 3200 | 95.6262 | 95.75 | 0.6184 | 0.63 |
| 17 | 7 | 2.61 | 2.63 | 4.82 | 4.79 | 7651 | 6400 | 96.7687 | 96.79 | 0.8654 | 0.81 |
| 18 | 3 | 2.732 | 2.73 | 4.87 | 5.10 | 11737 | 12800 | 96.4607 | 96.58 | 0.706 | 0.72 |
| 22 | 5 | 2.908 | 2.89 | 4.13 | 4.23 | 71915 | 51200 | 96.2050 | 96.4 | 0.883 | 0.90 |
A incubation time (h), B inoculum level (%v/v); Y 1 cell growth (OD600), Y 2 pH, Y 3 bacteriocin activity (AU/ml), Y 4 DPPH free radical scavenging activity and Y 5 Total titrable acidity (gm/ml)
apredicted values
bobserved values
Curd formulation under optimized conditions
Pasteurized milk (1000 ml) was inoculated with 2.17 % v/v of E. faecium MTCC 5695 and incubated for 26.48 h (optimized conditions) at 37 °C. Cell growth, pH and bacteriocin activity in the curd produced at optimized conditions was estimated. The experimental values of cell growth (4.29), pH (5.52), and bacteriocin production (102400 AU/ml) were found to be close to the values as predicted by the models (4.4, 5.512 and 184180 AU/ml, respectively).
Co cultivation studies and viable count against food borne pathogens
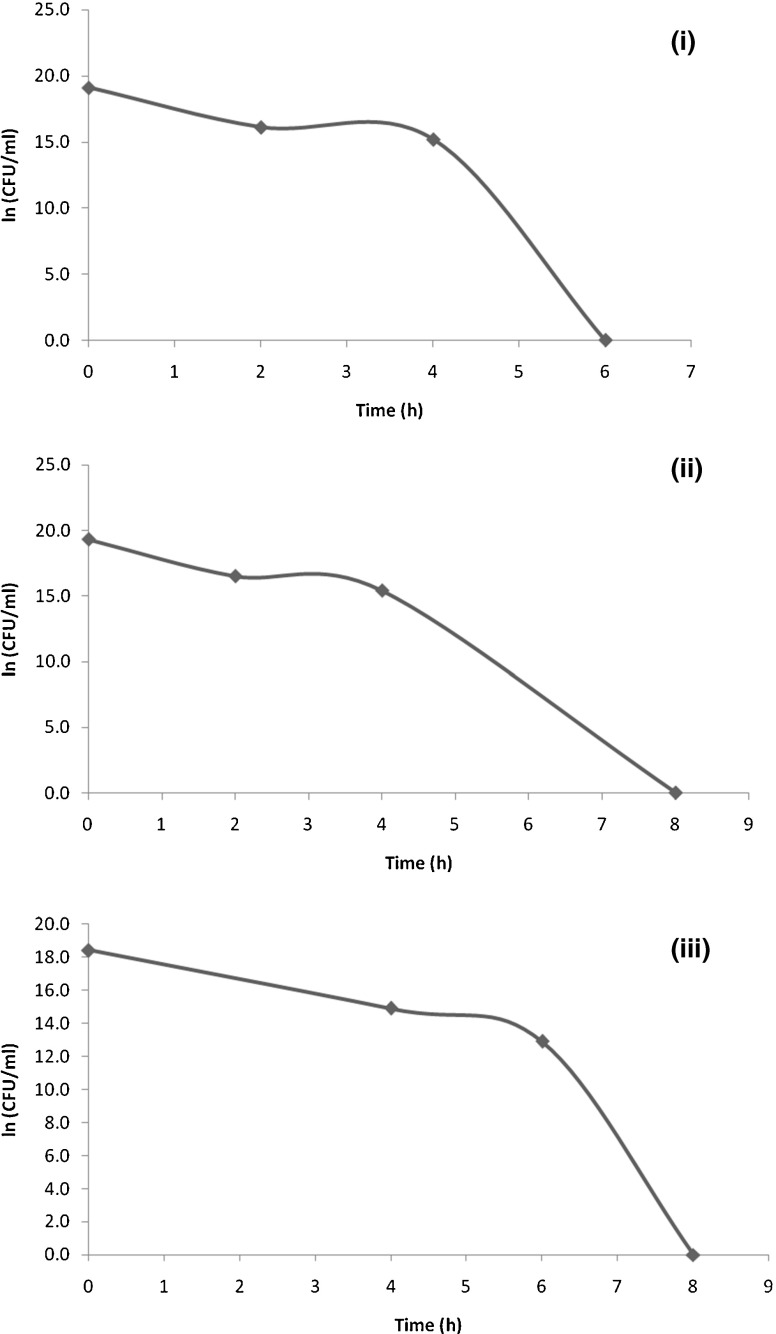
Co cultivation studies performed in curds fermented under optimized conditions using the starter culture E. faecium MTCC 5695 was found to be effective in reducing the viable counts of pathogens (L. monocytogenes Scott A, S. aureus FB271 and E. coli MTCC 118) tested, beyond a detectable level. A complete reduction in the viable counts of Listeria and Staphylococcus were observed by the 6th h (Fig. 4i and ii). Plating results showed growth on selective media up to 4th h. However, complete reduction in viable counts of E. coli was observed only by the 8th h, since it is a Gram negative bacterium having a lipopolysaccharide layer inbuilt in the cell wall (Fig. 4iii). L. monocytogenes and E. coli are capable of surviving at acidic conditions and hence may withstand the acidified conditions in fermented dairy products thereby resulting in food spoilage, Somkuti and Steinberg (2010). The outcome of this study revealed that enterocin produced by E. faecium MTCC 5695 played a crucial role in inhibiting the colonization of these pathogens in the optimized formulated curd. S. aureus is effectively controlled in an environment of pH 5.3 or less. Therefore, LAB are preferred as starter culture since upon growth, they reduce the pH of the medium by producing metabolites like lactic acid, acetic acid and diacetyl, resulting in the suppression of growth of S. aureus Ananou et al. (2005).
Fig. 4.
Survival of pathogens in formulated curd under optimized conditions; L. monocytogenes ScottA (i), Staphylococcus aureus FB271 (ii) and Escherichia coli MTCC118 (iii)
Food borne pathogens pose a serious threat to the food industry by causing diseases ranging from mild gastroenteritis to chronic disability and sequelae Vrinda et al. (2012). Fermented foods are generally prone to attack by food borne pathogens since they provide an efficient substratum for their growth and multiplication. These foods act as vehicles for transmission of food associated illness. Over 20 % of outbreaks of food-borne illnesses are caused by L. monocytogenes in developed countries where consumption of fermented dairy products are on the high De Buyser et al. (2001). E. coli cause wide range of gastrointestinal diseases like bloody diarrhoea in humans to calamitous maladies like hemorrhagic colitis and haemolytic uremic syndrome, Griffin and Tauxe (1991). S. aureus is a major pathogen that has the potential to cause a variety of toxin related diseases in humans and animals. It is considered to be one of the most common food borne pathogens responsible for large mortality rates in many countries Bean et al. (1996). The control of food borne pathogens in food has been carried out by various approaches that include the usage of antibiotics and chemical additives. This results in development of antibiotic resistance in these pathogens and also affects the food quality. A redressal to this problem would be incorporation of bacteriocin producing cultures like E. faecium MTCC 5695 as starter culture for fermented foods.
Sensory analysis
The results of sensory analysis showed that control curd had typical white color and curd like aroma. The optimized curd produced using E. faecium MTCC 5695 as starter was buff colored, lacked typical curd like flavor and had a fatty mouth feel. The overall quality scores for control curd and curd prepared using E. faecium MTCC 5695 were 9 and 8, respectively. It can be concluded from the study that the optimized formulated curd is acceptable.
Conclusions
Enterococci are widespread in nature and their capacity to produce enterocins active against a variety of food borne pathogens makes it of prime importance for use as starter culture in fermented foods. Optimization studies on growth and enterocin production by enterococci in fermented foods are scarce. In the present study, attempts were made for the utilization of enterocin producing culture E. faecium MTCC 5695 for the formulation of probiotic curd under optimized conditions. RSM was found to be an effective technique for the rapid screening of the significant influencing parameters and development of a polynomial model to optimize fermentation condition for the production of curd enriched with probiotic properties. The significance of this work is that, this curd can be used as an effective therapeutic as prophylactic agents for diseases like Traveler’s diarrhea and other gastroenteric disorders.
Acknowledgments
NB thankfully acknowledges Council of Scientific and Industrial Research (CSIR) for funding this work through Encouraging & Motivating World class Exploratory Research (EMPOWER) scheme. VR thanks University Grants Commission (UGC), Govt. of India for the senior research fellowship. Authors place on record their thanks to Director, CFTRI for encouragement and permission to publish the work.
Contributor Information
Prakash M. Halami, Phone: +91-821-2517539, FAX: +91-821-2517233, Email: prakashalami@cftri.res.in
Bhaskar Narayan, Phone: +91-821-2517539, FAX: +91-821-2517233, Email: bhasg3@yahoo.co.in.
References
- Allgeyer LC, Miller MJ, Lee SY. Sensory and microbiological quality of yogurt drinks with prebiotics and probiotics. J Dairy Sci. 2010;93:4471–4479. doi: 10.3168/jds.2009-2582. [DOI] [PubMed] [Google Scholar]
- Amit KR, Swapna HC, Bhaskar N, Halami PM, Sachindra NM. Effect of fermentation ensilaging on recovery of oil from fresh water fish viscera. Enzyme Microb Technol. 2010;46:9–13. doi: 10.1016/j.enzmictec.2009.09.007. [DOI] [Google Scholar]
- Ananou S, Maqueda M, Martinez-Bueno M, Galvez A, Valdivia E. Control of Staphylococcus aureus in sausages by enterocin AS-48. Meat Sci. 2005;71:549–556. doi: 10.1016/j.meatsci.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Aymerich T, Artigas MG, Garriga M, Monfort JM, Hugas M. Effect of sausage ingredients and additives on the production of enterocin A and B by Enterococcus faecium CTC492. Optimization of in vitro production and anti-listerial effect in dry fermented sausages. J Appl Microbiol. 2000;88:686–694. doi: 10.1046/j.1365-2672.2000.01012.x. [DOI] [PubMed] [Google Scholar]
- Badarinath V, Halami PM. Evaluation of bacteriocinogenic lactic acid bacteria isolated from fermented milk and idli batter for probiotic applications. Int J Prob Preb. 2009;4(1):33–40. [Google Scholar]
- Badarinath V, Halami PM. Molecular characterization of class IIa, heat-stable enterocin produced by Enterococcus faecium MTCC 5153. Ind J Biotechnol. 2011;10:307–315. [Google Scholar]
- Bean NH, Goulding JS, Lao C, Angulo FJ. Surveillance for food borne-disease outbreaks – United States, 1988–1992. Morbidity and Mortality Weekly Report (SS-5) 1996;45:1–66. [PubMed] [Google Scholar]
- Canzi E, Guglielmetti S, Mora D, Tamagnini I, Parini C. Condition affecting cell surface properties of human intestinal bifidobacteria. Antonie Van Leeuwenhoek. 2005;88:207–219. doi: 10.1007/s10482-005-6501-3. [DOI] [PubMed] [Google Scholar]
- Cruz AG, Faria JAF, Walter EHM, Andrade RR, Cavalcanti RN, Oliveira CAF, Granato D. Processing optimization of probiotic yogurt containing glucose oxidase using response surface methodology. J Dairy Sci. 2010;93(11):5059–5068. doi: 10.3168/jds.2010-3336. [DOI] [PubMed] [Google Scholar]
- De Buyser ML, Dufour B, Maire M, Lafarge V. Implication of milk and milk products in food-borne diseases in France and in different industrialized countries. Int J Food Microbiol. 2001;67:1–17. doi: 10.1016/S0168-1605(01)00443-3. [DOI] [PubMed] [Google Scholar]
- De Vuyst L, Foulquie Moreno MR, Revets H. Screening for enterocins and detection of hemolysin and vancomycin resistance in enterococci of different origins. Int J Food Microbiol. 2003;84:299–318. doi: 10.1016/S0168-1605(02)00425-7. [DOI] [PubMed] [Google Scholar]
- Dora IAP, Glenn RG. Cholesterol assimilation by lactic acid bacteria and bifidobacteria isolated from the human gut. Appl Environ Microbiol. 2002;68:4689–4693. doi: 10.1128/AEM.68.9.4689-4693.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MF, Boris S, Barbes C. Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J Appl Microbiol. 2003;94:449–455. doi: 10.1046/j.1365-2672.2003.01850.x. [DOI] [PubMed] [Google Scholar]
- Ganesan P, Kumar CS, Bhaskar N. Antioxidant properties of methanol extract and its solvent fractions obtain from selected Indian red see weeds. Biores Technol. 2008;99(8):2717–2723. doi: 10.1016/j.biortech.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Geis A, Singh J, Teuber M. Potential of lactic streptococci to produce bacteriocin. Appl Environ Microbiol. 1983;45:205–211. doi: 10.1128/aem.45.1.205-211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraffa G. Functionality of enterococci in dairy products. Int J Food Microbiol. 2003;88(2–3):215–222. doi: 10.1016/S0168-1605(03)00183-1. [DOI] [PubMed] [Google Scholar]
- Granato D, Branco GF, Cruz AG, Faria JAF, Shah NP. Compr Rev Food Sci Food Safety. 2010;9:455–470. doi: 10.1111/j.1541-4337.2010.00120.x. [DOI] [PubMed] [Google Scholar]
- Griffin P, Tauxe R. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic Escherichia coli and the associated haemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- Ibarra A, Acha R, Calleja MT, Chiralt-Boix A, Wittig E. Optimization and shelf life of a low-lactose yogurt with Lactobacillus rhamnosus HN001. J Dairy Sci. 2012;95(7):3536–3548. doi: 10.3168/jds.2011-5050. [DOI] [PubMed] [Google Scholar]
- Klaenhammer TR. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39–86. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Koh SP, Tan CP, Lai OM, Arifin N, Yusoff MSA, Long K. Enzymatic synthesis of medium- and long-chain triacylglycerols (MLCT): optimization of process parameters using response surface methodology. Food Bioprocess Technol. 2010;3:288–299. doi: 10.1007/s11947-008-0073-y. [DOI] [Google Scholar]
- Kristo E, Biliaderis CG, Tzanetakis N. Modelling of rheological, microbiological and acidification properties of a fermented milk product containing a probiotic strain of Lactobacillus paracasei. Int Dairy J. 2003;13:517–528. doi: 10.1016/S0958-6946(03)00074-8. [DOI] [Google Scholar]
- Mandenius CF, Brundin A. Bioprocess optimization using design-of-experiments methodology. Biotechnol Progr. 2008;24:1191–1203. doi: 10.1002/btpr.67. [DOI] [PubMed] [Google Scholar]
- Pereira DI, Gibson GR. Cholesterol assimilation by lactic acid bacteria and bifidobacteria isolated from the Human gut. Appl Environ Microbiol. 2002;68(9):689–4693. doi: 10.1128/AEM.68.9.4689-4693.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra P, Halami PM. Screening, selection and characterization of phytic acid degrading lactic acid bacteria from chicken intestine. Int J Food Microbiol. 2009;133(1–2):129–134. doi: 10.1016/j.ijfoodmicro.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Sarantinopoulos P, Leroy F, Leontopoulou E, Georgalaki M, Kalantzopoulos G, Tsakalidou E, De Vuyst L. Bacteriocin production by Enterococcus faecium FAIR-E 198 in view of its application as adjunct starter in Greek Feta cheese making. Int J Food Microbiol. 2002;72:125–136. doi: 10.1016/S0168-1605(01)00633-X. [DOI] [PubMed] [Google Scholar]
- Shih MC, Hou HJ, Chang KC. Process optimization for soft tofu. J Food Sci. 1997;64(4):833–837. doi: 10.1111/j.1365-2621.1997.tb15466.x. [DOI] [Google Scholar]
- Somkuti GA, Steinberg DH. Pediocin production in milk by Pediococcus acidilactici in co-culture with Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. J Ind Microbiol Biotechnol. 2010;37:65–69. doi: 10.1007/s10295-009-0648-2. [DOI] [PubMed] [Google Scholar]
- Statsoft (1999) Statistica for Windows. Statsoft Inc., Tulsa, USA
- Tamime AY. Fermented milks: a historical food with modern applications-a review. Eur J Clin Nutr. 2002;56:S2–S15. doi: 10.1038/sj.ejcn.1601657. [DOI] [PubMed] [Google Scholar]
- Vereecken KM, Impe JFV. Analysis and practical implementation of a model for combined growth and metabolite production of lactic acid bacteria. Int J Food Microbiol. 2002;73(2–3):239–250. doi: 10.1016/S0168-1605(01)00641-9. [DOI] [PubMed] [Google Scholar]
- Vijayendra SVN, Gupta RC (2011) Assessment of probiotic and sensory properties of dahi and yoghurt prepared using bulk freeze-dried cultures in buffalo milk. Ann Microbiol. doi:10.1007/s13213-011-0331-5
- Vinderola CG, Medici M, Perdigon G. Relationship between interaction sites in the gut, hydrophobicity, mucosal immunomodulation capacities and cell wall protein profiles in indigenous and exogenous bacteria. J Appl Microbiol. 2004;96:230–243. doi: 10.1046/j.1365-2672.2004.02158.x. [DOI] [PubMed] [Google Scholar]
- Vrinda R, Bhaskar N, Halami PM. Combined effect of enterocin and lipase from Enterococcus faecium NCIM5363 against food borne pathogens: mode of action studies. Curr Microbiol. 2012;65:162–169. doi: 10.1007/s00284-012-0138-z. [DOI] [PubMed] [Google Scholar]
- Yoon KY, Woodams EE, Hang YD. Production of probiotic cabbage juice by lactic acid bacteria. Biores Technol. 2006;97:1427–1430. doi: 10.1016/j.biortech.2005.06.018. [DOI] [PubMed] [Google Scholar]