Abstract
Methanolic extracts of four cultivated edible mushrooms of Pleurotus spp. namely Pleurotus florida, Pleurotus sajor-caju, Pleurotus cystidiosus and Pleurotus djamor along with the sporeless/low sporing mutants of Pleurotus florida, and Pleurotus sajor-caju were analyzed for their antioxidant activity using different chemical assays. The electrochemical behaviors of these extracts were also analyzed using cyclic voltammetry and differential pulse voltammetry. Results showed that scavenging effects on 2,2-diphenyl-1-picrylhydrazyl radicals were good (73.3–42.4 %) at 1.5 mg/ml. At 12 mg/ml, the reducing powers (2.54–1.71) and chelating effects on ferrous ions (56.0–78.5 %) were excellent. H2O2 scavenging abilities at 1.5 mg/ml showed a wide range (20.0–85.4 %). Scavenging of superoxide radicals were excellent and were found to be in the range of 61.1–90.0 % at 16 mg/ml concentration. FRAP results were in the range of 1.20 – 0.98 at 16 mg/ml. Total phenolic and total flavonoid contents of the methanolic extracts ranged from 22.67 to 36.03 mg/g and 1.19–2.94 μg/g respectively. The study assessed the amount of variation in antioxidant activities exhibited by different cultivated species and their sporeless/low sporing mutants.
Keywords: Edible mushrooms, Pleurotus spp., Mutants, Antioxidant activity, Total phenol content, Total flavonoid content, Electrochemical behavior
Introduction
Edible mushrooms belonging to Pleurotus species are most widely cultivated in different parts of Asia including India, Europe and Africa. They are highly nutritious and are known for their distinctive flavor and aroma (Chang 1999). Many compounds isolated from these mushrooms exhibited significant antioxidant, antitumor and anti-inflammatory activities (Jose et al. 2002). They were also found to be active against hypertension, lipid peroxidation and hypercholesterolemia (Jose and Janardhanan 2000; Gunde-Cimmerman et al. 1993 and 1999 & Wasser 2002). Research work carried out in Japan, China, Korea, USA and Europe over the last decade revealed the potent and health benefits of edible mushrooms.
However, in India, very few mushrooms have been investigated for their pharmacological and nutritional properties (Ajith and Janardhanan 2007). Recently, Antioxidative enzymatic profile of six mushrooms stored at low temperature was analysed (Dama et al. 2010). India with its vast reserve of lignocellulosic waste, predominantly subtropical climate (20–30 °C) and cheap labor is specially suited for the cultivation of species like Pleurotus. Further, its cultivation technique is simple without the use of compost, manure, limestone, casing or temperature shocks. Its adaptability to a wide range of temperature, high biological efficiency and good nutraceutical properties makes it the most suitable species for the Indian subcontinent. In such a scenario, it therefore forms a necessity to evaluate the antioxidant properties of these commercial strains of the cultivated mushrooms which are rapidly gaining popularity.
The current work involved evaluation of the antioxidant properties of commercial strains of the cultivated Pleurotus species namely Pleurotus ostreatus var florida (Jacq.et Fr.) Kummer, Pleurotus sajor-caju (Fr.) Singer, Pleurotus djamor (Rumph.) Boedijn, and Pleurotus cystidiosus O. K. Miller along with the sporeless mutant of Pleurotus ostreatus var florida and low spored mutant of Pleurotus sajor-caju (Fr.) Singer, developed by us earlier (Sandhya et al. 2006).
The cultivation technology for all the studied mushrooms had been standardized on paddy straw at Mushroom Lab, Indian Institute of Horticultural Research, Bangalore. The commercial package for some of them had already been released. The chemical composition of the mushrooms is known to vary considerably with difference in strains, substrates, cultivation techniques and methods of analysis (Beelman and Edwards 1989). Hence, the data available on the wild species may not be pertinent. It is therefore important to assess the antioxidant activity of these newer cultivable species before commercialising the cultivation technology.
An attempt was made in our laboratory to evaluate the difference in the antioxidant properties of these mushrooms belonging to Pleurotus spp. cultivated on paddy straw under similar laboratory conditions. This evaluation is important as the purpose is to bring out the variation in the antioxidant properties (if any) between the parent and the mutants developed and also to assess the antioxidant activities of the newly cultivated species. Taking into consideration the synergistic and additive effects that alter the total antioxidant capacities, all the studies were performed on the whole extracts that are found to be more beneficial than the isolated individual constituents (Liu 2003).
Material and methods
Reagents and chemicals
L-ascorbic acid, HRP type II (Horseradish peroxidase), TROLOX (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), BHT (butylated hydroxytoluene), riboflavin, DPPH (2,2-diphenyl-1-picrylhydrazyl), gallic acid, quercetin, NBT (nitroblue tetrazolium salt), ferrozine (Pyridyl]-5,6-diphenyl-1,2,4-triazine-4,4′-disulfonic acid.Na-salt), methionine, EDTA (Ethylenediaminetetraacetic acid) and homovanillic acid (3-Methoxy-4-hydroxyphenyl acetic acid) were all obtained from Sigma-Aldrich Chemicals (India) Ltd. All other chemicals used were of analytical grade. HPLC grade methanol was obtained from Merck India. Water was treated with Millipore Direct Q3 purification system and used for preparation of reagents. The strains of the mushroom species used in the current study are registered at IIHR as, Pleurotus florida(IIHR-Pfl1), Pleurotus sajor-caju(IIHR-Psc1), Pleurotus cystidiosus(IIHR-PCyst1), Pleurotus djamor(ARKA-OM1), Low sporing mutant(NBAIM-R-1), Sporeless mutant(IIHR-Psm1).
Mushroom cultivation
The spawn of all the oyster mushroom species and the mutants, collected from facility at IIHR Bangalore, was made on boiled and sterilized sorghum grains by inoculating culture pieces from 10 days old culture plates and incubated at 25 ± 2 °C (San Antonio 1984). Cultivation of the species was carried out using pasteurized paddy straw (steam pasteurization at 80 ± 2 °C) as substrate (Bano and Srivastava 1962). Pasteurized straw (2 Kg) was filled in Polypropylene bags (140 × 180 mm, 150 gauze thick) and thoroughly spawned at 4 % spawn dose (by wet weight). After inoculation, the bags were plugged with non absorbent cotton using PVC ring as neck. The inoculated bags were incubated at 25 ± 2 °C for spawn running which was completed in 18–20 days in P. djamor and sporeless mutant of P. florida, 20–22 days for P. florida and P. sajor-caju and 26–28 days for P. cystidiosus and low spored mutant of P. sajor-caju.
After complete spawn run, the bags were shifted into cropping room where a temperature of 25 ± 2 °C and humidity of 80–85 % was maintained and the bags were opened for fructification. Holes (25 mm) were made in the poly bags for sporophore induction. Fructification of all the species was obtained on pasteurized paddy straw-based substrate in polypropylene bags by the conventional oyster mushroom cultivation technique. The spawn running temperature was kept around 24 ± 2 °C. At the fruiting stage, the temperature of 24 ± 26 °C, relative humidity of 80–90 % was maintained.
Sample preparation
Sporophores (mushrooms) after harvest were cleaned and dried in a hot air tray drier at 40 ± 2 °C until crisp which took 10–12 h. Dried powders (100 g) were defatted by refluxing with light petrol (60–80 °C) for 6 h. The defatted material was then dried and extracted with 95 % ethanol (500 ml × 3) by refluxing for 6 h and filtered through Watman No. 4 filter paper. The combined ethanolic extracts were evaporated to dryness at 40 °C on a rotary evaporator and stored at 4 °C. For the analysis, dried extracts were redissolved in methanol at a concentration of 20 mg/ml and stored at 4 °C for further use.
Chemical assays
DPPH radical scavenging activity
The methanolic extracts in various concentrations (0.25–1.5 mg/ml, 2.5 ml) were mixed with methanolic DPPH solution (0.1 mM, 0.5 ml) and were shaken vigorously. The solutions were then allowed to stand for 30 min in dark. The absorbance was measured at 517 nm using a spectrophotometer (Hitachi spectrophotometer, U-2001). Methanol added to DPPH solution was taken as control. TROLOX was used as standard. The percentage of DPPH scavenged was calculated using the equation: 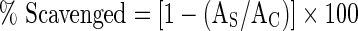 , where AC is the absorbance of control, and AS is the absorbance of sample extracts with DPPH (Aquino et al. 2001).
, where AC is the absorbance of control, and AS is the absorbance of sample extracts with DPPH (Aquino et al. 2001).
Chelating with ferrous ions
To study the ability of the mushroom extracts to chelate with the ferrous ions, the methanolic mushroom extracts (2–20 mg/ml, 0.4 ml) were added to a solution of 2 mM FeCl2 (0.2 ml). The reaction was then initiated by adding 5 mM ferrozine (0.4 ml). The mixture was made up to a final volume of 4 ml using methanol. The mixture was then shaken vigorously and allowed to stand for 10 min at room temperature. The absorbance at 562 nm was measured spectrophotometrically (Hitachi U-2001). Solution containing only FeCl2 and ferrozine was taken as control. Ascorbic acid was used as standard. The percentage of chelation was calculated by using the equation: 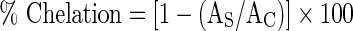 , where, AC is the absorbance of control, and AS is the absorbance of solution containing mushroom extracts (Ak and Gulcin 2008).
, where, AC is the absorbance of control, and AS is the absorbance of solution containing mushroom extracts (Ak and Gulcin 2008).
H2O2 scavenging activity
In this assay the oxidation of homovanillic acid (HVA) to its dimer in the presence of hydrogen peroxide and peroxidase is determined spectrofluorometrically (Pazdzioch-Czochra and Widenska 2002). The presence of antioxidants in the solution inhibits the formation of the dimer. Samples contained 0.5 ml of phosphate buffer (25 mM, pH 7.5), H2O2 (1 mM, 0.2 ml) and 0.1 ml of various concentrations of mushroom extracts (1–6 mg/ml). The samples were vortexed and incubated for 5 min at 20 °C. After incubation, 0.1 ml of HVA (1.25 mM) and 0.1 ml of Horseradish peroxidase (10U, type II) were added and incubated for another 5 min at 20 °C. The formation of the dimer was measured using luminescence spectrophotometer (Perkin Elmer, LS55) at an excitation wavelength of 315 nm and emission wavelength of 425 nm. Various concentrations of the extracts in the absence of H2O2 are taken for blank correction. TROLOX was used as standard.
Superoxide scavenging activity
Measurement of the superoxide scavenging activity of the mushroom extracts was based on the method described by Martinez et al. (2001) with slight modification. This assay is based on the photochemical reduction of nitroblue tetrazolium (NBT) in the riboflavin–light–NBT system. Each 3 ml reaction mixture contained 50 mM sodium phosphate buffer (pH 7.8), 13 mM methionine, 2 μM riboflavin, 100 μM EDTA, NBT (75 μM) and 1 ml of mushroom extracts of various concentrations (4–20 mg/ml). The formation of blue formazan was followed by monitoring the increase in absorbance at 560 nm after 40 min illumination from a fluorescent lamp. The percentage inhibition of the superoxide anion formed was calculated using the following equation: 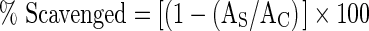 , where AC is the absorbance of control, and AS is the absorbance of solution containing sample extracts. TROLOX was used as standard.
, where AC is the absorbance of control, and AS is the absorbance of solution containing sample extracts. TROLOX was used as standard.
Reducing power
In this method, antioxidant compound forms a colored complex with potassium ferricyanide, trichloroacetic acid and ferric chloride, which is measured at 700 nm. Increase in absorbance of the reaction mixture directly indicates the reducing power of the samples (Jayaprakasha et al. 2001). Various concentrations (2–12 mg/ml) of methanolic extracts of mushrooms (2.5 ml) were mixed with 2.5 ml of sodium phosphate buffer (0.2 mM, pH 6.5) and 2.5 ml of 1 % potassium ferricyanide. The mixture was then incubated at 50 °C for 20 min. After adding 2.5 ml of 10 % tricloroacetic acid (w/v), the mixture was centrifuged at 1,000 rpm for 10 min (REMI R25). To the upper layer (2.5 ml), 2.5 ml of deionised water and 0.5 ml of 0.1 % ferric chloride were added, the contents were mixed thoroughly and the absorbance was measured at 700 nm using a spectrophotometer (Hitachi U-2001). TROLOX was used as standard (Oyaizu 1986)
Ferric Reducing Antioxidant Power (FRAP)
FRAP method involves spectroscopic determination of the complex formed when ferric tripyridyl triazene complex was reduced to the ferrous ion. The oxidant in the FRAP assay was prepared by mixing 2.5 ml of TPTZ (10 mM in 40 mM HCl), 25 ml of acetate buffer (0.3 M pH 3.6) and 2.5 ml of FeCl3 6H2O (20 mM) and warmed at 37 °C. To 3.6 ml of freshly prepared FRAP reagent, 120 μl of mushroom extracts in different dilutions (2–20 mg/ml) and 360 μl of water were added. The mixtures were incubated at 37 °C for 30 min before the absorbance was measured spectrophotometrically at 595 nm (Hitachi, U-2001). TROLOX was used as standard (Benzie and Strain 1996; Pulido et al. 2000).
Total phenolic content and total flavonoid content
Total phenolic content of the extracts was determined spectrophotometrically according to the method of Mau et al. (2002) with some modifications. The reaction mixture containing 0.1 ml of mushroom extracts (20 mg/ml) in 13 % HCl/MeOH (60:40, v/v) and 2 ml of 2 % sodium carbonate was incubated at room temperature for 3 min. To this 0.1 ml of Folin Ciocalteu reagent (50 %) was added and mixed thoroughly. The mixture was allowed to stand at room temperature for 30 min. The absorbance was measured at 750 nm. The results were expressed as mg of gallic acid equivalents (GAEs) per gram of mushroom extract, which was obtained from the calibration curve of gallic acid.
Total flavonoid content at 20 mg/ml concentration of the extracts was also measured using colorimetric assay. To 1 ml of mushroom extracts, 1 ml of 10 % AlCl3, potassium acetate (1 M, 0.1 ml) and 3.8 ml of MeOH were added and the mixture was allowed to stand for 40 min at room temperature. The solution was mixed well and absorbance was measured at 415 nm against prepared reagent blank (Öztürk et al. 2007). The results were expressed as μg of quercetin equivalents (CEs) per gram of mushroom extract, obtained from the calibration plot of quercetin.
Electrochemical studies
Instrumental setup
Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements were performed using electrochemical workstation (IVUM COMPACTSTAT) equipped with a closed standard three electrode cell. A glassy carbon (BAS, Ø = 0.3 cm) electrode was used as working electrode. Pt foil was used as counter electrode and Ag/AgCl 3 M HCl was used as reference electrode. The working electrode was polished in an aqueous suspension of 0.3 μm alumina on a polishing pad and rinsed with deionised water before analysis. After every analysis the electrode was sonicated in 6 M HCl and in methanol for 5 min (Barros et al. 2008).
Measurements
Various concentrations of mushroom extracts (1–12 mg/ml) were prepared freshly in methanol/acetate buffer 0.1 M (pH 4)/NaClO4 (70:28:2) solution and taken in a 10 ml reaction vessel. Calibration was done using different concentrations of gallic acid (0.02–0.2 mg/ml) as standard. To minimize the adsorption of the species onto the surface of the electrode, the electrochemical responses were measured immediately after immersing the working electrode. The antioxidative power of the extracts was evaluated by using DPV with the operating conditions set to 60 mV pulse amplitude and 0.030Vs−1 as scan rate. Current density was plotted as a function of each mushroom extract concentration and compared with that of gallic acid.
Statistical analysis
For preparing the methanolic extracts of mushrooms, samples from 10 different fruiting bags were mixed and extracted. The measurements in all the assays were carried out in triplicate. The experimental results were analysed using ANOVA (analysis of variance) followed by student’s t-test (Microcal Origin ver. 6.0). p values < 0.05 were regarded as significant.
Results and discussions
DPPH radical scavenging activity
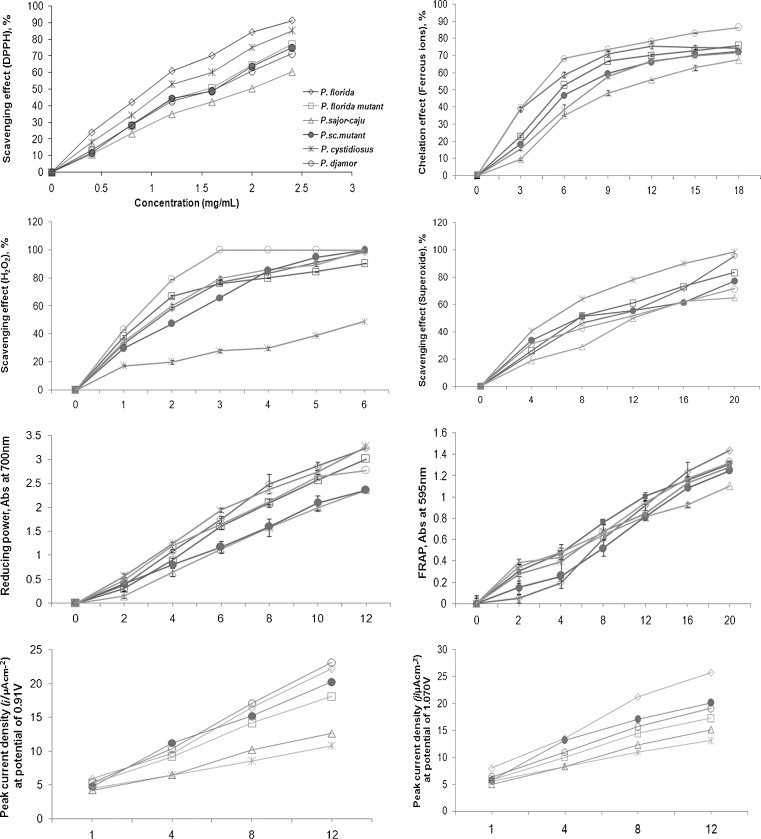
Many of the chronic diseases associated with DNA damage, mutagenesis, carcinogenesis and pathogenic bacterial growth can be reduced by the termination of free radical propagation in biological systems (Zhu et al. 2002). The results of the current study show that methanolic extracts of the cultivated oyster mushrooms had increasing scavenging effect with increase in concentration. The activity was moderate (70.3 % – 42.4 %) even at low concentration of 1.6 mg/ml (Fig. 1). However the scavenging effect of TROLOX at 6 μg/ml was 65.1 %. Among the 6 varieties studied, P. florida exhibited maximum scavenging activity (70.3 %) followed by P. cystidiosus (60.4 %). Least activity was exhibited by P.sajor-caju (42.4 %) whereas its low sporing mutant showed slight increase in activity (48.7 %). Lakshmi et al. (2004) reported that the ethyl acetate extracts of the wild varieties of P. rimosus at a concentration of 0.1 % showed 12.49 TEAC, whereas P. florida, P. sajor-caju, at 1 % concentration, showed AEAC values of 0.66, 0.40 respectively. The methanolic extracts of medicinal mushrooms have slightly higher radical scavenging properties ranging from 24.6 to 74.4 % at 0.64 mg/ml.
Fig. 1.
Changes in chemical quality parameters of methanolic extracts of various concentrations of different mushrooms (n = 3)
Ferrous ions chelating activity
Iron is known as pro-oxidant in lipid oxidation. The Fe (II) state accelerates the lipid oxidation by breaking down hydrogen and lipid peroxides to reactive free radicals (Öztürk et al. 2007). The study of the chelating effects on the ferrous ions has gained importance because ferrous ions are found to be most active pro-oxidants in the food system (Yamaguchi et al. 1988). Chelating effects of the studied methanolic extracts on ferrous ions increased with the increased concentrations and were 56.0–78.5 % at a concentration of 12 mg/ml (Fig. 1). However the chelating effect of L-ascorbic acid was 87.2 % at 0.5 μg/ml concentration. Among the methanolic extracts of all the mushrooms, P.dajmor exhibited excellent chelating ability (78.5 %) whereas P.sajor-caju exhibited moderate chelating ability (56.0 %). The results showed that the sporeless mutant of P.florida has slight reduction in its chelating ability compared to that of the parent whereas the low sporing mutant of P.sajor-caju mutant exhibited significant increase (10.4 %) in its chelating ability compared to that of its parent. Yang et al. (2002) reported that the methanolic extracts of several commercial mushrooms had the chelating effects in the range of 45.6 – 81.6 % at 1.6 mg/ml concentration and suggested that at higher concentrations of the extracts, higher chelating effect could be observed. Oyster mushrooms proved to be good as ferrous ion chelators (85.1 – 96.5 %) at 5 mg/ml of their methanolic extracts.
H2O2 scavenging activity
Hydroxyl radicals are the major active oxygen species that cause lipid oxidation and biological damage to the cell (Aurand et al. 1977). Though H2O2 itself is not very reactive, it can sometimes be toxic as it may sometimes give rise to hydroxyl radical in the cells. Therefore removal of excessive H2O2 from the cells is very important for their protection (Kumar et al. 2008). The assay for H2O2 scavenging activity is based on the incubation of a putative scavenger with H2O2 and analyzing the reaction mixture for the loss of H2O2. The study of H2O2 scavenging activity by the mushroom extracts showed a marked variation between the scavenging effects of the extracts. The activity ranged from 20.0 to 85.4 % at concentration of 2 mg/ml (Fig. 1). Of the species studied, P.djmor exhibited excellent scavenging activity (85.4 %) followed by P.sajor-caju (60.2 %) and P.florida mutant (66.8 %). Least activity was exhibited by P.cystidiosus (20.0 %).
Superoxide scavenging activity
Reactive oxygen species in biological systems are often produced by sunlight, ultra- violet, ionizing radiation, chemical reactions and metabolic processes. These have a wide variety of pathological effects resulting in DNA damage and cellular degeneration related to aging. In cellular oxidation reactions, superoxide radical is usually formed first, and its effects are further magnified by production of other kinds of cell-damaging free radicals and oxidizing agents (Zhao et al. 2007). The superoxide scavenging activities of various concentrations (4–20 mg/ml) of the methanolic expressed in terms of % scavenging activity, ranged from 61.1 to 90.0 % (Fig. 1). The results showed that P. cystidiosus has significantly stronger superoxide scavenging power compared to other species. The superoxide scavenging ability between the parents and their mutants was found to be negligible.
Reducing power
The high reducing power is indicative of the hydrogen donating ability of the active species present in the extracts (Shimada et al. 1992). Reducing powers of the methanolic extracts of Pleurotus spp. were excellent and increased steadily with the increase in concentration (Fig. 1). At 12 mg/ml concentration, the reducing powers were 3.36 –2.36. The reducing power of TROLOX at 150 μg/ml was 0.997. The reducing power of these mushrooms is found to be greater than that of the commercial mushrooms reported by Yang et al. (2002). Results show that P.cystidiosus and P.florida exhibited excellent reducing power (3.36 and 3.34) followed by P.florida mutant (3.01). There was negligible difference in the reducing power between the methanolic extracts of P.sajor-caju and its low sporing mutant.
Research conducted in Taiwan in 1999 showed that among methanolic extracts of the commercial mushrooms, abalone and tree oyster mushrooms showed good reducing powers of 1.00 and 1.19 at 10 mg/ml, respectively. Mau et al. (2002) reported that the methanolic extract from snow ears showed a high reducing power of 0.73–0.84 at 1.0– 5.0 mg/ml. Medicinal mushrooms like Ganoderma spp. are reported to have higher reducing powers. This was attributed to the higher reductone (Enediols with a carbonyl group adjacent to the enediol group) content that reacts with free radicals thereby terminating the chain reactions.
Ferric reducing antioxidant power
Ferric ions also react with peroxides to produce radicals although the rate is 10-fold less than that of ferrous ion (Kehrer 2000). This assay evaluates the hydrogen-donating capacity of the extracts and suppresses formation of the free radicals thereby acting as ‘preventive antioxidant’. All the methanolic extracts showed increased FRAP with the increase in concentration (Fig. 1). At 16 mg/ml concentration, the FRAP values measured as absorbance at 595 nm were in the range 1.20 – 0.98. However the FRAP of Trolox at 100 μg/ml concentration was 0.66. Results showed that all the species have more or less similar activity. P.sajor-caju exhibited least FRAP activity. Earlier studies reported that the ethyl acetate extracts of P. rimosus at 0.1 % concentration showed 0.31 TEAC, whereas P. florida, and P. sajor-caju, at same concentration showed AEAC values of 0.021 and 0.021 respectively (Lakshmi et al. 2004). The variation in the activities compared to that of the earlier reports may be because of the difference in the extraction procedures used.
Antioxidant components
Mushrooms are known to contain phenolics of known antioxidant properties which can be compared to that of BHT, tocopherols and gallate (Yang et al. 2002). These form the major component of mushroom extracts. The total phenol and flavonoid content of the methanolic extracts of the Pleurotus spp. are summarised in Table 1. Total phenols and flavonoids were found to be high in P. florida. This was reflected in the results found for DPPH scavenging ability, FRAP and reducing power. Methanolic extract of P. cystidiosus contained lesser flavonoids. This was reflected in its poor ability to scavenge hydrogen peroxide. The total phenols in the methanolic extracts ranged from 22.67–36.03 mg/g of the mushroom extracts and total flavonoid content ranged from 1.19 to 2.94 μg/g of the mushroom extracts.
Table 1.
Total flavonoids and total phenols, Electro-chemical parameters (from differential pulse voltammetry) and IC50 values (for various antioxidant assays) of methanolic extracts of different mushrooms
| P. florida | P. florida mutant | P.sajor-caju | P.sc.mutant | P. cystidiosus | P. djmor | |
|---|---|---|---|---|---|---|
| Total flavonoids (μg/g of extract) | 2.9 ± 0.07a | 2.3 ± 0.23b | 1.7 ± 0.08c | 1.7 ± 0.02c | 1.2 ± 0.02d | 2.1 ± 0.10e |
| Total phenols (mg/g of extract) | 36.0 ± 0.44a | 26.1 ± 6.59b | 22.7 ± 1.32b | 24.0 ± 2.90c | 27.1 ± 2.43d | 27.0 ± 4.89d |
| Electrochemical studies | ||||||
| E1/2/V | 0.99 | 1 | 0.98 | 0.98 | 1.01 | 0.99 |
| Slope/μAcm−2 mg−1 ml | 5.578 | 4.348 | 2.89 | 5.056 | 2.11 | 5.998 |
| E1/2/V | 1.16 | 1.14 | 1.15 | 1.13 | 1.14 | 1.17 |
| Slope/μAcm−2 mg−1 ml | 6.096 | 3.338 | 3.457 | 4.719 | 2.518 | 4.319 |
| IC50 values (mg/ml) | ||||||
| DPPH scavenging activity | 1.00 ± 0.022 | 1.38 ± 0.024 | 1.75 ± 0.060 | 1.42 ± 0.055 | 1.17 ± 0.063 | 1.46 ± 0.038 |
| Reducing power | 5.8 ± 0.73 | 6.5 ± 0.36 | 8.5 ± 0.88 | 8.1 ± 0.76 | 5.6 ± 0.28 | 6.3 ± 0.50 |
| Chelation with ferrous ions | 4.6 ± 0.24 | 6.1 ± 0.27 | 9.3 ± 0.27 | 6.8 ± 0.13 | 7.3 ± 0.37 | 4.3 ± 0.15 |
| FRAP | 09.8 ± 1.17 | 08.9 ± 0.79 | 11.1 ± 0.79 | 11.0 ± 1.24 | 09.8 ± 1.60 | 09.1 ± 1.19 |
| H2O2 scavenging activity | 2.0 ± 0.07 | 1.7 ± 0.07 | 1.7 ± 0.07 | 2.2 ± 0.20 | 6.5 ± 0.04 | 1.3 ± 0.34 |
| Superoxide scavenging activity | 10.1 ± 1.6 | 10.0 ± 1.8 | 12.4 ± 2.0 | 10.8 ± 2.7 | 07.3 ± 2.2 | 11.9 ± 2.4 |
*Each value is expressed as mean ± standard deviation (n = 3). Means with different letters within a row are significantly different (P < 0.05)
Electrochemical behavior of mushroom extracts
Cyclic voltammograms of the methanolic extracts of the Pleurotus spp. showed the electrochemical behavior the oxidation potentials were more positive than that of gallic acid and ascorbic acid, (around 0.91 V). Under the studied conditions, the results indicated the absence of these standard compounds in mushrooms in considerable amounts. Similarities in the oxidation potential of the extracts (Table 1) indicate that all the mushrooms of the Pleurotus species under study have similar composition of electroactive species.
Differential pulse voltammetry by Barros et al. (2008) was used to measure the electrochemical responses of the extracts under study. The differential pulse voltammograms for the mushroom extracts had two peaks except that of P. cystidiosus (Fig. 2(i)) which had an additional peak at 0.85 V. The peak current showed linear response with the increase in concentration of the extracts. A linear relation between the two parameters was observed though the slopes for the plots of peak current density vs extract concentration differed (Fig. 1 and Table 1). Barros et al. (2008) reported the oxidation potentials of various Agaricus spp. extracts to be around 0.9 V. The slopes for the plots peak current density vs extract concentration at this potential were in range 0.55–0.85.
Fig. 2.
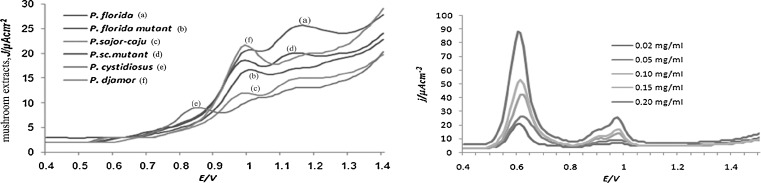
(i) Differential pulse voltammograms of 12 mg/ml methanolic extracts of different mushrooms in methanol/acetate buffer 0.1 M (pH 4)/NaClO4 (70:28:2) solutions and and (ii) Differential pulse voltammograms of gallic acid in methanol/acetate buffer 01 M (pH/NaClO4(70:28:2) solutions at different concentrations
Direct correlation between the slopes of the plot and the total phenolics present in the extracts were observed. Similar results were reflected in most of the chemical assays studied. The results indicate that this method can be used in assessment of the antioxidant properties of the mushroom extracts. The study also indicated that mutation in general reduced the total flavonoid content. The IC50 values were calculated from the plots for each assay and are summarised in Table 1.
In our earlier study, we had evaluated the antioxidant properties of edible mushrooms that were available in commercial Indian market (Rajesh Babu and Nageswar Rao 2011). Mushrooms are known to contain sterols (Ohnuma et al. 2000), phenolic compounds and antioxidant vitamins like tocopherols and ascorbic acid, (Ferreira et al. 2009; Josiana et al. 2011), and polysaccharides (Cheng et al. 2008). HPLC-MS analysis of edible mushrooms done by Barros et al. (2009) revealed the presence of diverse phenolic acids like protocatechuic, p-hydroxybenzoic, p-coumaric and vanillic acid isomers. A non phenolic compound, namely cinnamic acid was also detected.
Conclusions
In the present study we thoroughly investigated the antioxidant potential of the cultivated species of oyster mushrooms and the mutants, as they are both commercially and medicinally important. The study indicated that the mutants developed, have slight variation in their AOA compared to their parents and therefore the cultures of these mutant strains along with the strains of P.djmor and P.cystediosys can therefore be released for commercial cultivations. Further, the chemical constituents responsible for the antioxidant properties will have to be investigated and identified.
Acknowledgements
Authors express deepest gratitude to their Divine Chancellor, Bhagavan Sri Sathya Sai Baba. Authors are thankful to the Director, Indian Institute of Horticultural Research, Bangalore for necessary collaboration. Authors also thank Prof. TN Lakhanpal for his constant guidance.
References
- Ajith TA, Janardhanan KK. Indian medicinal mushrooms as a source of antioxidant and antitumor agents. J Clin Biochem Nut. 2007;40:157–162. doi: 10.3164/jcbn.40.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ak T, Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Aquino R, Morelli S, Lauro MR, Abdo S, Saija A, Tomaino A. Phenolic constituents and antioxidant activity of an extract of Anthurium versicular leaves. J Nat Prod. 2001;64:1019–1023. doi: 10.1021/np0101245. [DOI] [PubMed] [Google Scholar]
- Aurand LW, Boonme NH, Gidding GG. Superoxide and singlet oxygen in milk lipid peroxidation. J Dairy Sci. 1977;60:363–369. doi: 10.3168/jds.S0022-0302(77)83874-5. [DOI] [Google Scholar]
- Bano Z, Srivastava HC. Cultivation of Pleurotus species on paddy straw. Food Sci. 1962;11:363–365. [Google Scholar]
- Barros L, Falcao S, Baptista P, Freire C, Vilas-Boas M, Ferreira ICFR. Antioxidant activity of Agaricus sp. mushrooms by chemical, biochemical and electrochemical assays. Food Chem. 2008;111:61–66. doi: 10.1016/j.foodchem.2008.03.033. [DOI] [Google Scholar]
- Barros L, Dueñas M, Ferreira CFRI, Baptista P, Santos-Buelga C. Phenolic acids determination by HPLC–DAD–ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem Toxicol. 2009;47:1076–1079. doi: 10.1016/j.fct.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Beelman RB, Edwards CG. Variability in the composition and nutritional value of the cultivated mushroom Agaricus bisporus. Mush News. 1989;37:20–26. [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "Antioxidant Power". The FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Chang ST. Global impact edible and medicinal mushrooms on human welfare in the 21st century: non green evolution. Int J Med Mushr. 1999;1:1–7. doi: 10.1615/IntJMedMushrooms.v1.i1.10. [DOI] [Google Scholar]
- Cheng JJ, Lin CY, Lur HS, Chen HP, Lu MK. Properties and biological functions of polysaccharides and ethanolic extracts isolated from medicinal fungus, Formitopsis pinicola. Process Biochem. 2008;43:829–834. doi: 10.1016/j.procbio.2008.03.005. [DOI] [Google Scholar]
- Dama CL, Kumar S, Brijesh KM, Kunj Bihari S, Sudha M, Doshi A. Antioxidative enzymatic profile of mushrooms stored at low temperature. J Food Sci Technol. 2010;47:650–655. doi: 10.1007/s13197-010-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira ICFR, Barros L, Abreu RMV. Antioxidants in wild mushrooms. Curr Med Chem. 2009;16:1543–1560. doi: 10.2174/092986709787909587. [DOI] [PubMed] [Google Scholar]
- Gunde-Cimmerman N. Medicinal value of the genus Pleurotus (Fr.) P. Kaest. (Agaricales s.l., Basidiomycetes) Int J Med Mushr. 1999;1:69–80. doi: 10.1615/IntJMedMushrooms.v1.i1.50. [DOI] [Google Scholar]
- Gunde-Cimmerman N, Freidrich J, Cimmerman A, Benicki N. Screening fungi for the production of an inhibitor of HMG CoA reductase, production of mevinolin by the fungi of the genus Pleurotus. FEMS Microbiol Lett. 1993;111:203–206. doi: 10.1111/j.1574-6968.1993.tb06386.x. [DOI] [Google Scholar]
- Jayaprakasha GK, Singh RP, Sakariah KK. Antioxidant activity of grape seed extracts on peroxidation models in-vitro. Food Chem. 2001;73:285–290. doi: 10.1016/S0308-8146(00)00298-3. [DOI] [Google Scholar]
- Jose N, Janardhanan KK. Antioxidant and antitumor activity of Pleurotus florida. Curr Sci. 2000;79:941–943. [Google Scholar]
- Jose N, Ajith TA, Janardhanan KK. Antioxidant, anti-inflammatory and antitumor activities of culinary medicinal mushroom Pleurotus pulmonarius (Fr.) Quel. (Agaricomycetideae) Int J Med Mushr. 2002;4:329–335. doi: 10.1615/IntJMedMushr.v4.i4.60. [DOI] [Google Scholar]
- Josiana AV, Barros L, Martins A, Santos-Buelga C, Vasconcelos H, Ferreira lCFR. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011;126:610–616. doi: 10.1016/j.foodchem.2010.11.063. [DOI] [Google Scholar]
- Kehrer JP. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43–50. doi: 10.1016/S0300-483X(00)00231-6. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kumar D, Manjusha SK, Singh N, Vashistha B. Antioxidant and free radical scavenging potential of Citrullus colocynthis (L.) Schrad methanolic fruit extract. Acta Pharm. 2008;58:215–220. doi: 10.2478/v10007-008-0008-1. [DOI] [PubMed] [Google Scholar]
- Lakshmi B, Tilak JC, Adhikari S, Devasagayam TPA, Janardhanan KK. Evaluation of antioxidant activity of selected Indian mushrooms. Pharm Biol. 2004;42:179–185. doi: 10.1080/13880200490514023. [DOI] [Google Scholar]
- Liu RH. Health benefits of fruits and vegetables are from additive and synergistic combination of phytochemicals. Am J Clin Nutr. 2003;78:517–520. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- Martinez AC, Marcelo EL, Marco AO, Moacyr M. Differential responses of superoxide dismutase in freezing resistant Solanum curtibolum and freezing sensitive Solanum tuberosum subjected to oxidative and water stress. Plant Sci. 2001;160:505–515. doi: 10.1016/S0168-9452(00)00418-0. [DOI] [PubMed] [Google Scholar]
- Mau JL, Lin HC, Chen CC. Antioxidant properties of several medicinal mushrooms. J Agric Food Chem. 2002;50:6072–6077. doi: 10.1021/jf0201273. [DOI] [PubMed] [Google Scholar]
- Ohnuma N, Amemyia K, Kakuda R, Yaoita Y, Machida K, Kikuchi M. Sterol constituents from two edible mushrooms, Lentinula edodes and Tricholoma matsutake. Chem Pharm Bull. 2000;48:749–751. doi: 10.1248/cpb.48.749. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Öztürk M, Aydoğmuş-Öztürk F, Duru ME, Topçu G. Antioxidant activity of stem and root extracts of Rhubarb (Rheum ribes): an edible medicinal plant. Food Chem. 2007;103:623–630. doi: 10.1016/j.foodchem.2006.09.005. [DOI] [Google Scholar]
- Pazdzioch-Czochra M, Widenska A. Spectrofluorimetric determination of hydrogen peroxide activity. Anal Chim Acta. 2002;452:177–184. doi: 10.1016/S0003-2670(01)01455-6. [DOI] [Google Scholar]
- Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem. 2000;48:3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- Rajesh Babu D, Nageswar Rao G (2011) Antioxidant properties and electrochemical behavior of cultivated commercial Indian edible mushrooms. J Food Sci Technol. doi:10.1007/s13197-011-0338-8 [DOI] [PMC free article] [PubMed]
- San Antonio JP. Origin and improvement of spawn of the cultivated mushroom Agaricus brunnescens Peck. Hortic Rev. 1984;6:85–118. [Google Scholar]
- Sandhya R, Meera P, Tewari RP, Krishna V. Development of sporeless/low sporing strains of Pleurotus through mutation. World J Microbiol Biotechnol. 2006;22:1021–1025. doi: 10.1007/s11274-005-2891-7. [DOI] [Google Scholar]
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soyabean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:475–498. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- Wasser SP. Medicinal mushrooms as source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;60:258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R, Tatsumi MA, Kato K, Yoshimitsu C. Effect of metal salts and fructose on the autoxidation of methyl linoleate in emulsions. Agric Biol Chem. 1988;52:849–850. doi: 10.1271/bbb1961.52.849. [DOI] [Google Scholar]
- Yang JH, Lin HC, Mau JL. Antioxidant properties of several commercial mushrooms. Food Chem. 2002;77:229–235. doi: 10.1016/S0308-8146(01)00342-9. [DOI] [Google Scholar]
- Zhao L, Zhao G, Ming D, Zhao Z, Xiao L, Xiaosong H (2007) Effect of selenium on increasing free radical scavenging activities of polysaccharide extracts from a Se-enriched mushroom species of the genus Ganoderma. Eur Food Res Technol. doi:10.1007/s00217-007-0562-7
- Zhu QY, Hackman RM, Ensunsa JL. Antioxidative activities of oolong tea. J Agric Food Chem. 2002;50:6929–6934. doi: 10.1021/jf0206163. [DOI] [PubMed] [Google Scholar]