Abstract
The influences of cooking methods (steaming, pressure-cooking, microwaving, frying and boiling) on total phenolic contents and antioxidant activities of fruit body of Boletus mushrooms (B. aereus, B. badius, B. pinophilus and B. edulis) have been evaluated. The results showed that microwaving was better in retention of total phenolics than other cooking methods, while boiling significantly decreased the contents of total phenolics in samples under study. Effects of different cooking methods on phenolic acids profiles of Boletus mushrooms showed varieties with both the species of mushroom and the cooking method. Effects of cooking treatments on antioxidant activities of Boletus mushrooms were evaluated by in vitro assays of hydroxyl radical (OH·) -scavenging activity, reducing power and 1, 1-diphenyl-2-picrylhydrazyl radicals (DPPH·) -scavenging activity. Results indicated the changes of antioxidant activities of four Boletus mushrooms were different in five cooking methods. This study could provide some information to encourage food industry to recommend particular cooking methods.
Keywords: Boletus mushrooms, Cooking methods, Phenolics, Antioxidant activity
Introduction
Many species of mushrooms are traditionally used as food and medicine (Mdachi et al. 2004). They are appreciated not only for their texture and high content of flavor components but also because they are rich in proteins and amino acids and poor in calories. Some mushrooms have been reported as therapeutic foods, useful in preventing diseases such as hypertension, hypercholesterolemia, atherosclerosis and/or cancer (Wasser and Weis 1999; Sun et al. 2011).
The genus Boletus mushrooms has a worldwide distribution comprising of 24 species (Hall et al. 1998). Heleno et al. (2011) studied the contents of proteins, carbohydrates, fatty acids,sugars, vitamins and phenolic acids of six wild Boletus species and determined their antioxidant properties. The extract of Boletus scaber demonstrated antiulcer and antitumor activities and low acute toxicity (Ryzhova et al. 1997). The methanol extracts from the fruiting body of the mushroom Boletus calopus showed free radical-scavenging activity (Kim et al. 2006a). Boletus aereus, Boletus badius, Boletus pinophilus and Boletus edulis are dominant wild mushrooms belonging in Boletus species, in China. Although the edible wild mushrooms command higher prices than cultivated mushrooms, people prefer to consume them due to their flavor and texture (Elmastas et al. 2007). Nevertheless, Boletus mushrooms are becoming more and more important in our diet for their nutritional and pharmacological characteristics.
Cooking, such as boiling, microwaving, pressure-cooking, griddling, baking, steaming and frying, induces significant changes in the texture and chemical composition (Osman et al. 2010; Wolosiak et al. 2010; Mandge et al. 2011; Medoua and Oldewage-Theron 2011). The most mushrooms are commonly cooked before being consumed. Therefore, antioxidant activities of mushrooms are closely linked not only to species but also to cooking methods. Lower antioxidant activities were reported in cooked mushrooms (Manzi et al. 2004). While, the polyphenol concentration and antioxidant properties were increased by cooking treatments in L. edodes (Choi et al. 2006), However, the effects were reported to depend on the duration of cooking (Soler-Rivas et al. 2009). This supported the need to thoroughly evaluate the effects of cooking on antioxidant activities of edible mushrooms in view to develop the best cooking methods possible.
Because modern day consumers seek to avoid aggressive cooking methods which may affect the functioning of the food, there is growing interest in the phytochemical profiles and antioxidant activities of cooked mushroom. However, there are few investigations on the effects of cooking treatments on Boletus mushrooms. Therefore, the present work aimed at evaluating the effects of cooking on the total phenolic contents, phenolic acids profiles and antioxidant properties.
Materials and methods
Sampling
Four fresh mushrooms fruit body (B. aereus, B. badius, B. pinophilus and B. edulis) were provided from the MuShuiHua fresh market in Kunming, in China, and identified by Yunnan Academy of Agriculture Sciences. They were cleaned from soil and substrate with a stainless knife. Three experiments were carried out, using 180 g of mushroom in each. The amount was divided into six variants (30 g each), raw and prepared for cooking in five methods. Mushrooms were then cut along their vertical axes into six pieces and the cuts were divided into six groups of 30 g within each variant, cut to slices 1–2 mm thick.
Cooking treatment
Steaming: Mushroom pieces (30 g) were placed in a pirex cylindrical bowl without additional water, and the bowl was placed in a domestic steam cooker. Then the samples were cooked for 8 min under atmospheric pressure.
Pressure-cooking: Mushroom pieces (30 g) were placed in a pirex cylindrical bowl without additional water, and the bowl was placed in a domestic high pressure cooker. Then the samples were cooked at high pressure in for 5 min until tender.
Microwaving: Mushroom pieces (30 g) were placed in a glass dish without additional water, and cooked in a domestic microwave oven at 900 watts for 1.5 min until tender.
Frying: Mushroom pieces (30 g) were placed in a frying pan with 100 mL hot peanut oil (160°C), and stirred for 3 min until the sample became crisp-tender.
Boiling: Mushroom pieces (30 g) were poured on a pot containing 300 mL boiling water. Then, they were cooked for 10 min and placed on a filter paper to drain water excess.
All samples, including raw and thermal processed, were freeze-dried to use in subsequent experiments.
Methanolic extracts
1 g freeze-dried sample was extracted by stirring with 10 mL 50 % methanol at room temperature for 24 h, and filtered through Whatman no. 1 paper (Savioe et al. 2008). The filtrates were concentrated with a rotary vacuum evaporator at 40 °C. The resultant extracts were stored at −20 °C until were used.
Total phenolic content determination
Total phenolics were determined according to the method of Mau et al. (2001) with some modifications. In brief, phenolic content was estimated by mixing 200 μL of deionized water, 50 μL of the diluted extracts, and 50 μL of Folin-Ciocalteau’a reagent. After 6 min, 500 μL of 7.5 % sodium carbonate solution were added to the mixture, which was adjusted to 1.3 mL with distilled water and allowed to stand at room temperature for 60 min. Then, the absorbance was read at 765 nm. Gallic acid (ranging from 0–100 μg/mL) was used to construct the calibration curve (R2 = 0.9982). The results were expressed as μg of gallic acid equivalents (GAE)/g fresh weight (FW).
Phenolic acids profiles determination
Samples preparation for analysis of phenolics followed Kim et al. (2006b). The 20 μL of extract was loaded on an Agilent 1100 HPLC system with a photodiode array detector equipped with an autoinjector (Agilent Technologies, Palo Alto, CA, USA). The separations were performed on a reversed-phase Zorbax SB-C18 HPLC column (250 mm × 4.6 mm i.d., 5 μm, Agilent Technologies) with a gradient elution consisting of methanol (eluent A) and aqueous 0.3 % phosphoric acid (eluent B) at a flow rate of 1 mL/min. The gradient pattern was a linear gradient increasing from 0 % to 100 % A in 60 min and keeping 100 % A for 10 min.
Eleven standard phenolics, p-coumaric acids, catechin, gallic acid, p-hydroxybenzioc acid, vanillin, luteolin, chlorogenic acid, protocatechuic, cinnamic acid, quercetin and benzoic acid, were used for calibration. Standard stock solutions (50, 100, 250, and 500 μg/g) were made, and all standard calibration curves showed high degrees of linearity (R2 > 0.99). Identification of the phenolic compounds in samples was done by comparison of the retention times and UV spectra with those of standard materials, and the quantification were done by comparing their peak areas with those of standard curves.
Hydroxyl radical (OH·) -scavenging activity assay
OH·-scavenging activity was assessed using the previous method (Smirnoff and Cumbes 1989) with a slight modification. 1 mL of extracts, 0.3 mL of 8 mM freshly prepared ferrous sulfate solution, 0.25 mL of 20 mM hydrogen peroxide, and 1 mL of 3 mM salicylic acid were injected into the test tube and incubated for 30 min at 37 °C; 0.45 mL of distilled water was then added to the test tube. The mixture obtained was centrifuged for 10 min at 3,000 × g. Distilled water was used instead of extracts as a control. OH·-scavenging activity was calculated according to the Eq. (1) and IC50 was expressed as the concentration of 50 % of OH·-scavenging activity.
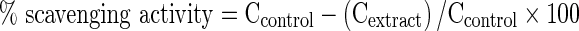 |
1 |
Reducing power assay
The reducing power of extracts was determined by the method of Benjakul et al. (2005). Different concentrations of extract in 3.5 mL of phosphate buffer (0.2 M, pH 6.6) were mixed with 2.5 mL of 1 % potassium ferricyanide in test tubes. The mixtures were incubated for 20 min at 50 °C. At the end of the incubation, 2.5 mL of 10 % trichloroacetic acid were added to the mixtures, followed by centrifugation for 10 min at 650 × g. 2.5 mL of supernatant fluid was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1 % ferric chloride, and the absorbance of the mixture was measured at 700 nm. The increase in the absorbance of the reaction mixture indicated the reducing power of extracts. EC50 was defined as the concentration of 0.500 of absorbance.
1, 1-diphenyl-2-picrylhydrazyl radicals (DPPH·) -scavenging activity assay
DPPH·-scavenging activity of extracts was determined by a modified method of Jing and Kitts (2004). Reaction mixture containing 2 mL of 0.1 mM DPPH· methanol solution and 0.4 mL of extracts was kept at room temperature in dark for 30 min. The absorbance at 517 nm was recorded. The DPPH·-scavenging activity was calculated according to the Eq. (1), and IC50 was expressed as the concentration of 50 % of DPPH·-scavenging activity.
Statistical analysis
Determinations were performed in triplicate in two independent experimental assays. Experimental results were expressed as mean ± standard deviation. Data were analyzed by one-way analysis of variance (ANOVA) using SPSS (version 11.0, Chicago, U.S.A.). A p value of <0.05 was taken as the level of statistical significance.
Results and discussion
Effects of cooking on total phenolic contents
Phenolics are the major contributors to the total antioxidant capacity of fruits, vegetables, and mushrooms (Heo et al. 2007). Previous researches showed that the relations between total phenolic contents of plants and their antioxidant activities were linear (Isabelle et al. 2010; Annegowda et al. 2011). In this study, total phenolic contents of raw mushrooms of B. aereus, B. badius, B. edulis and B. pinophilus were 209.5, 150.5, 184.5 and 220.0 μg of GAE/g FW, respectively. The effects of different cooking methods on the total phenolic contents of samples were shown in Table 1. For B. aereus, all cooking methods significantly decreased the total phenolic contents, and the loss during the boiling treatment was significantly higher than that of other cooking methods (p < 0.05). Compared with raw mushrooms, microwaving and steaming treatments showed insignificant effects on total phenolic content of B. badius. For B. edulis, the effect of microwaving treatment on total phenolic content was significantly higher than that of other cooking methods (p < 0.05). All cooking methods could keep total phenolic contents of B. pinophilus, except boiling, which significantly decreased its content (p < 0.05). In short, four mushrooms showed the highest retention of phenolics during the microwaving treatment compared with the other cooking methods, while boiling significantly decreased the total phenolic contents. Several reports on the retention of total phenolics in cooked mushroom are available in recent years. Khalil and Mansour (1995) stated that cooking treatments significantly decreased the phenolic contents. Barros et al. (2007) indicated that the cooking procedure with heating could destroy the structures of phenolics and decrease their contents. Ju et al. (2010) showed that steaming with pressure could increase the amounts of soluble phenolic acids of the Chaga mushroom (Inonotus obliquus).
Table 1.
Effect of different cooking methods on total phenolic contentsof Boletus mushroom (μg of gallic acid equivalents/g FW)
| Cooking methods | Raw | Steaming | Pressure-cooking | Microwaving | Frying | Boiling |
|---|---|---|---|---|---|---|
| B. aereus | 209.5 ± 5.52a | 126.5 ± 1.04b | 122.5 ± 1.51b | 149.0 ± 4.95b | 123.0 ± 1.48b | 70.5 ± 1.46c |
| B. badius | 150.5 ± 11.04a | 139.5 ± 14.53a | 127.0 ± 6.99b | 146.0 ± 8.02a | 117.5 ± 6.00b | 57.0 ± 2.03c |
| B. edulis | 184.5 ± 8.02a | 137.0 ± 2.53c | 140.0 ± 1.45c | 161.0 ± 3.54b | 91.5 ± 1.35d | 65.0 ± 1.54e |
| B. pinophilus | 220.0 ± 6.04a | 217.5 ± 9.46a | 217.0 ± 5.45a | 216.5 ± 6.00a | 217.5 ± 6.06a | 102.0 ± 1.45b |
Values not sharing a common letter are significantly different at p < 0.05
Effects of cooking on phenolic acids profiles
The phenolic acids profiles of raw and cooked mushrooms were presented in Table 2. For B. aereus, seven phenolics were identified. The content of gallic acid was the highest, showing 101.4 μg/g FW, and the content of p-coumaric acid was lowest. Microwaving treatment significantly increased the content of gallic acid (p < 0.05), while pressure, frying and boiling treatments significantly decreased its content (p < 0.05). All cooking methods significantly decreased the contents of chlorogenic acid and p-coumaric acid (p < 0.05). Microwaving treatment also increased the content of protocatechuic acid, depicting a 3-fold variation of the raw sample. In addition, p-hydroxybenzioc acid and p-coumaric acid were not detected in the fried and boiled samples. For B. badius, eight phenolics were identified. The content of gallic acid was the highest, showing 144.3 μg/g FW. The frying treatment could keep the content of gallic acid, and other cooking methods significantly decreased its content (p < 0.05). The content of catechin in raw sample was 54.9 μg/g FW, however, cooking treatment significantly decreased its content (p < 0.05). Frying treatment could significantly increase the contents of p-hydroxybenzioc acid, vanillin acid and protocatechuic acid (p < 0.05). p-hydroxybenzioc acid was not detected in the boiled sample. For B. edulis, only five phenolics were identified, including quercetin, chlorogenic acid, gallic acid, vanillin acid and protocatechuic acid. The content of protocatechuic acid was highest, showing 74.4 μg/g FW, followed by gallic acid, being 42.1 μg/g FW. All cooking treatments could significantly decrease the content of protocatechuic acid and gallic acid in B. edulis (p < 0.05). The frying treatment could significantly increase the content of chlorogenic acid and vanillic acid (p < 0.05). In addition, quercetin was not detected in the fried and boiled samples. For B. pinophilus, seven phenolics were identified. The contents of p-hydroxybenzioc acid and gallic acid were 56.0 and 58.4 μg/g FW, respectively. All cooking methods decreased the content of p-hydroxybenzioc acid. Microwaving treatment could increase the content of gallic acid, which was similar to the B. aereus, but different from B. badius and B. edulis. This might due to their different gene species and structures. Steaming and microwaving treatments could increase the content of p-coumaric acid and keep the content of catechin. However, catechin and chlorogenic acid were not detected in pressur-cooked and boiled samples.
Table 2.
Effect of different cooking methods on phenolic acids profiles of Boletus mushroom. (μg/g FW)
| Cooking methods | Raw | Steaming | Pressure-cooking | Microwaving | Frying | Boiling |
|---|---|---|---|---|---|---|
| B. aereus | ||||||
| p-hydroxybenzioc acid | 6.9 ± 0.03c | 9.0 ± 0.07b | 12.8 ± 0.92a | 4.1 ± 0.01d | nd | nd |
| p-coumaric acid | 2.7 ± 0.52a | 1.5 ± 0.31b | 0.1 ± 0.00c | 1.1 ± 0.26b | nd | nd |
| chlorogenic acid | 54.3 ± 1.61a | 25.6 ± 1.94c | 22.6 ± 1.54c | 44.3 ± 2.94b | 26.2 ± 2.87c | 39.2 ± 4.26b |
| gallic acid | 101.4 ± 7.63b | 97.8 ± 3.64b | 52.8 ± 2.13d | 120.5 ± 7.42a | 64.6 ± 1.69c | 55.1 ± 2.02d |
| vanillin acid | 21.1 ± 2.89a | 16.6 ± 1.04b | 16.6 ± 0.69b | 21.8 ± 2.27a | 25.6 ± 2.43a | 13.7 ± 0.92c |
| protocatechuic acid | 20.9 ± 1.23c | 38.8 ± 5.06b | 22.6 ± 1.65c | 68.6 ± 3.65a | 32.1 ± 3.49b | 21.4 ± 1.98c |
| B. badius | ||||||
| p-hydroxybenzioc | 10.1 ± 1.81b | 12.5 ± 1.03b | 13.7 ± 1.47b | 4.8 ± 0.01c | 19.3 ± 0.45a | nd |
| Cinnamic acid | 0.3 ± 0.01c | 0.3 ± 0.02b | 0.3 ± 0.01c | 0.2 ± 0.01c | 0.4 ± 0.01a | 0.3 ± 0.04c |
| p-coumaric acid | 0.1 ± 0.01d | 3.9 ± 0.03c | 5.3 ± 0.82b | 3.9 ± 0.01c | 10.8 ± 0.54a | 6.3 ± 0.75b |
| catechin | 54.9 ± 2.22a | 7.5 ± 0.51c | 7.9 ± 0.07c | 8.4 ± 0.94bc | 4.2 ± 0.06d | 9.5 ± 2.03b |
| chlorogenic acid | 4.4 ± 0.63c | 11.8 ± 1.85ab | 8.7 ± 0.81b | 5.5 ± 0.74c | 14.9 ± 0.59a | 13.3 ± 0.81a |
| gallic acid | 144.3 ± 7.56a | 50.7 ± 4.94b | 52.7 ± 1.42b | 56.7 ± 2.15b | 131.3 ± 8.29a | 32.9 ± 0.96c |
| vanillin acid | 13.7 ± 2.11b | 11.1 ± 0.59b | 10.5 ± 1.91b | 12.3 ± 0.86b | 23.5 ± 1.62a | 9.3 ± 2.08b |
| protocatechuic acid | 3.2 ± 0.61b | 1.3 ± 0.01d | 3.7 ± 0.47b | 4.6 ± 0.51b | 19.4 ± 0.68a | 2.7 ± 0.13c |
| B. edulis | ||||||
| quercetin | 1.1 ± 0.21a | 1.5 ± 0.11a | 1.0 ± 0.01b | 1.0 ± 0.01b | nd | nd |
| chlorogenic acid | 15.5 ± 0.49b | 6.2 ± 0.55d | 8.2 ± 0.18c | 2.7 ± 0.19e | 29.3 ± 1.41a | 7.8 ± 0.77cd |
| gallic acid | 42.1 ± 1.28a | 7.1 ± 0.06d | 11.1 ± 0.83c | 9.7 ± 0.37c | 29.0 ± 1.08b | 9.6 ± 0.48c |
| Vanillin acid | 14.9 ± 0.08b | 13.7 ± 0.15b | 13.8 ± 0.09b | 13.5 ± 0.17b | 26.4 ± 0.19a | 11.6 ± 0.11c |
| protocatechuic acid | 74.4 ± 4.81a | 50.0 ± 2.89b | 41.1 ± 3.22bc | 37.6 ± 3.01cd | 44.3 ± 2.83b | 32.2 ± 2.21d |
| B. pinophilus | ||||||
| p-hydroxybenzioc | 56.0 ± 2.02a | 32.7 ± 1.49b | 11.7 ± 0.91c | 35.3 ± 1.72b | 35.4 ± 1.46b | 7.4 ± 0.11d |
| p-coumaric acid | 38.1 ± 2.11b | 44.6 ± 3.21a | 10.2 ± 0.92d | 45.8 ± 3.42a | 28.9 ± 0.96c | 6.1 ± 0.03e |
| catechin | 5.0 ± 0.50a | 5.3 ± 0.47a | nd | 4.0 ± 0.54a | nd | nd |
| chlorogenic acid | 10.9 ± 0.69a | 3.1 ± 0.31c | nd | 3.4 ± 0.22c | 6.6 ± 0.12b | nd |
| gallic acid | 58.4 ± 3.85b | 49.5 ± 2.53c | 73.4 ± 3.21a | 48.6 ± 0.96c | 61.3 ± 1.93b | nd |
| vanillin acid | 21.0 ± 1.03b | 15.3 ± 0.67c | 13.2 ± 1.26cd | 18.6 ± 0.86b | 31.7 ± 1.02a | 11.1 ± 1.34d |
| protocatechuic acid | 21.0 ± 0.25a | 15.0 ± 1.18b | 6.5 ± 0.05c | 16.5 ± 0.47b | 14.4 ± 1.91b | 3.0 ± 0.02d |
Values not sharing a common letter are significantly different at p < 0.05
nd not detected
Some literature reports indicated that increasing molecular weight of phenolic compounds lead to enhanced antioxidant activity of the tested compounds. These powerful antioxidant compounds may be explained by the fact that they possess a great number of hydroxyl groups. In addition, antioxidant activities of phenolic compounds also depend on their phenolic acids composition. Jacobo-Velzquez and Cisneros-Zevallos (2009) reported that the specific antioxidant activity of pure quercetin (Q) and chlorogenic acid (C) were 2,638 and 1,470 μg Trolox/mg phenolic, respectively, while mixtures of Q/C (75/25) and Q/C (25/75) resulted in specific antioxidant activity values of 2,172 and 1,907 μg Trolox/mg phenolic, respectively. However, there are great variations in the antioxidant activities of the different phenolic acids reported by different authors, due to the methods of analysis. Yokozawa et al. (1998) reported the IC50 values of gallic acid and ellagic acid were 8.14 and 4.60 μmol/L. Thitilertdecha et al. (2010) indicated that the IC50 values of gallic acid and ellagic acid of 2.49 and 1.64 μmol/L. Furthermore, some phenolic compounds were unstable and easily became non antioxidative under heating. Heat used in the cooking procedure could destroy the hydroxyl groups of phenolics (Barros et al. 2007) or transform phenolics with high antioxidant activity into different phenolics with low antioxidant activity (Jacobo-Velzquez and Cisneros-Zevallos 2009), and then cause decrease in their antioxidant activity. Therefore, cooking not only decreased the total phenolic contents, but also changed the type and relative amounts of phenolics (Sun et al. 2011).
Effects of cooking on antioxidant activities
OH·-scavenging activities
The OH·-scavenging activities of four mushrooms submitted to steaming, pressure-cooking, microwaving, frying and boiling with respect to raw samples were shown in Table 3. For B. aereus, all cooking methods significantly decresed the OH·-scavenging activity (p < 0.05), and the IC50 values of boiled and pressured samples reached 599.5 and 586.4 μg/mL, which were 2.99 and 2.92 folds of the raw samples, respectively. For B. badius, all cooking methods kept OH·-scavenging activities, except boiling, which significant decreased OH·-scavenging activities of B. badius (p < 0.05). For B. edulis, IC50 values of microwaved, steamed and pressured sapmles showed no significantly difference with that of raw mushroom, but boiling and frying treatments significantly decreased its OH·-scavenging activity (p < 0.05). For B. pinophilus, microwaving and steaming treatments kept OH·-scavenging activities. However, boiling significantly increased the IC50 value (p < 0.05), while pressure-cooking and frying increased its OH·-scavenging activity (p < 0.05).
Table 3.
Effect of different cooking methods on antioxidant activities of Boletus mushroom
| Cooking methods | Raw | Steaming | Pressure-cooking | Microwaving | Frying | Boiling |
|---|---|---|---|---|---|---|
| IC50 of scavenging OH· activity (μg/mL) | ||||||
| B. aereus | 200.8 ± 6.53d | 450.6 ± 4.76c | 586.4 ± 5.69a | 387.3 ± 5.22b | 410.2 ± 4.63b | 599.5 ± 7.31a |
| B. badius | 302.3 ± 5.75b | 318.9 ± 5.11b | 311.4 ± 6.18b | 310.5 ± 5.25b | 307.9 ± 6.33b | 421.3 ± 5.81a |
| B. edulis | 102.3 ± 5.20c | 105.8 ± 2.75c | 102.0 ± 3.26c | 98.6 ± 3.08c | 410.3 ± 3.44a | 305.9 ± 3.45b |
| B. pinophilus | 520.4 ± 5.56b | 512.6 ± 6.02b | 421.3 ± 4.60c | 509.5 ± 5.68b | 386.1 ± 5.01c | 645.8 ± 5.84a |
| EC50 of reducing power (μg/mL) | ||||||
| B. aereus | 49.3 ± 1.38b | 98.8 ± 2.76a | 100. 8 ± 2.03a | 89.7 ± 2.47a | 99.7 ± 2.03a | 122.3 ± 2.33a |
| B. badius | 114.8 ± 3.14c | 123.2 ± 3.06b | 129.7 ± 3.00b | 98.4 ± 2.92d | 128.4 ± 2.2c | 187.1 ± 3.8a |
| B. edulis | 41.9 ± 1.43c | 78.0 ± 2.46a | 74.9 ± 3.07a | 76.9 ± 2.21a | 75.9 ± 2.43b | 75.6 ± 2.11a |
| B. pinophilus | 66.5 ± 2.10a | 58.7 ± 2.78b | 49.7 ± 1.89c | 63.4 ± 3.07ab | 60.9 ± 1.47b | 70.7 ± 2.27a |
| IC50 of scavenging DPPH· activity (μg/mL) | ||||||
| B. aereus | 135.4 ± 4.67b | 139. 8 ± 3.02a | 144.3 ± 4.00a | 137.9 ± 5.17a | 145.3 ± 4.13b | 147.1 ± 5.22a |
| B. badius | 120.7 ± 5.01b | 125.0 ± 5.02b | 137.4 ± 3.38c | 106.8 ± 4.11a | 134.2 ± 3.02c | 222.8 ± 5.84a |
| B. edulis | 91.0 ± 2.68b | 97.4 ± 2.78b | 106.2 ± 3.89a | 97.3 ± 3.12b | 101.7 ± 4.67a | 93.2 ± 3.02b |
| B. pinophilus | 102.8 ± 3.08a | 98.9 ± 4.14a | 66.0 ± 2.11c | 80.5 ± 3.02b | 64.9 ± 2.32c | 108.3 ± 2.37a |
Values not sharing a common letter are significantly different at p < 0.05
OH: Hydroxyl radical; DPPH·: 1, 1-diphenyl-2-picrylhydrazyl radicals
Reducing powers
Reducing powers of four samples submitted to steaming, pressure-cooking, microwaving, frying and boiling with respect to raw samples are shown in Table 3. For B. aereus, all cooking method showed the significant decreases in reducing power (p < 0.05). For B. badius, microwaving treatment significantly increased reducing power (p < 0.05), and boiling significantly decreased its activities (p < 0.05). For B. edulis, effects of cooking methods on the reducing power was similar to that of B. aereus and all cooking method significantly decreased the reducing power of B. edulis (p < 0.05). All cooking methods kept reducing powers of B. pinophilus, except pressure-cooking, which increased its activity with significant difference (p < 0.05).
DPPH·-scavenging activities
Table 3 showed the DPPH·-scavenging activities of mushrooms submission to different cooking methods. The IC50 values of DPPH·-scavenging activity of B. aereus cooked by five methods showed no significant difference. For B. badius, microwaving treatment significantly increased DPPH·-scavenging activities (p < 0.05), and boiling significantly decreased its activity (p < 0.05). It was similar to the effects of cooking treatments on its reducing power. For B. edulis, boiling, microwaving and steaming kept DPPH·-scavenging activities, however, pressure-cooking and frying decreased its activity with significant difference (p < 0.05). Microwaving, pressure-cooking and frying significantly increased DPPH·-scavenging activities of B. pinophilus (p < 0.05), and the IC50 values of pressured and fried samples were 66.0 and 64.9 μg/mL, which were lower than that of raw mushroom significantly (p < 0.05).
Some possibilities are suggested for the increase in antioxidant activity of some fruits, vegetables, and mushrooms after cooking. On the one hand, the thermal destruction of cell wall and sub cellular compartment could effectively cause liberation of high amounts of antioxidant components, such as microwaving treatment could accelerate release of phenolic compounds (Hayat et al. 2009). On the other hand, thermal chemical reaction could product stronger radical-scavenging antioxidants (Jimnez-Monreal et al. 2009). Ju et al. (2010) studied steaming of high pressure could increase the antioxidant activity of the Chaga mushroom. Third, new non-nutrition antioxidants or the formation of novel compounds were produced in the thermal process, such as Maillard reaction products with antioxidant activities (Morales and Babel 2002). It has been shown that the thermal processing of sweet corn, tomato and other vegetables increasing antioxidant activity, perhaps as a result of Maillard reaction production (Nindo et al. 2003). However, it is not clear to what extent each possible factor contributes to the increase antioxidant activities of fruits, vegetables, and mushrooms.
Conclusion
This study evaluated the effects of five cooking methods (steaming, pressure-cooking, microwaving, frying and boiling) on the total phenolic centents, phenolic acids profiles and antioxidant activities (OH·-scavenging activity, reducing power and DPPH·-scavenging activity) of four Boletus mushrooms (B. aereus, B. badius, B. pinophilus and B. edulis). Total phenolic contents of four mushrooms were all decreased during the five cooking treatments. However, microwaving treatment was better in retention of total phenolics than others. The effects of five treatments on the phenolic acids profiles showed varieties with both the species of mushroom and the cooking method. The changes of antioxidant activities during the five cooking treatments were also varieties with both the species of mushroom and the cooking method. The results of the study mainly provided some information on the effects of different cooking methods on the phenolics and antioxidant activities of Boletus mushrooms, which might encourage the food industry to recommended particular cooking methods. However, further study on effect of cooking on bioaccessibility in vivo should be carried out in the future.
Acknowledgments
This work was financially supported by Yunnan Natural Science Foundation (2010ZC032)
References
- Annegowda HV, Bhat R, Tze LM, Karim AA, Manor SM (2011) The free radical scavenging and antioxidant activities of pod and seed ectract of Clitoria fairchildiana (Howard)-an underutilized legume. J Food Sci Technol. doi:10.1007/s13197-011-0370-8 [DOI] [PMC free article] [PubMed]
- Barros L, Baptista P, Correia DM, Morais JS, Perreira ICFR. Effects of conservation treatment and cooking on the chemical composition and antioxidant activity of Portuguese wild edible mushrooms. J Agric Food Chem. 2007;55:4781–4788. doi: 10.1021/jf070407o. [DOI] [PubMed] [Google Scholar]
- Benjakul S, Lertittikul W, Bauer F. Antioxidant activity of Maillard reaction products from a porcine plasma protein-sugar model system. Food Chem. 2005;93:189–196. doi: 10.1016/j.foodchem.2004.10.019. [DOI] [Google Scholar]
- Choi Y, Lee SM, Chun J, Lee HB, Lee J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006;99:381–387. doi: 10.1016/j.foodchem.2005.08.004. [DOI] [Google Scholar]
- Elmastas M, Isildak O, Turkekul I, Temur N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J Food Compos Anal. 2007;20:337–345. doi: 10.1016/j.jfca.2006.07.003. [DOI] [Google Scholar]
- Hall IR, Lyon AJE, Wang Y, Sinclair L. Ectomycorrhizal fungi with edible fruiting bodies, Boletus edulis. Econ Bot. 1998;52:44–56. doi: 10.1007/BF02861294. [DOI] [Google Scholar]
- Hayat K, Hussain S, Abbas S, Farooq U, Ding B, Xia S, Jia C, Zhang X, Xia W. Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity in vitro. Sep Purif Technol. 2009;70:63–67. doi: 10.1016/j.seppur.2009.08.012. [DOI] [Google Scholar]
- Heleno SA, Barros L, Sousa MJ, Martins A, Santos-Buelga C, Ferreira ICFR. Targeted metabolites analysis in wild Boletus species. LWT Food Sci Technol. 2011;44:1343–1348. doi: 10.1016/j.lwt.2011.01.017. [DOI] [Google Scholar]
- Heo HJ, Kim YJ, Chung D, Kim DO. Antioxidant capacities of individual and combined phenolics in a model system. Food Chem. 2007;104:87–92. doi: 10.1016/j.foodchem.2006.11.002. [DOI] [Google Scholar]
- Isabelle M, Lee BL, Lim MT, Koh WP, Huang DJ, Ong CN. Antioxidant activity and profiles of common vegetables in Singapore. Food Chem. 2010;120:993–1003. doi: 10.1016/j.foodchem.2009.11.038. [DOI] [Google Scholar]
- Jacobo-Velzquez DA, Cisneros-Zevallos L. Correlations of antioxidant activity against phenolic content revisited: a new approach in data analysis for food and medicinal plants. J Food Sci. 2009;74:R107–R113. doi: 10.1111/j.1750-3841.2009.01352.x. [DOI] [PubMed] [Google Scholar]
- Jimnez-Monreal AM, Garcao-Diz L, Martnez-Tom M, Mariscal M, Murcia MA. Influence of cooking methods on antioxidant activity of vegetables. J Food Sci. 2009;74:H97–H103. doi: 10.1111/j.1750-3841.2009.01091.x. [DOI] [PubMed] [Google Scholar]
- Jing H, Kitts DD. Antioxidant activity of sugar–lysine Maillard reaction products in cell free and cell culture systems. Arch Biochem Biophy. 2004;429:154–163. doi: 10.1016/j.abb.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Ju HK, Chung HW, Hong SS, Park JH, Lee J, Kwon SW. Effect of steam treatment on soluble phenolic content and antioxidant activity of the Chaga mushroom (Inonotus obliquus) Food Chem. 2010;119:619–625. doi: 10.1016/j.foodchem.2009.07.006. [DOI] [Google Scholar]
- Khalil H, Mansour EH. The effect of cooking, autoclaving and germination on the nutritional quality of faba beans. Food Chem. 1995;54:177–182. doi: 10.1016/0308-8146(95)00024-D. [DOI] [Google Scholar]
- Kim EH, Kim SH, Chung JI, Chi HY, Kim JA, Chung IM. Analysis of phenolic compounds and isoflavones in soybean seeds (Glycine max (L.) Merill) and sprouts grown under different conditions. Eur Food Res Technol. 2006;222:201–208. doi: 10.1007/s00217-005-0153-4. [DOI] [Google Scholar]
- Kim JW, Yoo ID, Kim WG. Free radical-scavenging δ-lactones from Boletus calopus. Planta Medica. 2006;72:1431–1432. doi: 10.1055/s-2006-951722. [DOI] [PubMed] [Google Scholar]
- Mandge HM, Sharma S, Dar BN (2011) Instant multigrain porridge: effect of cooking treatment on physicochemical and functional properties. J Food Sci Technol. doi:10.1007/s13197-011-0461-6 [DOI] [PMC free article] [PubMed]
- Manzi P, Marconi S, Aguzzi A, Pizzoferrato L. Commercial mushrooms: Nutritional quality and effect of cooking. Food Chem. 2004;84:201–206. doi: 10.1016/S0308-8146(03)00202-4. [DOI] [Google Scholar]
- Mau JL, Chao GR, Wu KT. Antioxidant properties of methanolic extracts from several ear mushrooms. J Agric Food Chem. 2001;49:5461–5467. doi: 10.1021/jf010637h. [DOI] [PubMed] [Google Scholar]
- Mdachi SJM, Nkunya MHH, Nyigo VA, Urasa IT. Amino acid composition of some Tanzanian wild mushrooms. Food Chem. 2004;86:179–182. doi: 10.1016/j.foodchem.2003.08.030. [DOI] [Google Scholar]
- Medoua GN, Oldewage-Theron WH (2011) Effect of drying and cooking on nutrition value and antioxidant capacity of morogo (Amarantbus bybridus) a traditional leafy vegetable grown in South Africa. J Food Sci Technol. doi:10.1007/s13197-011-0560-4 [DOI] [PMC free article] [PubMed]
- Morales FJ, Babel MB. Antiradical efficiency of Maillard reactin mixtures in a hydrophilicmedia. J Agric Food Chem. 2002;50:2788–2792. doi: 10.1021/jf011449u. [DOI] [PubMed] [Google Scholar]
- Nindo CI, Sun T, Wang SW, Tang J, Powers JR. Evaluation of drying technologies for retention of physical quality and antioxidants in asparagus (Asparagus officinalis, L.) LWT. 2003;36:507–516. doi: 10.1016/S0023-6438(03)00046-X. [DOI] [Google Scholar]
- Osman NM, Ahmed IAM, Babiker EE. Fermentation and cooking of sicklepod (Cassia obtusifolia) leaves: changes in chemical and amino acid composition, antinutrients and protein fractions and digestibility. Int J Food Sci Technol. 2010;45:124–132. doi: 10.1111/j.1365-2621.2009.02111.x. [DOI] [Google Scholar]
- Ryzhova GL, Kravtsova SS, Matasova SA, Gribel NV, Pashinskii VG, Dychko KA. Chemicalmand pharmacological properties of dry extract of the birch mushroom. Khim Farmat Zh. 1997;31:44–47. [Google Scholar]
- Savioe JM, Minvielle N, Largeteau ML. Radical-scavenging properties of extracts from the white button mushroom, Agaricus bisporus. J Sci Food Agric. 2008;88:970–975. doi: 10.1002/jsfa.3175. [DOI] [Google Scholar]
- Smirnoff N, Cumbes OJ. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28:1057–1060. doi: 10.1016/0031-9422(89)80182-7. [DOI] [Google Scholar]
- Soler-Rivas C, Ramrez-Anguiano A, Reglero G, Santoyo S. Effect of cooking, in vitro digestion and Caco-2 cells absorption on the radical scavenging activities of edible mushrooms. Int J Food Sci Technol. 2009;44:2189–2197. doi: 10.1111/j.1365-2621.2009.02059.x. [DOI] [Google Scholar]
- Sun LP, Zhuang YL, Bai X. Effects of boiling and microwaving treatments on nutritional characteristics and antioxidant activities of Agaricus blazei Murril. Int J Food Sci Technol. 2011;46:1209–1215. doi: 10.1111/j.1365-2621.2011.02602.x. [DOI] [Google Scholar]
- Thitilertdecha N, Teerawutgulrag A, Kilburn JD, Rakariyatham N. Identification of major phenolic compounds from Nephelium lappaceum L. and their antioxidant activities. Molecules. 2010;15:1453–1465. doi: 10.3390/molecules15031453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser SP, Weis AL. Medicinal properties of substances occurring in higher Basidiomycetes mushrooms. Current perspectives (Review) Int J Med Mushroom. 1999;1:31–62. doi: 10.1615/IntJMedMushrooms.v1.i1.30. [DOI] [PubMed] [Google Scholar]
- Wolosiak R, Worobiej E, Piecyk M, Druzynska B, Nowak D, Lewicki PP. Activities of amine and phenolic antioxidants and their changes in broad beans (Vicia faba) after freezing and steam cooking. Int J Food Sci Technol. 2010;45:29–37. doi: 10.1111/j.1365-2621.2009.02099.x. [DOI] [Google Scholar]
- Yokozawa T, Chen CP, Dong E, Tanaka T, Nonaka GI, Nishioka I. Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2-picrylhydrazyl radical. Biochem Pharmacol. 1998;56:213–222. doi: 10.1016/S0006-2952(98)00128-2. [DOI] [PubMed] [Google Scholar]


