Abstract
The raw and processed (cooked and cooked + solid-state fermented with Rhizopus oligosporus) split beans of two landraces of coastal sand dune wild legumes (Canavalia cathartica and Canavalia maritima) of the southwest coast of India were examined for bioactive compounds (total phenolics, tannins and vitamin C) and antioxidant potential (total antioxidant activity, ferrous-ion chelating capacity, DPPH free radical-scavenging activity and reducing activity). One-way ANOVA revealed significant elevation of bioactive compounds as well as antioxidant activities in fermented beans compared to raw and cooked beans in both legumes (p < 0.001). The EC50 values in fermented beans of both legumes were significantly lowest compared to raw and cooked beans (p < 0.001). In principal component analysis, total phenolics along with antioxidant activities (total antioxidant, ferrous-ion chelating and free radical-scavenging activities) of fermented beans of C. cathartica, while total antioxidant and free radical-scavenging activities of fermented beans of C. maritima were clustered. The present study demonstrated that split beans of coastal sand dune Canavalia fermented by R. oligosporus endowed with high bioactive principles as well as antioxidant potential and thus serve as future nutraceutical source.
Keywords: Antioxidant activity, Bioactive compounds, Canavalia, Fermentation, Rhizopus oligosporus, Seeds
Introduction
Animal and plant cells are often exposed to several challenges those are responsible for oxidative stress leading to production of oxygen free radicals and reactive oxygen species responsible for oxidative damage. Such damages are prevented by natural antioxidants, which are effective against chronic diseases like atherosclerosis, neurodegenerative disorders and cancer (Sulaiman et al. 2011). Antioxidants are known to protect the cells by reducing the formation of free radicals, scavenging the free radicals, converting existing free radicals into less harmful molecules and interrupting the radical chained reactions (Du et al. 2009). The antioxidant activity of various fruits, vegetables and legumes are due to the combined effect of various bioactive compounds (e.g. polyphenols, vitamin C, vitamin E and carotenoids) (Blomhoff et al. 2006). Seeds of legumes possess economically viable nutritional constituents such as proteins, carbohydrates and fibres (Vietmeyer 1986; Kris-Etherton et al. 2002; Lewis et al. 2005; Das et al. 2012). Several studies suggest that regular dietary intake of food legumes are associated with health-promoting effects due to the presence of various bioactive compounds (e.g. Bhat and Karim 2009; Sridhar and Seena 2006; Vadivel and Biesalski 2011). Besides major nutrients, wild legumes serve as source of nutraceuticals by reducing the risks of diabetes, cardiovascular diseases and cancer (Kris-Etherton et al. 2002; Vadivel and Biesalski 2010).
Indigenous landraces of wild legumes belonging to the genus Canavalia (C. cathartica and C. maritima) in coastal regions of the Southwest India exhibit fast growth, high seed yield, tolerance to salinity and resistance to diseases (Seena and Sridhar 2006). Several studies on the seeds of Canavalia have demonstrated their nutritional adequacy especially in proteins, amino acids, fatty acids and fibre (Seena and Sridhar 2006; Sridhar and Bhagya 2007). However, a few bioactive compounds have been reported from the seeds of coastal Canavalia (Seena and Sridhar 2006). Roasted seed powder of C. maritima serves as substitute for coffee, while leaves are endowed with L-betonicine and roots are useful in treating ciguatera poisoning (Bourdy et al. 1992; Bhagya and Sridhar 2009). Seeds of Canavalia are consumed by the coastal dwellers of Southwest India after processing (e.g. soaking, boiling and removal of coat and testa of ripened beans). Leaves, roots and seeds of coastal Canavalia are traditionally used in curing skin diseases as well as skin burns (Chock 1968; Bhagya and Sridhar 2009). Although some studies have been performed on antioxidant activities of other Canavalia spp. (C. ensiformis and C. gladiata) (e.g. Doss et al. 2010; Sowndhararajan et al. 2011; Vadivel et al. 2011, 2012), so far no studies are available on the antioxidant potential of coastal Canavalia landraces (Canavalia cathartica and C. maritima). Rhizopus oligosporus is commonly employed to produce fermented oriental foods as it is Generally Regarded As Safe (GRAS). Besides enhancement of nutritional quality, solid-state fermented foods showed enhanced antioxidant properties (Sheih et al. 2000; Starzyńska-Janiszewska et al. 2008; Cai et al. 2012). There is no literature on impact of fungal fermentation on the nutritional and bioactive compounds of Canavalia of coastal sand dunes. Therefore, the present study envisaged evaluating bioactive compounds and antioxidant potential of raw, cooked and R. oligosporus fermented split beans of Canavalia of coastal sand dunes of Southwest India.
Materials and methods
Seed samples and fermentation
The seed samples of Canavalia cathartica Thouars and Canavalia maritima Thouars were sampled from coastal sand dunes of Someshwara, Southwest India (12°47′ N, 74°52′ E) during summer season (February–March, 2010). Healthy undamaged seeds were selected from dry pods and sun-dried for 2 days. Each seed was cut twice longitudinally using a nut-cracker to have four pieces of split beans and dehusked. Split beans were subjected to two treatments: 1) pressure-cooking, and 2) fermentation of pressure cooked split beans. The split beans (25 g) were transferred to conical flask (250 mL), soaked in distilled water (1:3 w/v) followed by pressure-cooking (6.5 L, Deluxe stainless steel; TTK PrestigeTM, Prestige Ltd., India). The cooked split beans were spread on aluminum foil, dried at 42 ± 2 °C, milled (Wiley Mill, 30 mesh) and stored in air-tight glass containers. Another set of split beans (25 g) was cooked in conical flasks (250 mL capacity), cooled to laboratory temperature, inoculated with two 5 mm plugs of 3-day-old cultures (grown on potato dextrose agar medium) of Rhizopus microsporus var. oligosporus (MTCC # 556; strain designation # 22959; Institute of Microbial Technology, Chandigarh, India) and allowed for solid-state fermentation up to 1 week at 37 °C. The fermented beans formed tight cake on fermentation and it was spread on aluminum sheets, dried at 42 ± 2 °C, powdered and stored in glass containers.
Assessment of bioactive principles
Total phenolics
The total phenolic content was determined according to the method outlined by Rosset et al. (1982). Fifty mg of split bean flours were extracted in 5 mL of 50 % methanol in water bath (95 ± 1 °C) for 10 min, centrifuged (1,500 rpm) and the supernatant was collected. The extraction procedure was repeated for the remaining flour pellet, supernatant were pooled and made up to 10 mL. An aliquot 0.5 mL extract was mixed with 0.5 mL distilled water and treated with 5 mL Na2CO3 (in 0.1 N NaOH). After 10 min of incubation, 0.5 mL Folin-Ciocalteu’s reagent (diluted, 1:2 v/v) was added and the absorbance was read at 725 nm (UV–VIS Spectrophotometer-118, SYSTRONICS, Ahmedabad, Gujarat, India). Tannic acid was used to prepare standard curve and the results were expressed as mg of tannic acid equivalents (TAEs) per gram of the sample (mg TAEs/g).
Tannins
Vanillin-HCl method was employed to determine tannins of split bean flours (Burns 1971). One g of flour was extracted with 50 mL methanol (28 °C, 20–28 h), centrifuged and supernatant was collected. One mL of extract was treated with 5 mL of vanillin hydrochloride reagent mixture (4 % vanillin in methanol and 8 % concentrated HCl in methanol; 1:1). After 20 min, the color developed was read at 500 nm with 50–250 μg catechin (Sigma Aldrich, 98 % HPLC grade, USA) as standard. The tannin content was expressed as catechin equivalents (CEs) in milligram per gram of the sample (mg CEs/g).
Vitamin C
The vitamin C content of split bean flours was estimated according to Roe (1954) with minor modifications. One gram of sample was extracted in 10 mL of 5 % trichloroaceticacid (TCA). An aliquot (0.2 mL) was made up to 1 mL in 5 % TCA followed by addition of 1 mL of 2,4-dinitrophenylhydrazine (DNPH). The reaction mixture was boiled for 10 min, cooled to laboratory temperature, 4 mL of 65 % sulphuric acid was added, incubated up to 30 min at laboratory temperature and the absorbance was measured at 540 nm. Ascorbic acid (Sisco Research Laboratories, Mumbai, India; purity, 99.8 %) was used to prepare standard curve. The vitamin C content was expressed as ascorbic acid equivalents (AAEs) in milligram per gram of the sample (mg AAEs/g).
Antioxidant assays
Antioxidant properties have been evaluated by four assays in our study: 1) total antioxidant activity (Reduction of Mo(VI) to Mo(V) by antioxidant compounds); 2) Fe2+ ion chelating capacity (Detected by Ferrous ion-Ferrozine complex formation); 3) radical-scavenging activity (DPPH radical absorption on exposure to radical scavengers); 4) reducing power (conversion of Fe3+/ferricyanide complex to the ferrous form). Split bean flour extract was prepared at a concentration of 1 mg/mL (W/V) using methanol. A known amount of the extract was used for the antioxidant assays.
Total antioxidant activity
To determine the total antioxidant activity (TAA), 0.1 mL of extract was mixed with 1 mL of reagent mixture (sulfuric acid, 0.6 M + sodium phosphate, 28 mM + ammonium molybdate, 4 mM) (Prieto et al. 1999). The samples were incubated at 95 °C for 90 min, cooled to laboratory temperature followed by measurement of absorbance of phosphomolybdenum complex at 695 nm with methanol as blank. The TAA was expressed as μM equivalent of ascorbic acid per gram of the seed powder.
Ferrous-ion chelating capacity
The Fe2+ chelating capacity was determined according to the method by Hsu et al. (2003). To 1 mL of the extracts, 0.1 mL each of 2 mM FeCl2 and 0.2 mL of 5 mM ferrozine were added. The final volume was made up to 5 mL using methanol. The mixture was incubated for 10 min at laboratory temperature followed by determination of absorbance of Fe2+-ferrozine complex at 562 nm. The sample without the extract served as control and ferrous ion chelating capacity was calculated:
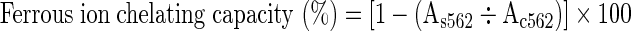 |
1 |
(Where, Ac is absorbance of the control and As is absorbance of sample)
DPPH free radical-scavenging activity
Free radical-scavenging activity of the extracts was measured according to the procedure by Singh et al. (2002). Different concentrations (0.2–1 mL: 200–1,000 μg) of the test samples were made up to 1 mL using methanol. Four mL of 0.01 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH) was added and allowed to react at room temperature for 20 min. Reagents without addition of sample served as control and absorbance of the reaction mixture was measured at 517 nm to calculate free radical-scavenging activity:
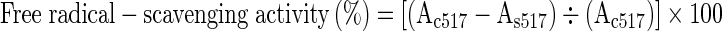 |
2 |
(Where, Ac is absorbance of the control and As is absorbance of sample)
The effective concentration (EC50; concentration of sample necessary to scavenge 50 % of the DPPH radicals) (μg extract mL−1) was obtained by plotting per cent radical-scavenging activity against concentration of the extracts.
Reducing activity
Reducing activity of the extract was determined employing the method outlined by Oyaizu (1986) with a slight modification. Different concentrations (0.2–1 mL: 200–1,000 μg) of split bean flour extracted in methanol were taken, 0.2 M phosphate buffer (pH 6.6, 2.5 mL) was added followed by addition of 2.5 mL 1 % potassium ferricyanide. The contents were mixed and incubated at 50 °C for 20 min. After incubation, 2.5 mL of 10 % TCA was added and centrifuged (3,000 rpm) for 10 min, 2.5 mL of supernatant was mixed with equal volume of distilled water. To the above mixture, 5 mL of 0.1 % ferric chloride was added and the absorbance was measured at 700 nm. Increase in the absorbance of the reaction mixture indicated increased reducing power.
Data analysis
The difference in quantity of bioactive compounds (total phenolics, tannins and vitamin C) among raw and processed (cooked and cooked + fermented) split beans was assessed by One-way ANOVA (SigmaPlot 11; Systat Software Inc., USA). Relationship between bioactive compounds (total phenolics, tannins and vitamin C) and antioxidant capacities (total antioxidant activity, Fe2+ chelating capacity and DPPH radical-scavenging activity) was tested by One-way ANOVA. Principal component analysis (PCA) was performed for total phenolics of raw and processed (cooked and cooked + fermented) split beans against antioxidant assays (total antioxidant activity, Fe2+ chelating capacity and DPPH radical-scavenging activity) (SPSS 16.0: www.spss.com). The PCA score plot was used to determine whether total phenolics of raw, cooked and fermented split beans of C. cathartica and C. maritima vs. antioxidant activities (total antioxidant, Fe2+ chelating and DPPH radical-scavenging activities) could be grouped in to different classes.
Results and discussion
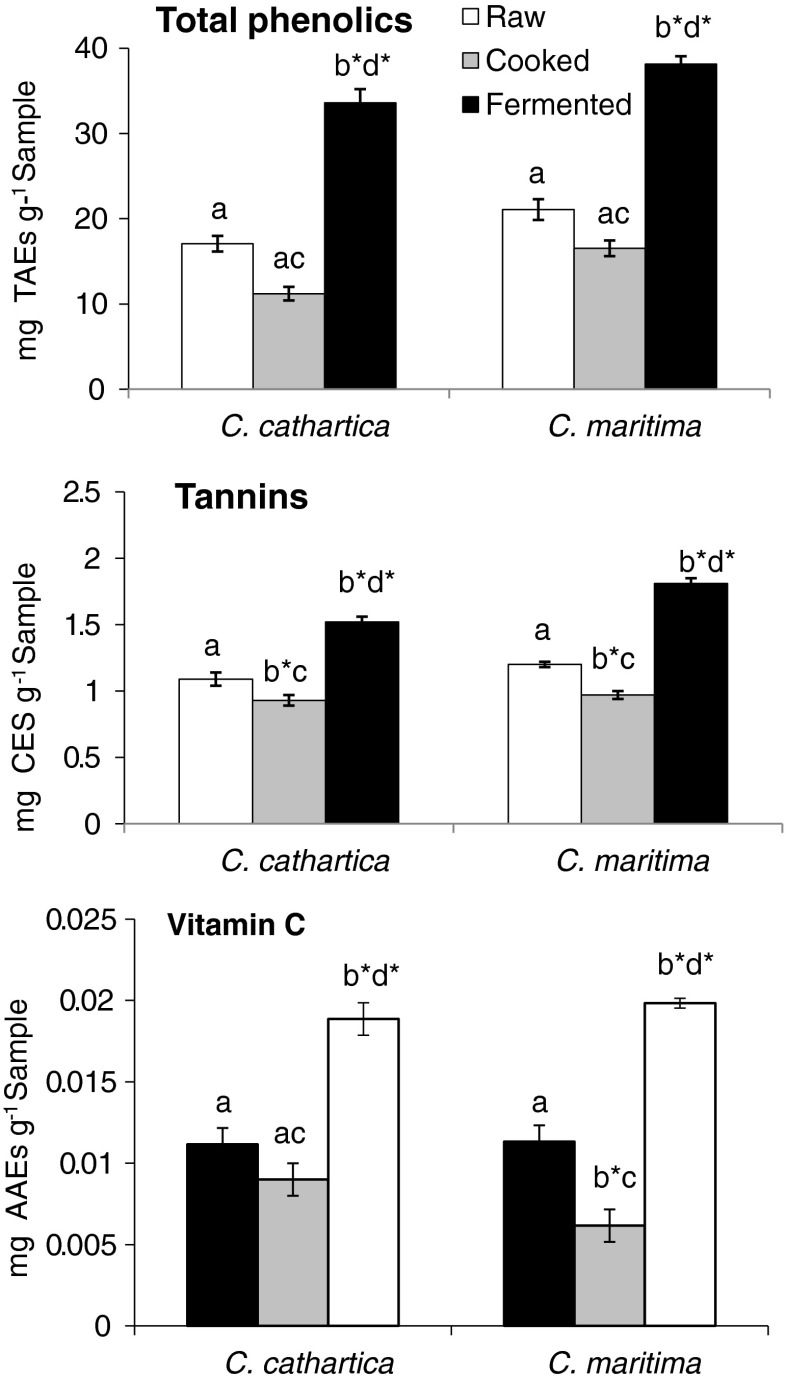
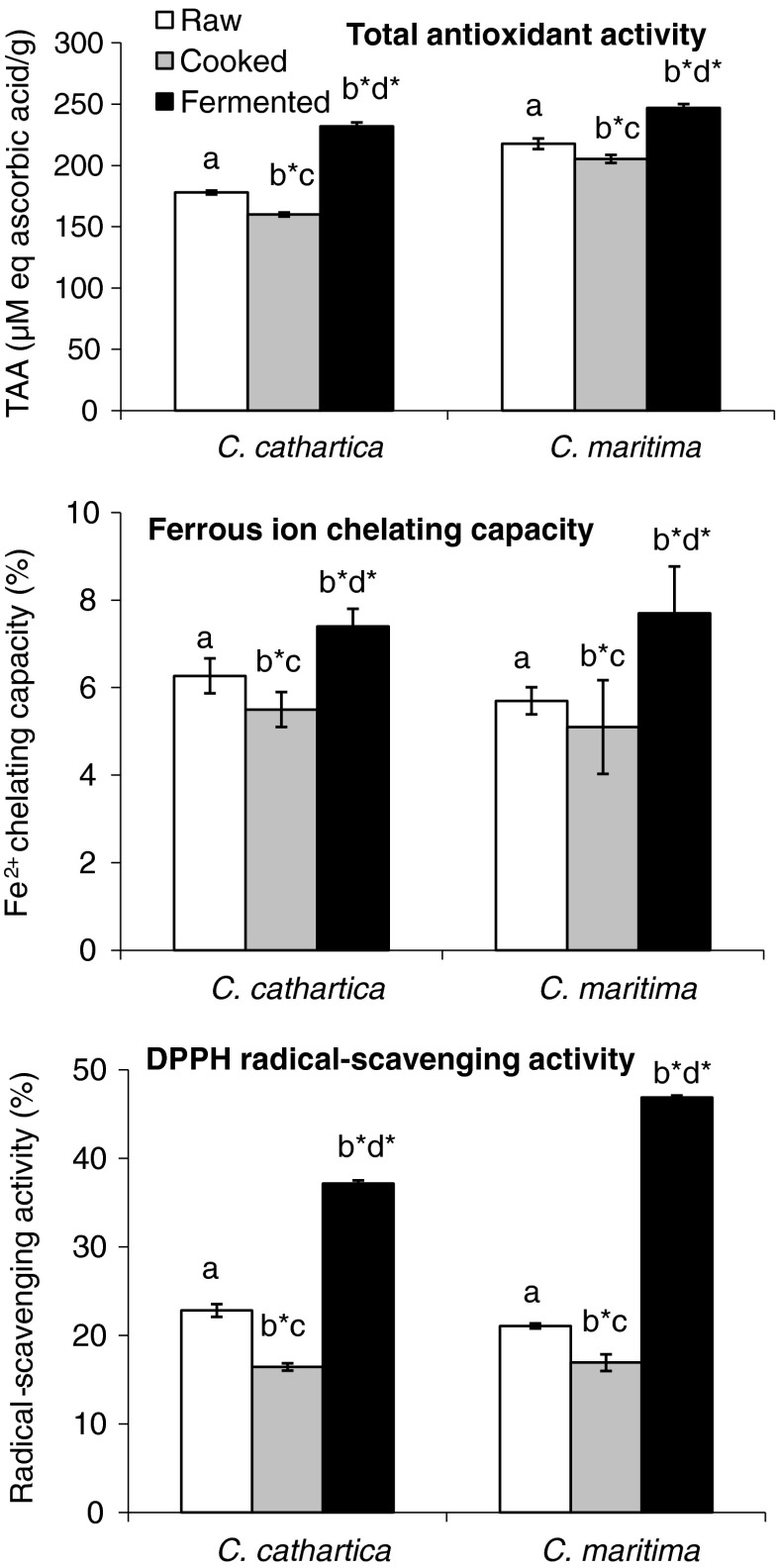
Health-promoting and disease-resistance value of antioxidants of plant origin attracted major attention than the synthetic antioxidants (e.g. butylated hydroxytoluene and butylated hydroxyanisole) (Annegowda et al. 2011). Phenolic compounds in biological materials serve as primary antioxidants or free radical terminators. Epidemiological studies showed a strong relationship between the consumption of natural product rich in phenols with low incidence of cancer, coronary heart disease and atherosclerosis (Randhir et al. 2004; Alothman et al. 2009). Total phenolics and tannin content of raw beans of both legumes in our study decreased on cooking, while it was significantly elevated in R. oligosporus fermented beans (p < 0.001) (Fig. 1). The reduction in phenolic content of cooked beans has been attributed to the loss due to leaching and formation of complex with proteins during thermal treatment leading to poor extractability. The cooked beans of both legumes showed decreased total antioxidant activity (TAA) (p < 0.001), while the fermented beans showed elevated TAA (p < 0.001) (Fig. 2). Ferrous ion chelating capacity as well as DPPH free radical-scavenging activity were also showed elevation in fermented beans than the raw and cooked beans (p < 0.001). The total phenolics of raw beans of C. cathartica and C. maritima were about 8 to 9–folds higher than the raw beans of other Canavalia spp. (C. ensiformis and C. gladiata) (17.1–21.1 vs. 2.21–2.45 mg g−1), which resulted in relatively high EC50 of DPPH free radical-scavenging activities in C. ensiformis and C. gladiata (18–32.2 vs. 69.8–91.2 μg mL−1) (Doss et al. 2010). Our observations on low yield of phenolics and tannins in cooked beans are in agreement with earlier studies on seeds and leaves of other legumes (Siddhuraju 2006; Xu and Chang 2008; Osman et al. 2010). Similarly, cooking, soaking + cooking and open-pan roasting significantly decreased total free phenolics in Canavalia seeds (Seena and Sridhar 2006; Sridhar and Niveditha 2011; Vadivel et al. 2011, 2012). Although cooking sprouted seeds of C. cathartica and C. maritima decreased the total phenolics (D’Cunha et al. 2009a, b), in C. ensiformis and C. gladiata sprouting + oil-frying significantly elevated the extractable free phenolics, antioxidant potential and inhibition of type II diabetes-related enzymes (α-amylase and α-glucosidase) (Vadivel et al. 2011, 2012). On the contrary, extraction of total phenolics and tannins in soaked + autoclaved seeds of C. ensiformis were higher than raw seeds (phenolics: 2.2 vs. 1.7 %; tannins: 1.2 vs. 1 %) (Sowndhararajan et al. 2011). Similarly, sprouting wheat, buckwheat, corn and oat seeds for 2 days + autoclaving elevated the total free phenolics by 9 %, 20 %, 27 % and 50 %, respectively (Randhir et al. 2008). This suggests variation of extraction of phenolics and tannins is dependent on the legume species, edaphic factors and methods of extraction. Gazzani et al. (1998) predicted that environmental factors (climatic, growth conditions, ripening stage, temperature and duration of storage) and thermal treatment might influence the antioxidant activity. Although total phenolics and tannins were decreased in cooked beans of both legumes in our study, fermentation of cooked beans with R. oligosporus resulted in significant elevation of total phenolics and tannins. A good correlation was seen with the total phenolics, antioxidant and β-glucosidase activities in soybean fermented with R. oligosporus (McCue and Shetty 2003). Phenolics are conjugated with sugar moiety through hydroxyl group to form glycosides, which reduces their antioxidant potential due to lack of free hydroxyl groups on the phenolic rings (Robbins 1980). Increased activity of β-glucosidase enzyme during solid state fermentation releases free aglycones (Woodward 1982; Vatten and Shetty 2002), which is clearly evident in our study by highest quantity of extractable phenolics with enhanced antioxidant activity in fermented beans.
Fig. 1.
Total phenolics (TAEs, tannic acid equivalents), tannins (CEs, catechin equivalents) and vitamin C (AAEs, ascorbic acid equivalents) in raw, cooked and fermented beans of C. cathartica and C. maritima (n = 5, mean ± SD) (different letters on the bars represent significant difference: *, p < 0.001, one-way ANOVA)
Fig. 2.
Total antioxidant activity, Fe2+ chelating capacity (200 μg mL−1) and DPPH radical-scavenging activity (200 μg mL−1) of raw, cooked and fermented beans of C. cathartica and C. maritima (n = 5, mean ± SD) (different letters on the bars represent significant difference: *, p < 0.001, one-way ANOVA)
Vitamin C is known as a potential antioxidant, pro-oxidant and potent radical scavenger simultaneously forms its own ascorbyl radical to promote oxidative reactions (Podmore et al. 1998). Loss of vitamin C in thermally processed samples occurs primarily by chemical degradation involving oxidation of ascorbic acid to dehydroascorbic acid (DHAA) and 2, 3-diketogulonic acid (Gregory 1996). Heat also speeds up the oxidation of ascorbic acid in fruits and vegetables resulting in the loss of vitamin C content. In our study, even though cooking decreased the vitamin C in beans, fermentation by R. oligosporus significantly elevated its concentration in both legumes (p < 0.001) (Fig. 1), which resulted in higher antioxidant potential (Fig. 2).
The DPPH free radical-scavenging assay has been widely employed to evaluate the ability of compounds to serve as radical scavengers or hydrogen donors in plant extracts. The EC50 of DPPH free radical-scavenging activity of both Canavalia beans was lowest in fermented than raw and cooked beans (p < 0.001) (Table 1). The radical-scavenging activity was significantly decreased in Canavalia beans on cooking in our study corroborates with thermal treatment of C. ensiformis and C. gladiata seeds (Vadivel et al. 2011, 2012). However, sprouting + oil-frying elevated radical-scavenging activity in C. ensiformis and C. gladiata seeds (Vadivel et al. 2011, 2012). On the contrary, thermally processed seeds (soaking + autoclaving) of C. ensiformis showed elevation of radical-scavenging potential (Sowndhararajan et al. 2011). The R. oligosporus fermented beans were more efficient in antioxidant activities (TAA and radical-scavenging activity) compared to raw and cooked beans, which is comparable with earlier study on seeds of two cultivars of Lathyrus sativus (Starzyńska-Janiszewska et al. 2008). In our study, the fermented beans showed significant elevation of TAA, Fe2+ chelating capacity, DPPH free radical-scavenging activity and reducing power (p < 0.001) shows the value-addition by the fermentation (Fig. 2). The EC50 values were least in fermented beans followed by raw beans and cooked beans (Table 1). The EC50 values of raw beans of C. cathartica and C. maritima are better than C. ensiformis and C. gladiata (18–32.2 vs. 69.8–91.2 μg mL−1) (Doss et al. 2010). Reducing activity in raw beans of C. ensiformis and C. gladiata was elevated with increasing concentration, but the absorbance was higher in C. ensiformis and C. gladiata compared to C. cathartica and C. maritima (0.31–0.56 vs. 0.08–0.09).
Table 1.
Comparison of EC50 (μg mL−1) values for DPPH free radical-scavenging activity of Canavalia spp. (n = 5, mean ± SD)
| Standard and sample | Radical-scavenging activity |
|---|---|
| Ascorbic acid (50 μg mL−1) | 5.2 ± 0.29 |
| Canavalia cathartica | |
| Raw | 18.0 ± 0.50a |
| Cooked | 49.2 ± 0.29b*c |
| Fermented | 16.7 ± 0.29b*d* |
| Canavalia maritima | |
| Raw | 32.2 ± 2.02a |
| Cooked | 49.8 ± 0.29b*c |
| Fermented | 11.8 ± 0.29b*d* |
Values in the column with different alphabets represent significant difference: *, p < 0.001, one-way ANOVA
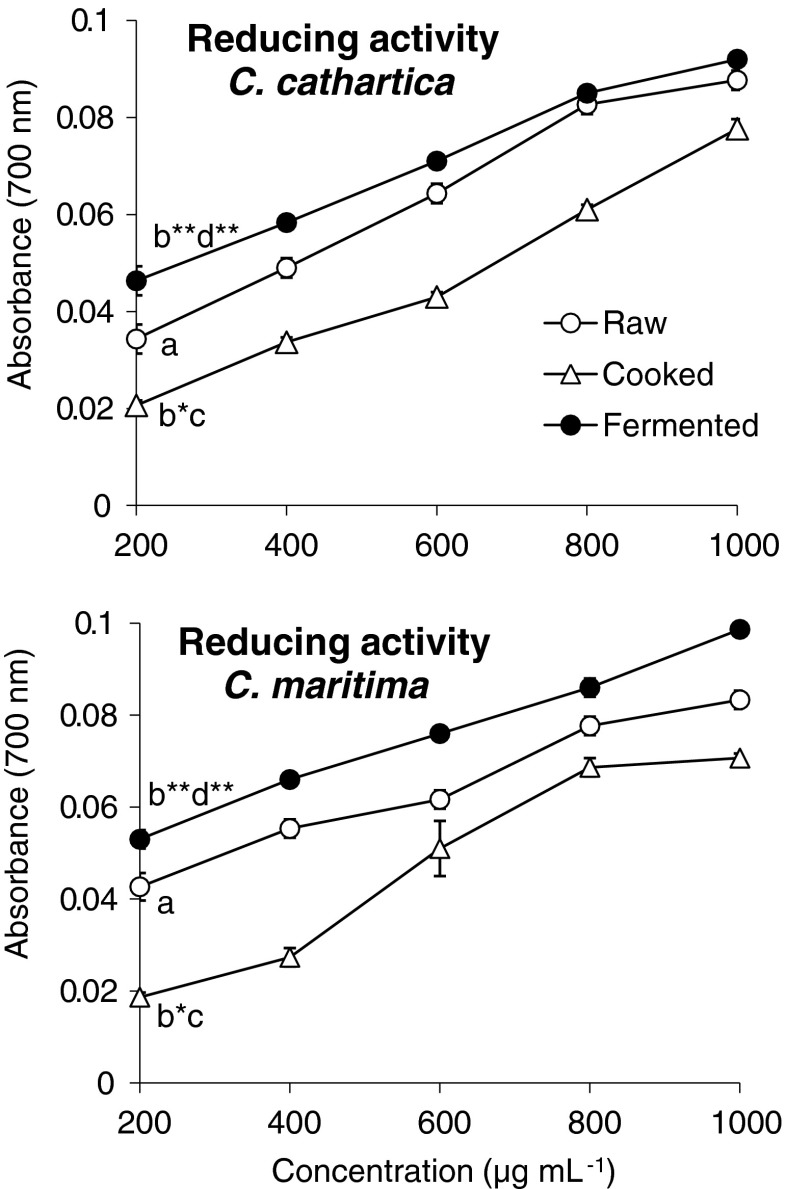
Study of metal ion chelating capacity is valuable because catalytic activity of metal ions are known to cause lipid peroxidation resulting in the deterioration of food and also cause arthritis and cancer (Gordon 1990; Halliwell et al. 1995). The significant decrease in Fe2+ chelating capacity in cooked beans of C. cathartica and C. maritima corroborates with studies on raw and thermally processed seeds of C. ensiformis (Sowndhararajan et al. 2011). Fermentation of black bean (Glycine max) using Rhizopus azygosporus resulted in the improved metal chelating activity compared to non-fermented beans (Lee et al. 2008). Our observations also showed significant elevation of Fe2+ chelating capacity in R. oligosporus fermented beans corroborating the studies on black beans by Lee et al. (2008). Gülçin et al. (2004) reported that chelating agents form σ-bonds with metal ions and act as secondary antioxidants by reducing the redox potential of metal ions. Elevation of reducing activity on fermentation might be due to the production of reductants during fermentation (Yang et al. 2000). Such reductants act as intracellular antioxidants and enhance the reducing activity of fermented beans than raw and cooked beans. Various studies reported that fermentation elevates reducing activity in legume seeds and their products (Wang et al. 2004; Lin et al. 2006; Lee et al. 2008). The reducing power of fermented beans of Canavalia was higher than raw and cooked beans (p < 0.01) (Fig. 3).
Fig. 3.
Reducing power of raw, cooked and fermented beans of C. cathartica and C. maritima (n = 5, mean ± SD) (different letters above 200 μg on each curve represent significant difference: *, p < 0.001, one-way ANOVA)
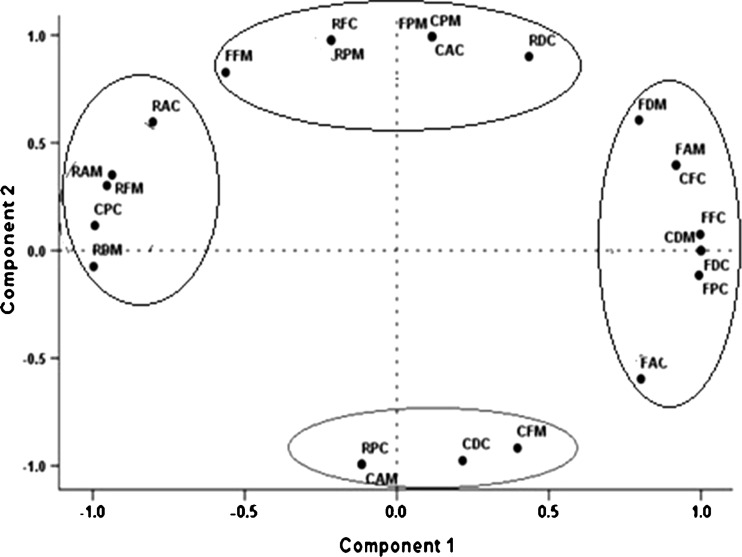
The PCA performed on total phenolics vs. antioxidant activities resulted two components (Eigenvalue, < 1), which accounted for 100 % variance. Total phenolics of raw (RPC and RPM), cooked (CPC and CPM) and fermented (FPC and FPM) beans of C. cathartica (C) and C. maritima (M) with antioxidant activities (total antioxidant activity: RAC, RAM, CAC, CAM, FAC and FAM; ferrous-ion chelating capacity: RFC, RFM, CFC, CFM, FFC and FFM; free radical-scavenging activity: RDC, RDM, CDC, CDM, FDC and FDM) revealed 50.94 % variance for component 1 and 49.06 % variance for component 2 (Fig. 4). The plots in axis 1 and axis 2 showed two groups. In group 1, total phenolics and all the four antioxidant activities tested for fermented C. cathartica were clustered indicating the relationship between the bioactive compound and antioxidant activities. Total antioxidant and ion chelating activities of cooked C. maritima were clustered together in group 2. The PCA plot showed that the total phenolics and antioxidant activities of raw beans of C. maritima, and total phenolics, total antioxidant and ion chelating activities of C. cathartica are located to the left in the score plot, which indicates the low antioxidant potential. It is noteworthy that total phenolics and most of the antioxidant activities of both fermented seeds located on right of the plot indicating their nutraceutical potential.
Fig. 4.
Score plot of principal component analysis (PC1, 50.94 %; PC2, 49.06 %) for the total phenolics of raw, cooked and fermented beans against total antioxidant activity, Fe2+ chelating capacity and DPPH radical-scavenging activity of Canavalia cathartica and C. maritima [first letter: raw (R), cooked (C) and fermented (F) beans; second letter: total phenolics (P), antioxidant (A), Fe2+ chelating (F), DPPH radical-scavenging (D) activities; third letter: C. cathartica (C) and C. maritima (M)]
Besides the bioactive components we tested in the present study, the antioxidant activity of Canavalia seeds might have been influenced by other potential compounds like flavonoids, phytates, amino acids, peptides, vitamin E, some fatty acids and minerals (Sridhar and Seena 2006; Vadivel and Biesalski 2010; Sridhar and Niveditha 2011). Further in depth knowledge on bioactive principles and their antioxidant potential is necessary to access benefit from the less known coastal sand dune Canavalia spp. distributed widely in pantropical habitats. Further evaluation of antioxidant potential and value addition of coastal sand dune Canavalia beans by fermentation with R. oligosporus is in progress in our laboratory.
Conclusions
Solid-state fermentation of Canavalia cathartica and C. maritima with Rhizopus oligosporus exhibited significantly highest quantity of bioactive compounds (total phenolics, tannins and vitamin C) and total antioxidant activity (Fe2+ chelating capacity, DPPH free radical-scavenging activity, reducing power and the lowest EC50 values) suggesting fermentation as an efficient strategy to improve the nutraceutical value. Based on analysis of variance, the antioxidant activity was strongly linked with total phenolics, tannins and vitamin C contents in fermented beans of Canavalia. The principal component analysis for total phenolics against antioxidant activities revealed that fermented beans are superior in bioactive compounds as well as antioxidant potential.
Acknowledgments
Authors are grateful to Mangalore University for permission to carry out studies on Canavalia of coastal sand dunes in the Department of Biosciences. We are thankful to Dr. Damodar Shenoy, Institute of Microbial Technology, Chandigarh, India, Dr. Hemachandra, Department of Zoology, St. Aloysius College, Mangalore, India and Mr. M.P. Krishna, Department of Applied Zoology, Mangalore University, Mangalore, India for helpful suggestions.
References
- Alothman M, Bhat R, Karim AA. Effects of radiation processing on hytochemicals and antioxidants in plant produce. Trends Food Sci Technol. 2009;20:201–212. doi: 10.1016/j.tifs.2009.02.003. [DOI] [Google Scholar]
- Annegowda HV, Bhat R, Tze LM, Karim AA, Mansor SM (2011) The free radial scavenging and antioxidant activities of pod and seed extract of Clitoria fairchildiana (Howard)–an underutilized legume. J Food Sci Technol. doi:10.1007/s13197-011-0370-8 [DOI] [PMC free article] [PubMed]
- Bhagya B, Sridhar KR. Ethnobiology of coastal sand dune legumes of Southwest India. Indian J Tradit Knowl. 2009;9:611–620. [Google Scholar]
- Bhat R, Karim AA. Exploring the nutritional potential of wild and underutilized legumes. Compr Rev Food Sci Food Saf. 2009;8:305–331. doi: 10.1111/j.1541-4337.2009.00084.x. [DOI] [Google Scholar]
- Blomhoff R, Carlsen MH, Andersen LF, Jacobs DR. Health benefits of nuts: potential role of antioxidants. Br J Nutr. 2006;96:S52–S60. doi: 10.1017/BJN20061864. [DOI] [PubMed] [Google Scholar]
- Bourdy G, Cabalion P, Amade P, Laurent D. Traditional remedies used in the Western Pacific for the treatment of ciguatera poisoning. J Ethanopharmacol. 1992;36:163–174. doi: 10.1016/0378-8741(92)90017-L. [DOI] [PubMed] [Google Scholar]
- Burns R. Methods for estimation of tannins in grain sorghum. Agron J. 1971;63:511–512. doi: 10.2134/agronj1971.00021962006300030050x. [DOI] [Google Scholar]
- Cai S, Gao F, Zhang X, Wang O, Wu W, Zhu S, Zhang D, Zhou F, Ji B (2012) Evaluation of γ- aminobutyric acid, phytate and antioxidant activity of tempeh-like fermented oats (Avena sativa L.) prepared with different filamentous fungi. J Food Sci Technol. doi:10.1007/s13197-012-0748-2 [DOI] [PMC free article] [PubMed]
- Chock AK. Hawaiian ethnobotanical studies 1: native food and beverage plants. Econ Bot. 1968;22:138–221. doi: 10.1007/BF02861956. [DOI] [Google Scholar]
- D’Cunha M, Sridhar KR, Bhat R. Nutritional quality of germinated seeds of Canavalia maritima of coastal sand dunes. In: Bellinghouse VC, editor. Food processing: methods, techniques and trends. New York: Nova Science Publishers Inc; 2009. pp. 363–384. [Google Scholar]
- D’Cunha M, Sridhar KR, Young C-C, Arun AB. Nutritional evaluation of germinated seeds of coastal sand dune wild legume Canavalia cathartica. Int Food Res J. 2009;16:249–260. [Google Scholar]
- Das L, Bhaumik E, Raychaudhuri U, Chakraborty R. Role of nutraceuticals in human health. J Food Sci Technol. 2012;49:173–183. doi: 10.1007/s13197-011-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss A, Pugalenthi M, Rajendrakumaran D, Vadivel V. Phenols, flavonoids and antioxidant activity of under-utilized legume seeds. Asian J Exp Biol Sci. 2010;1:700–705. [Google Scholar]
- Du G, Li M, Ma F, Liang D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 2009;113:557–562. doi: 10.1016/j.foodchem.2008.08.025. [DOI] [Google Scholar]
- Gazzani G, Papetti A, Massolini G, Daglia M. Anti and pro-oxidant activity of water soluble components of some common diet vegetables and the effect of thermal treatment. J Agric Food Chem. 1998;46:4118–4122. doi: 10.1021/jf980300o. [DOI] [Google Scholar]
- Gordon MH. The mechanism of antioxidant action in vitro. In: Hudson BJF, editor. Food antioxidants. London: Elsevier Applied Science; 1990. pp. 1–18. [Google Scholar]
- Gregory JF. Vitamins. In: Fennema OR, editor. Food chemistry. 3. New York: Dekker; 1996. pp. 531–616. [Google Scholar]
- Gülçin I, Beydemir S, Alici HA, Elmasta M, Büyükokuroglu ME. In vitro antioxidant properties of morphine. Pharmacol Res. 2004;49:59–66. doi: 10.1016/j.phrs.2003.07.012. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Murcia HA, Chico S, Aruoma OI. Free radicals and antioxidants in food an in vivo: what they do and how they work. CRC Crit Rev Food Sci Nutr. 1995;35:7–20. doi: 10.1080/10408399509527682. [DOI] [PubMed] [Google Scholar]
- Hsu CL, Chen W, Weng YM, Tseng CY. Chemical composition, physical properties and antioxidant activities of yam flours as affected by different drying methods. Food Chem. 2003;83:85–92. doi: 10.1016/S0308-8146(03)00053-0. [DOI] [Google Scholar]
- Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113:71S–88S. doi: 10.1016/S0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- Lee HI, Hung HY, Chou CC. Solid state fermentation with fungi to enhance the antioxidant activity, total phenolics and anthocyanin contents of black bean. Int J Food Microbiol. 2008;121:150–156. doi: 10.1016/j.ijfoodmicro.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Lewis G, Schrire B, Mackinder B, Lock M. Legumes of the world. Kew: Royal Botanical Gardens; 2005. [Google Scholar]
- Lin CH, Wei YT, Chou CC. Enhanced antioxidative activity of soybean koji prepared with various filamentous fungi. Food Microbiol. 2006;23:628–633. doi: 10.1016/j.fm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- McCue P, Shetty K. Role of carbohydrate-cleaving enzymes in phenolic antioxidant mobilization from whole soybean fermented with Rhizopus oligosporus. Food Biotechnol. 2003;17:27–37. doi: 10.1081/FBT-120019982. [DOI] [Google Scholar]
- Osman NM, Ahmed IAM, Babiker EE. Fermentation and cooking of sicklepod (Cassia obsusifolia) leaves: changes in chemical and amino acid composition, antinutrients and protein fractions and digestibility. Food Sci Technol. 2010;45:124–132. doi: 10.1111/j.1365-2621.2009.02111.x. [DOI] [Google Scholar]
- Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. Vitamin C exhibits pro-oxidant properties. Nature. 1998;392:559–560. doi: 10.1038/33308. [DOI] [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Randhir R, Lin YT, Shetty K. Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Proc Biochem. 2004;39:637–646. doi: 10.1016/S0032-9592(03)00197-3. [DOI] [PubMed] [Google Scholar]
- Randhir R, Kwon YI, Shetty K. Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts and seedlings. Innov Food Sci Emerg Technol. 2008;9:355–364. doi: 10.1016/j.ifset.2007.10.004. [DOI] [Google Scholar]
- Robbins R. Medical and nutritional aspects of citrus bioflavonoids. In: Nagy S, Attaway J, editors. Citrus nutritional quality. Washington: American Chemical Society; 1980. pp. 43–50. [Google Scholar]
- Roe JH. Chemical determination of ascorbic, dehydroascorbic and diketogluconic acids. In: Glick D, editor. Methods of biochemical analysis, volume 1. New York: Interscience; 1954. pp. 115–139. [DOI] [PubMed] [Google Scholar]
- Rosset J, Bärlocher F, Oertli JJ. Decomposition of conifer needles and deciduous leaves in two Black Forest and two Swiss Jura streams. Int Rev Ges Hydrobiol. 1982;67:695–711. [Google Scholar]
- Seena S, Sridhar KR. Nutritional and microbiological features of little known legumes, Canavalia cathartica Thouars and C. maritima Thouars of the southwest coast of India. Curr Sci. 2006;90:1638–1650. [Google Scholar]
- Sheih I-C, Wu H-Y, Lai Y-J, Lin C-F. Preparation of high free radical scavenging tempeh by newly isolated Rhizopus sp. R-69 from Indonesia. Food Sci Agric Chem. 2000;10:35–40. [Google Scholar]
- Siddhuraju P. The antioxidant activity and free radical-scavenging capacity of phenolics of raw and dry heated moth bean [(Vigna aconitifolia) (Jacq.) Marechal] seed extracts. Food Chem. 2006;99:149–157. doi: 10.1016/j.foodchem.2005.07.029. [DOI] [Google Scholar]
- Singh RP, Murthy CKN, Jayaprakasha GK. Studies on antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro methods. J Agric Food Chem. 2002;50:81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- Sowndhararajan K, Siddhuraju P, Manian S. Antioxidant activity of the differentially processed seeds of jack bean (Canavalia ensiformis L DC) Food Sci Biotechnol. 2011;20:585–591. doi: 10.1007/s10068-011-0083-9. [DOI] [Google Scholar]
- Sridhar KR, Bhagya B (2007) Coastal sand dune vegetation: a potential source of food, fodder and pharmaceuticals. Livest Res Rural Dev 19: Article # 84: http://www.cipav.org.co/lrrd/lrrd19/6/srid19084.htm (accessed: March 20, 2012)
- Sridhar KR, Niveditha VR. Wild legume Canavaia cathartica–an overview on nutritional and bioactive potential. In: Medina DA, Laine AM, editors. Food quality: control, analysis and consumer concerns. New York: Nova Science Publishers Inc; 2011. pp. 269–302. [Google Scholar]
- Sridhar KR, Seena S. Nutritional and antinutritional significance of four unconventional legumes of the genus Canavalia—a comparative study. Food Chem. 2006;99:267–288. doi: 10.1016/j.foodchem.2005.07.049. [DOI] [Google Scholar]
- Starzyńska-Janiszewska A, Stodolak B, Jamróz M. Antioxidant properties of extracts from fermented and cooked seeds of Polish cultivars of Lathyrus sativus. Food Chem. 2008;109:285–292. doi: 10.1016/j.foodchem.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Sulaiman SF, Yusoff NAM, Eldeen IM, Seow EM, Sajak AAB, Supriatno, Ooi KL. Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.) J Food Compos Anal. 2011;24:1–10. doi: 10.1016/j.jfca.2010.04.005. [DOI] [Google Scholar]
- Vadivel V, Biesalski HK. HPLC analysis of bioactive compounds in ten different wild type under-utilized legume grains. Inst Integr Omics Appl Biotechnol J. 2010;1:17–24. [Google Scholar]
- Vadivel V, Biesalski HK (2011) Effect of certain indigenous processing methods on the bioactive compounds of ten different wild type legume grains. J Food Sci Technol. doi:10.1007/s13197-010-0223-x [DOI] [PMC free article] [PubMed]
- Vadivel V, Stuetz W, Scherbaum V, Biesalski HK. Total free phenolic content and health relevant functionality of Indian wild legume grains: effect of indigenous processing methods. J Food Compos Anal. 2011;24:935–943. doi: 10.1016/j.jfca.2011.04.001. [DOI] [Google Scholar]
- Vadivel V, Cheong JN, Biesalski HK (2012) Antioxidant and type II diabetes related enzyme inhibition properties of methanolic extract of an underutilized food legume, Canavalia ensiformis (L.) DC: effect of traditional processing methods. LWT Food Sci Technol, http://dx.doi.org/10.1016/j.lwt.2012.01.014
- Vatten DA, Shetty K. Soild-state production of phenolic antioxidant from cranberry pomace by Rhizopus oligosporus. Food Biotechnol. 2002;16:189–210. doi: 10.1081/FBT-120016667. [DOI] [Google Scholar]
- Vietmeyer ND. Lesser-known plants of potential use in agriculture and forestry. Science. 1986;232:1379–1384. doi: 10.1126/science.232.4756.1379. [DOI] [PubMed] [Google Scholar]
- Wang YC, Yu RC, Chou CC. Viability of lactic acid bacteria and bifidobacteria in fermented soymilk after drying, subsequent rehydration and storage. Int J Food Microbiol. 2004;93:209–217. doi: 10.1016/j.ijfoodmicro.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Woodward J. Fungal and other β-D-glucosidase–their properties and applications. Enzym Microb Technol. 1982;4:73–79. doi: 10.1016/0141-0229(82)90084-9. [DOI] [Google Scholar]
- Xu B, Chang SKC. Effect of soaking, boiling, and steaming on total phenolic content and antioxidant activities of cool season food legumes. Food Chem. 2008;110:1–13. doi: 10.1016/j.foodchem.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Yang JH, Mau JL, Ko PT, Huang LC. Antioxidant properties of fermented soybean broth. Food Chem. 2000;71:249–254. doi: 10.1016/S0308-8146(00)00165-5. [DOI] [Google Scholar]