Abstract
Present study was conducted to evaluate the effect of addition of different levels of Moringa oleifera leaves extract (MLE) and butylated hydroxytoluene (BHT) in raw and cooked pork patties during refrigerated storage. Five treatments evaluated include: Control (without MLE/BHT), MLE 300 (300 ppm equivalent M. oleifera leaves phenolics), MLE 450 (450 ppm equivalent M. oleifera leaves phenolics), MLE 600 (600 ppm equivalent M. oleifera leaves phenolics) and BHT 200 (200 ppm BHT). Total phenolic content ranged from 60.78 to 70.27 mg per gram. A concentration dependent increase in reducing power and 1,1-diphenyl 2-picrylhydrazyl (DPPH) radical scavenging activity of both MLE and BHT was noticed. Higher (P < 0.001) a* and lower thiobarbituric acid reactive substances values were observed in MLE 600 and BHT 200 compared to control. Addition of MLE did not affect the sensory attributes or microbial quality. These results showed that M. oleifera leaves can be used as a potential source of natural antioxidants to inhibit lipid oxidation in ground pork patties.
Keywords: BHT, Lipid oxidation, Meat colour, Metmyoglobin, Moringa oleifera leaves, Natural antioxidants, Pork
Introduction
Microbial spoilage, enzymatic changes and lipid/protein oxidation are some of the important factors influencing the shelf-life of foods. Among muscle foods, pork products are more susceptible to oxidation than beef or lamb due to relatively high content of unsaturated fatty acids (Warriss 2010). Further, grinding and thermal processing disrupt the integrity of muscle membranes and expose lipid membranes to metal ions and facilitate the interaction of pro-oxidants with unsaturated fatty acids resulting in generation of free radicals and propagation of oxidative reaction (Asghar et al. 1988). Oxidation induces modification of muscle lipids and proteins and, therefore, affects the organoleptic and nutritional properties of meat and meat products. This is reflected in economic losses and health disorders.
Lipid oxidation may be delayed by the addition of antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA) and tert-butylhydroquinone (TBHQ). However, these synthetic antioxidants are considered to be potential promoters of carcinogens (Tang et al. 2001). With increased consumer concerns on the use of synthetic antioxidants, use of natural antioxidants in muscle foods is becoming highly relevant in the food industry.
Many herbs and spices are known to contain varieties of phytochemicals, which are potential sources of natural antioxidants and antimicrobial compounds that includes polyphenols, flavonoids, carotenoids, tannins and phenolic acids (Nair and Subramanian 1962; Sherwin 1998; Naveena et al. 2008; Devatkal et al. 2010). The antioxidant and antimicrobial activities of various phytochemicals from black berry (in vitro study; Annuk et al. 1999), grape seed (Pork; Carpenter et al. 2007), grape pomace (Pork; Garrido et al. 2011) and green tea (mutton; Kumudavally et al. 2008) have been reported. The Moringa oleifera commonly known as drumstick, is native to India, Africa, Arabia, Southeast Asia and South America (Sengupta and Gupta 1970) and traditionally being used for dietary purposes as vegetable. M. oleifera is of special interest in food preservation because in addition to contributing taste and aroma to foods, it also contains a variety of bioactive substances, which are of considerable use in extending shelf life. Leaves of M. oleifera have been reported to contain natural antioxidants and flavonoid pigments such as kaempferol, rhamnetin, isoquercitrin, and kaempferitrin (Nair and Subramanian 1962). These compounds are effective free radical scavengers (Salah et al. 1995) and also effective metal chelators (Shahidi et al. 1992). However, wide variation was observed in antioxidant properties between plants due to varied agro climatic locations. Further, the antioxidants were shown to behave differently in raw and cooked pork patties (McCarthy et al. 2001). Few researchers have reported that M.oleifera leaves extract showed antioxidant activity in various model systems (Reddy et al. 2005). However, so far little has been reported about its use in meat and meat products (Hazra et al. 2012). Hence, this study was conducted to determine the antioxidant potential of aqueous extract of M. oleifera leaves and effect of incorporation of extract on colour, lipid oxidation, sensory attributes and microbial quality of both raw and cooked pork patties during refrigerated storage.
Materials and methods
Materials
Fresh pork procured from local market of Hyderabad was utilized. After removing fat and connective tissues, lean meat was stored at 4 °C for 24 h before use. The moisture, protein and fat content (%) of pork samples were 72.95, 20.22 and 5.75, respectively. Fresh pork samples were separately obtained for each of the six replications on different occasions. Butylated hydroxytoluene (BHT), 2-thiobarbituric acid, trichloroacetic acid (Merk, Mumbai, India), tannic acid, 1,1-diphenyl 2-picrylhydrazyl (DPPH) (Sigma–Aldrich, New Delhi, India) used in the study were of analytical grade. Peptone and plate count agar were procured from Himedia, Mumbai, India.
Preparation of aqueous M. oleifera leaves extract (MLE)
Fresh M. oleifera leaves obtained from local market were washed well with water to remove the adhering dust. They were air dried and ground into powder in a heavy duty grinder (Soni Appliances, Mumbai, India) and sieved using a 60 mesh sieve and packed and stored at room temperature in high density poly ethylene bags until extraction. About 20 g of dried powder was mixed with 100 mL boiled distilled water and left for 5 min. The extract was obtained by filtration and analyzed for pH, total phenolic content, reducing power and DPPH radical scavenging activity. Freshly prepared extract was used for each replication on the basis of phenolic content.
Preparation of pork patties
Chilled pork samples were cut into small cubes and minced twice (13 mm plate followed by 6 mm plate) using a meat mincer (Model X70, Scharfen, 58413 Witten, Germany). The minced pork was randomly assigned into two batches (one for raw and another for cooked) of 5 different treatments: Control (no MLE/BHT), MLE 300 (300 ppm equivalent M. oleifera leaves phenolics), MLE 450 (450 ppm equivalent M. oleifera leaves phenolics), MLE 600 (600 ppm equivalent M. oleifera leaves phenolics) and BHT 200 (200 ppm BHT). The volume of extract added was replaced with distilled water in control and BHT samples. The BHT was dissolved in 5 mL vegetable oil before addition and the equal quantity of oil was added to other samples to maintain uniformity. Immediately after adding all ingredients, samples were thoroughly hand-mixed for 5 min and formed into 90 g patties. One batch of patties were kept uncooked (raw) and another set of patties were cooked in microwave oven (Model MC-767 w/w, LG Electronics India Pvt. Ltd., New Delhi, India) at 720 MHZ for about 4 min till the internal temperature attains 80 °C. During cooking, the patty was turned upside down to avoid color difference between two surfaces. Both the raw and cooked patties (after cooling) were aerobically packaged in a low density polyethylene pouches and stored at 4 °C and analyzed for pH, instrumental color, metmyoglobin content (raw patties only), thiobarbituric acid reactive substances (TBARS), sensory attributes (cooked patties only) and microbial count (total plate counts and psychrophilic counts) at periodical intervals. The raw patties were analysed on 0, 3, 5, 7 and 9 days of storage, whereas cooked patties were analysed at 5 days interval up to 15 days.
Analysis of samples
Total phenolics
The concentration of total phenolics in MLE was determined by the Folin–Ciocalteus (F–C) assay (Escarpa and Gonzalez 2001) with slight modifications. Suitable aliquots of extracts were taken in a test tube and the volume was made to 0.5 mL with distilled water followed by the addition of 0.25 mL F–C (1 N) reagent and 1.25 mL sodium carbonate solution (20 %). The tubes were vortexed and the absorbance recorded at 725 nm (Model UV-1700 PharmaSpec, Shimadzu, Japan) after 40 min. The total phenolics content was calculated using tannic acid as standard and results were expressed as μg tannic acid equivalent.
1,1-diphenyl 2-picrylhydrazyl (DPPH) radical scavenging activity
The method of Singh et al. (2002) was employed to assess the ability of MLE and BHT to scavenge DPPH radical. The MLE and BHT (50 and 100 μg phenolics) diluted with 0.1 M Tris–HCl buffer (pH 7.4) was mixed with 1 mL of DPPH (250 μM) and stored in the dark at room temperature for 20 min and the absorbance was measured at 517 nm. The scavenging activity was calculated by the following equation:
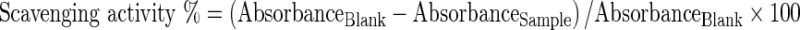 |
Reducing power
Reducing power was quantified as per the method described by Oyaizu (1986). Ten milligram phenolics from MLE and BHT were mixed with 2.5 mL phosphate buffer (0.2 M, pH 6.6) and incubated with 2.5 mL potassium ferricyanide (1 % w/v) at 50 °C for 20 min. At the end of incubation, 2.5 mL of 10 % trichloroacetic acid solution was added and centrifuged at 3,000 g for 10 min. The supernatant was mixed with 2.5 mL distilled water and 0.5 mL ferric chloride (0.1 % w/v) solution. The absorbance was measured at 700 nm.
pH
The pH of the raw and cooked patties was determined by blending 10 g sample with 50 mL distilled water for 60 s in a homogenizer (Model Miccra D8-Si, ART Moderne Labortechnik, Germany). The pH values were measured using a standardized electrode attached to a digital pH meter (Model 420A+, Thermo Orion, USA).
Instrumental color
Instrumental color analysis was performed using Hunter Lab Miniscan XE Plus colorimeter (Hunter Associates Laboratory Inc., Reston, VA, USA) with 25 mm aperture set for illumination D65, 100 standard observer angle. CIE L* (lightness), a* (redness) and b* (yellowness) were measured on the surface of raw and cooked pork patties from five randomly chosen spots.
Metmyoglobin (MMb) formation
Modified procedure of Warris (1979) was employed for extraction of myoglobin from raw pork patties. Samples were blended with 5 volumes of cold 0.04 M phosphate buffer at pH 6.8 for 10 s in a homogenizer. After keeping at 1 °C for 1 h, the mixtures were centrifuged at 3,500 g (Model CPR-24, Remi Instruments, Mumbai, India) and 4 °C for 30 min. The supernatant was filtered through Whatman No. 1 filter paper and the absorbance of filtrate was measured at 525, 572, and 700 nm using a UV–VIS spectrophotometer (Model UV-1700 PharmaSpec, Shimadzu, Japan). The % metmyoglobin (MMb) was calculated by the following equation:
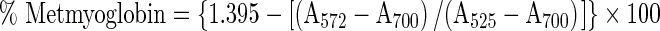 |
Thiobarbituric acid reactive substances (TBARS) value
The TBARS value (mg malonaldehyde/kg) of both raw and cooked pork patties was determined using the extraction method described by Witte et al. (1970) with slight modifications as the slurry was centrifuged at 3,000 g for 10 min instead of filtration through Whatman No. 42 filter paper. Four gram sample was homogenized with 20 mL of 20 % trichloroacetic acid solution and the slurry was centrifuged (Model CPR-24, Remi Instruments, Mumbai, India). Two mL of supernatant was mixed with equal volume of freshly prepared 0.1 % thiobarbituric acid in glass test tubes and heated in water bath at 100 °C for 30 min followed by cooling under tap water. The absorbance of the mixture was measured at 532 nm and the TBARS values were calculated using a TBA standard curve and expressed in mg malonaldehyde/kg.
Microbiological quality
Microbial quality in terms of aerobic plate count (APC) and psychrotrophic count was determined as per the procedure of International Commission of Microbiological Specifications for Foods (ICMSF) (1983). For determination of microbial counts, 10 g of meat sample was homogenized with 90 mL of 0.1 % sterile peptone water. Serial 10 fold dilutions were prepared by diluting 1 mL of homogenate in 9 mL of 0.1 % peptone water. Appropriate serial dilutions were duplicate plated (pour plate method) with plate count agar for aerobic plate count (APC) and psychrotrophic count (PPC). Plates were incubated at 37 °C for 48 h for APC and 7 °C for 10 days for PPC.
Sensory evaluation
An experienced 6 member panel of scientists of the institute evaluated cooked pork patties on day 0. The panelists rated each sample on an 8-point descriptive scale (8, extremely desirable; 1, extremely undesirable) for colour and appearance, flavour and overall acceptability (Keeton 1983). Patties were warmed in a microwave oven for 20 s just before sensory evaluation and coded samples were served at room temperature in separate booths. Water was served for cleansing the mouth between samples.
Statistical analysis
Mean values for various parameters were calculated and compared by analysis of variance using SPSS (SPSS version 13.0 for windows; SPSS, Chicago, IL, USA). The phenolic content, DPPH scavenging activity, reducing power and sensory attributes were analyzed using one-way ANOVA. A 4 × 4 factorial design with six replicates was employed for storage data (pH, instrumental color, TBARS value, MMb, APC and PPC) with treatments and storage time as main effects using two-way ANOVA. Statistical significance was determined at 95 % confidence level (P < 0.05).
Results and discussion
Antioxidant potential of MLE
Total phenolic content (mg/g M. oleifera leaves powder as tannic acid equivalent) was ranged from 60.78 to 70.27. Iqbal and Bhanger (2006) reported 88.2–127.9 mg of total phenolics per gram of ethonolic extracts of M. oleifera leaves powder. The scavenging effects of 50, 100, 150, 200 and 250 μg of total phenols of MLE on the DPPH radical were 21.92, 33.49, 43.35, 57.14 and 72.41 %, respectively. Butylated hydroxytoluene at similar concentrations had scavenging effects of 8.86, 33.25, 57.64, 80.05 and 86.69 %, respectively. Increase in the concentration of the plant extract, progressively increased the radical scavenging activity. This correlates well with the concentration of total phenolic compounds per unit volume of leaves powder. Negi and Jayaprakasha (2003) and Naveena et al. (2008) have reported an increase in radical scavenging activity with an increase in the concentration of pomegranate peel extract and pomegranate rind powder extract, respectively.
Reducing power of different levels of MLE and BHT were assessed using potassium ferricyanide method. The reducing power of 50, 100, 150, 200 and 250 μg of total phenols of MLE were 0.40, 0.62, 0.85, 1.13 and 1.33, respectively. Similarly, the reducing power of BHT increased from 0.30 to 0.64, when the concentration raised from 50 to 250 μg. The reducing properties are generally associated with the presence of reductones and the antioxidative action of reductones is based on the breaking of free radical chains by the donation of hydrogen atom (Gordon 1990). A concentration dependent increase in reducing power of MLE and BHT was noticed. A significant increase in reducing power of aqueous extracts of Lichens with increase in concentration from 50 to 400 ppm have been reported by Negi and Jayaprakasha (2003). The MLE exhibited higher reducing power compared to BHT at all levels.
Effect of MLE and BHT on quality attributes of raw and cooked pork patties
The changes in pH of raw and cooked pork patties during refrigerated storage are given in Tables 1 and 2 respectively. There was no difference (P > 0.05) in pH among the control and treated groups in both raw and cooked pork patties due to incorporation of antioxidants. The mean pH was similar in all the groups on all the days of analysis. Similarly, McCarthy et al. (2001) and Carpenter et al. (2007) reported no difference in the pH of control and test antioxidants like grape seed, bearberry and rosemary extracts incorporated raw and cooked pork meat products. However, there was an increase (P < 0.001) in pH among all the groups in both raw and cooked pork patties during storage period. The pH of products gradually increased during storage and increase (P < 0.001) was observed after 5th day of storage in raw pork patties and 10th day in cooked pork patties. The increase in pH may be attributed to the microbial metabolites. As stated by Gill (1983), bacteria on exhaustion of stored glucose, utilize amino acids released during protein breakdown and ammonia accumulates as a product of amino acid degradation and the pH rises.
Table 1.
Changes in pH, Hunter Lab L*, a*, b* values and % metmyoglobin of raw pork patties incorporated with M. oleifera leaves extract (MLE) and butylated hydroxyl toluene (BHT) at different concentrations during storage (4 °C)
| Parameter | Days of storage | Treatments | Overall mean (days) | ||||
|---|---|---|---|---|---|---|---|
| Control | MLE 300 | MLE 450 | MLE 600 | BHT 200 | |||
| pH | 0 | 6.4 ± 0.21 | 6.3 ± 0.08 | 6.3 ± 0.26 | 6.3 ± 0.08 | 6.3 ± 0.18 | 6.3v |
| 3 | 6.4 ± 0.14 | 6.4 ± 0.18 | 6.4 ± 0.22 | 6.4 ± 0.11 | 6.4 ± 0.24 | 6.4vw | |
| 5 | 6.5 ± 0.15 | 6.5 ± 0.22 | 6.5 ± 0.18 | 6.5 ± 0.22 | 6.5 ± 0.20 | 6.5w | |
| 7 | 6.6 ± 0.12 | 6.6 ± 0.14 | 6.5 ± 0.15 | 6.5 ± 0.17 | 6.6 ± 0.36 | 6.6x | |
| 9 | 6.7 ± 0.22 | 6.7 ± 0.16 | 6.6 ± 0.23 | 6.6 ± 0.20 | 6.6 ± 0.32 | 6.6y | |
| Overall mean (Treatments) | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | ||
| L* | 0 | 45.1 ± 1.33 | 45.0 ± 1.69 | 45.1 ± 1.20 | 44.7 ± 1.28 | 44.5 ± 1.48 | 44.9v |
| 3 | 46.2 ± 0.97 | 46.0 ± 1.81 | 45.7 ± 1.27 | 45.1 ± 1.29 | 44.9 ± 1.44 | 45.6wx | |
| 5 | 46.8 ± 0.69 | 46.7 ± 1.67 | 46.2 ± 1.27 | 45.3 ± 1.27 | 45.2 ± 1.47 | 46.0wx | |
| 7 | 47.4 ± 0.68 | 47.3 ± 1.71 | 46.6 ± 1.19 | 45.5 ± 1.26 | 45.4 ± 1.48 | 46.4x | |
| 9 | 47.7 ± 0.71 | 47.7 ± 1.66 | 47.0 ± 1.24 | 45.6 ± 1.27 | 45.6 ± 1.52 | 46.7y | |
| Overall mean (Treatments) | 46.6b | 46.5b | 46.1b | 45.2a | 45.1a | ||
| a* | 0 | 11.2 ± 0.56 | 11.0 ± 0.53 | 11.2 ± 0.44 | 11.6 ± 0.72 | 11.9 ± 0.63 | 11.4y |
| 3 | 9.4 ± 0.63 | 9.6 ± 0.58 | 9.9 ± 0.47 | 10.4 ± 0.76 | 10.8 ± 0.61 | 10.0 x | |
| 5 | 8.2 ± 0.86 | 8.6 ± 0.57 | 8.9 ± 0.47 | 9.5 ± 0.73 | 9.7 ± 0.46 | 9.0 w | |
| 7 | 7.5 ± 0.87 | 8.0 ± 0.50 | 8.2 ± 0.46 | 8.9 ± 0.56 | 9.1 ± 0.47 | 8.3 v | |
| 9 | 7.0 ± 0.81 | 7.4 ± 0.52 | 7.7 ± 0.54 | 8.4 ± 0.67 | 8.7 ± 0.37 | 7.9 v | |
| Overall mean (Treatments) | 8.7a | 8.9a | 9.2ab | 9.8bc | 10.1c | ||
| b* | 0 | 21.6 ± 1.78 | 21.2 ± 1.61 | 19.1 ± 1.34 | 19.4 ± 0.73 | 19.3 ± 0.77 | 20.1 |
| 3 | 21.5 ± 1.52 | 20.8 ± 1.41 | 19.4 ± 0.97 | 19.3 ± 0.51 | 19.6 ± 0.41 | 20.1 | |
| 5 | 20.9 ± 1.12 | 20.5 ± 1.59 | 19.0 ± 0.62 | 18.9 ± 0.59 | 19.4 ± 0.35 | 19.7 | |
| 7 | 20.0 ± 1.27 | 20.8 ± 1.29 | 19.2 ± 0.72 | 19.0 ± 0.52 | 19.0 ± 0.40 | 19.6 | |
| 9 | 20.1 ± 0.93 | 20.6 ± 1.18 | 19.1 ± 0.84 | 18.7 ± 0.59 | 18.9 ± 0.40 | 19.5 | |
| Overall mean (Treatments) | 20.8b | 20.8b | 19.2a | 19.0a | 19.2a | ||
| % MMb | 0 | 15.9 ± 1.08 | 15.3 ± 1.03 | 13.9 ± 1.14 | 11.8 ± 0.93 | 8.8 ± 0.52 | 13.1v |
| 3 | 26.5 ± 1.17 | 25.0 ± 1.28 | 22.3 ± 1.37 | 19.8 ± 1.28 | 13.5 ± 0.79 | 21.4w | |
| 5 | 39.5 ± 1.39 | 37.6 ± 1.76 | 35.0 ± 1.63 | 33.1 ± 1.18 | 22.7 ± 1.17 | 33.6x | |
| 7 | 52.3 ± 2.79 | 50.3 ± 2.47 | 47.6 ± 2.09 | 44.7 ± 2.95 | 31.0 ± 1.43 | 45.2y | |
| 9 | 62.3 ± 2.52 | 60.6 ± 2.55 | 57.8 ± 2.16 | 54.4 ± 2.00 | 41.7 ± 1.53 | 55.4z | |
| Overall mean (Treatments) | 39.3b | 37.8b | 35.3b | 32.8b | 23.6a | ||
Overall means with same superscripts in a row (a,b,c) and in a column (v,w,x,y,z) are not significantly different (P > 0.05) (n = 6). Control, no antioxidant; MLE 300, 300 μg M. oleifera leaves phenolics; MLE 450, 450 μg M. oleifera leaves phenolics; MLE 600, 600 μg M. oleifera leaves phenolics; BHT 200, 200 μg BHT
MLE M. oleifera leaves extract; BHT Butylated hydroxyl toluene; MMb Metmyoglobin; L* Lightness; a* Redness; b* Yellowness
Table 2.
Changes in pH and Hunter Lab L*, a* and b* values of cooked pork patties incorporated with M. oleifera leaves extract (MLE) and butylated hydroxyl toluene (BHT) at different concentrations during storage (4 °C)
| Parameter | Days of storage | Treatments | Overall mean (Days) | ||||
|---|---|---|---|---|---|---|---|
| Control | MLE 300 | MLE 450 | MLE 600 | BHT 200 | |||
| pH | 0 | 6.4 ± 0.14 | 6.4 ± 0.11 | 6.3 ± 0.08 | 6.3 ± 0.11 | 6.4 ± 0.06 | 6.4x |
| 5 | 6.5 ± 0.12 | 6.4 ± 0.09 | 6.4 ± 0.13 | 6.4 ± 0.27 | 6.4 ± 0.14 | 6.4xy | |
| 10 | 6.6 ± 0.11 | 6.5 ± 0.14 | 6.5 ± 0.12 | 6.5 ± 0.34 | 6.5 ± 0.11 | 6.5yz | |
| 15 | 6.6 ± 0.13 | 6.5 ± 0.12 | 6.5 ± 0.21 | 6.5 ± 0.08 | 6.5 ± 0.17 | 6.5z | |
| Overall mean (Treatments) | 6.5 | 6.5 | 6.4 | 6.4 | 6.4 | ||
| L* | 0 | 55.9 ± 1.85 | 54.6 ± 2.11 | 54.3 ± 2.92 | 54.2 ± 3.10 | 54.1 ± 2.48 | 54.6x |
| 5 | 56.4 ± 1.99 | 55.1 ± 1.97 | 54.5 ± 2.80 | 54.4 ± 3.16 | 54.3 ± 2.67 | 55.0xy | |
| 10 | 56.7 ± 1.97 | 55.5 ± 1.82 | 54.8 ± 2.48 | 55.1 ± 3.01 | 54.7 ± 2.63 | 55.4yz | |
| 15 | 57.7 ± 2.01 | 55.7 ± 1.83 | 55.4 ± 2.63 | 55.2 ± 2.90 | 54.9 ± 2.48 | 55.8z | |
| Overall mean (treatments) | 56.7c | 55.2b | 54.8ab | 54.7ab | 54.5a | ||
| a* | 0 | 7.3 ± 0.27 | 7.3 ± 0.25 | 7.5 ± 0.16 | 7.6 ± 0.14 | 7.8 ± 0.11 | 7.5y |
| 5 | 7.1 ± 0.25 | 7.1 ± 0.20 | 7.4 ± 0.13 | 7.6 ± 0.12 | 7.7 ± 0.07 | 7.4xy | |
| 10 | 7.0 ± 0.29 | 7.0 ± 0.27 | 7.4 ± 0.11 | 7.5 ± 0.12 | 7.60 ± 0.09 | 7.3xy | |
| 15 | 6.9 ± 0.24 | 7.0 ± 0.25 | 7.3 ± 0.13 | 7.4 ± 0.09 | 7.6 ± 0.11 | 7.2x | |
| Overall mean (treatments) | 7.0a | 7.1a | 7.4ab | 7.5b | 7.7b | ||
| b* | 0 | 22.1 ± 1.45 | 22.5 ± 1.27 | 19.8 ± 0.58 | 20.2 ± 0.60 | 19.6 ± 0.35 | 20.8y |
| 5 | 22.0 ± 1.48 | 22.4 ± 1.14 | 19.7 ± 0.71 | 19.9 ± 0.74 | 19.6 ± 0.41 | 20.7xy | |
| 10 | 21.7 ± 1.59 | 22.2 ± 1.14 | 19.3 ± 0.58 | 19.9 ± 0.94 | 19.4 ± 0.35 | 20.5xy | |
| 15 | 21.2 ± 1.41 | 21.7 ± 1.37 | 19.0 ± 0.48 | 19.6 ± 0.60 | 19.0 ± 0.40 | 20.1 x | |
| Overall mean (treatments) | 21.7b | 22.2b | 19.5a | 19.9a | 19.4a | ||
Overall means with same superscripts in a row (a,b,c) and in a column (v,w,x,y,z) are not significantly different (P > 0.05) (n = 6). Control, no antioxidant; MLE 300, 300 μg M. oleifera leaves phenolics; MLE 450, 450 μg M. oleifera leaves phenolics; MLE 600, 600 μg M. oleifera leaves phenolics; BHT 200, 200 μg BHT
MLE M. oleifera leaves extract; BHT Butylated hydroxyl toluene; L* Lightness; a* Redness; b* Yellowness
Colour is an important sensory attribute, which determines the products acceptability rate. The red colour of the meat is mainly due to the myoglobin content. Myoglobin is highly susceptible to oxidation into metmyoglobin (MMb) due to lipid oxidation and microbial spoilage. The effects of addition of antioxidants and storage time on Hunter Lab colour and relative percentage of metmyoglobin in raw pork patties are presented in Table 1. Among the treatments, MLE 600 and BHT 200 had lower (P < 0.05) Hunter lab L* (lightness) values on the surface of raw patties compared to control patties. Naveena et al. (2008) reported a reduction in L* value due to addition of pomegranate rind powder extract in chicken patties, whereas Carpenter et al. (2007) did not see any alteration in L* and b* (yellowness) value of raw pork patties due to addition of grape seed extract or bearberry. However, Rojas and Brewer (2008) have reported that L* value increased initially and remained constant later due to incorporation of natural antioxidants.
Redness values of both raw and cooked pork patties were affected by addition of antioxidants storage time and their interactions. The significant decrease (P < 0.001) in a* value during storage indicates the change in color from red to brown, which is due to the formation of metmyoglobin. Progressive reduction in the redness value of pork patties was also reported by Carpenter et al. (2007) during refrigerated display condition. Higher (P < 0.001) a* values of MLE 600 and BHT 200 compared to control exhibited the evidence that colour was stabilized by the added antioxidants. Higher a* values due to addition of pomegranate rind powder extract in chicken patties was reported by Naveena et al. (2008). However, McBride et al. (2007) reported that rosemary extract (0.1 %) did not improve meat redness in aerobically stored beef samples compared with BHA/BHT or vitamin E incorporated samples. Treated raw pork samples exhibited a gradual decrease in a* values over storage period, whereas control samples showed around 20 % reduction in a* values at the end of 3 days of storage. Among the treatments, MLE 600 and BHT 200 exhibited a* value of around 8.5 even on 9th day of storage, whereas the a* value of 8.5 was reached even at 5th day of storage in control group. Cooked pork patties had comparatively lower redness value than raw patties. As internal cooking temperature increases, percent myoglobin denaturation increases with reduction in redness value (John et al. 2005). Similarly, McCarthy et al. (2001) noticed Hunter a* values ranging from 5 to 8 for raw pork and 3–5 in cooked pork patties.
Both cooked and raw patties showed gradual reduction in b* value during storage. Among the treatments, MLE 450, MLE 600 and BHT 200 had lower (P < 0.01) Hunter lab b* (yellowness) values on the surface of both raw and cooked patties compared to control and MLE 300 patties. Similarly, Rojas and Brewer (2008) noticed decrease in b* value of beef patties containing natural antioxidants.
The effect of MLE and BHT treatment on MMb formation during storage is presented in Table 1. Both control and treated samples, upon storage showed an increase (P < 0.001) in MMb levels (from 13.13 % to 55.37 %). The MMb values confirmed the results of instrumental colour values. O’ Sullivan et al. (2004) observed a negative correlation between a* values and metmyoglobin content during storage. A non significant reduction (P > 0.05) in mean MMb value with increase in the concentration of MLE was observed. According to An et al. (2004), raw beef patties stored in aerobic packaging showed a rapid colour deterioration that cannot be stabilized by the sole addition of ascorbate, green tea extract (GTE) or grape seed extract. Similarly, Jo et al. (2003) reported that a high dose of green tea ethanolic extract (1,000 mg GTE/kg) can delay the loss of redness in raw pork patties throughout storage. However, Formanek et al. (2003) observed better a color retention in rosemary extracts incorporated irradiated minced beef as evidenced by decreased metmyoglobin concentration and increased oxymyoglobin values during storage.
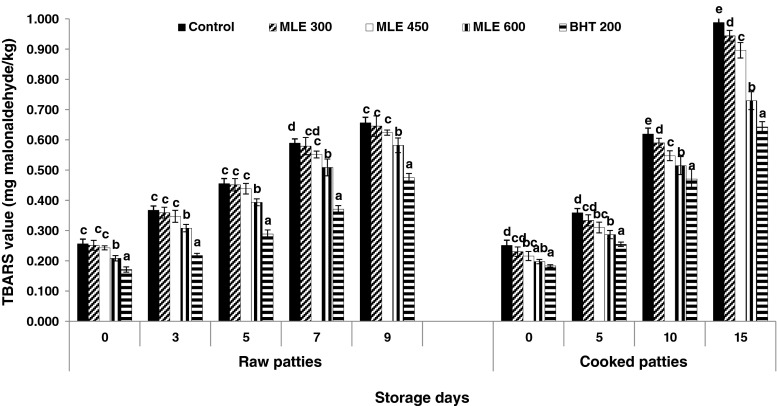
The influence of inclusion of MLE and BHT on lipid oxidation in terms of TBARS values in raw pork patties stored for 9 days and cooked pork patties stored for 15 days at 4 °C is presented in Fig. 1. The TBARS values at day 0 indicated a noticeable degree of initial lipid oxidation in all groups. Meat products can exhibit high oxidation levels when meat was not processed under vacuum. In general, increase (P < 0.05) in TBARS values in all the samples during storage indicate persistent formation of aldehydes in the pork products. However, the TBARS values for all antioxidant containing treatments were consistently lower than that for the control. Additionally, the TBARS values were affected (P < 0.001) through interactive effects of duration of storage and antioxidants treatments. Among the treatments, MLE 600 was more (P < 0.05) effective in reducing lipid oxidation compared to MLE 450 and MLE 300, but less effective compared to BHT 200 in both raw and cooked pork patties. In case of raw patties, there was no significant difference among control, MLE 300 and MLE 450 except on 7th day, where MLE 450 showed significantly lower TBARS value than control. However, in case of cooked patties, MLE 450 showed significantly lower TBARS value than control from 0 day onwards and from 10 day of storage, even MLE 300 had lower TBARS value than control. Antioxidants are believed to break free radical chains of oxidation by donation of hydrogen from the phenolic groups, thereby terminate free radical chain reactions and forming a stable end product (Sherwin 1998). These results are in agreement with those obtained by Juntachote et al. (2006) and Naveena et al. (2007), who reported the effectiveness of both synthetic and natural antioxidants in controlling lipid oxidation in meat products. McBride et al. (2007) reported that rosemary extract (0.1 %) was more effective in controlling lipid oxidation in aerobically stored beef samples compared with BHA/BHT or vitamin E incorporated samples.
Fig. 1.
Changes in TBARS value (mg malonaldehyde/kg) of raw and cooked pork patties incorporated with M. oleifera leaves extract (MLE) and butylated hydroxyl toluene (BHT) at different concentrations during storage (4 °C) (n = 6). Control, no antioxidant; MLE 300, 300 μg M. oleifera leaves phenolics; MLE 450, 450 μg M. oleifera leaves phenolics; MLE 600, 600 μg M. oleifera leaves phenolics; BHT 200, 200 μg BHT. Standard error bars are indicated. Bars bearing different letters are significantly different (P < 0.05) between treatment within a storage period. MLE M. oleifera leaves extract; BHT Butylated hydroxyl toluene; MMb Metmyoglobin; TBARS Thiobarbituric acid reactive substances
The Table 3 shows the effects of antioxidants and storage time on microbial quality of raw and cooked pork patties. Aerobic plate count and psychrotrophic count increased (P < 0.001) throughout storage, in both control and treated samples. Addition of MLE did not reduce (P > 0.05) the microbial load in both raw and cooked meat patties. This might be due to currently used doses of MLE, which may not be sufficient to impart an antimicrobial effect on growth of aerobic and psychrotrophic bacteria. Davidson (1997) reported that the efficacy of antibacterial component in foods, is expected to be often much lower than in vivo, since the active components could bind with food ingredients like proteins and fats and thus exhibit decreased efficiency. Carpenter et al. (2007) also reported no effect or improvement in the microbial status of grape seed extract or bearberry incorporated pork patties relative to their respective controls. The aerobic plate counts of above 6 log/g were found in both control and treated raw pork patties on day 9. This is in accordance with Mitsumoto et al. (2005) that the shelf life of raw meat is limited by microbial spoilage and usually have a shelf life of around 7 days in refrigeration depending on hygiene and preservation conditions.
Table 3.
Changes in aerobic plate count (APC-log CFU/g) and psychrophilic count (PPC-log CFU/g) of raw and cooked pork patties incorporated with M. oleifera leaves extract (MLE) and butylated hydroxyl toluene (BHT) at different concentrations during storage (4 °C)
| Parameter | Days of storage | Treatments | Overall mean (days) | ||||
|---|---|---|---|---|---|---|---|
| Control | MLE 300 | MLE 450 | MLE 600 | BHT 200 | |||
| APC (raw patties) | 0 | 3.4 ± 0.17 | 3.3 ± 0.15 | 3.2 ± 0.20 | 3.2 ± 0.14 | 3.2 ± 0.16 | 3.2w |
| 3 | 4.4 ± 0.15 | 4.6 ± 0.15 | 4.3 ± 0.22 | 4.2 ± 0.26 | 4.3 ± 0.20 | 4.3x | |
| 5 | 5.3 ± 0.25 | 5.2 ± 0.24 | 5.1 ± 0.28 | 5.1 ± 0.28 | 5.2 ± 0.16 | 5.2y | |
| 7 | 6.0 ± 0.12 | 5.9 ± 0.32 | 5.8 ± 0.24 | 5.7 ± 0.18 | 5.8 ± 0.28 | 5.9y | |
| 9 | 7.1 ± 0.28 | 6.9 ± 0.29 | 6.7 ± 0.34 | 6.6 ± 0.28 | 6.8 ± 0.32 | 6.8z | |
| Overall mean (Treatments) | 5.2 | 5.1 | 5.0 | 5.0 | 5.1 | ||
| PPC (raw patties) | 0 | 2.7 ± 0.14 | 2.7 ± 0.20 | 2.7 ± 0.08 | 2.6 ± 0.16 | 2.7 ± 0.09 | 2.7w |
| 3 | 3.8 ± 0.22 | 3.7 ± 0.28 | 3.6 ± 0.24 | 3.5 ± 0.20 | 3.8 ± 0.17 | 3.7x | |
| 5 | 4.3 ± 0.25 | 4.2 ± 0.32 | 4.1 ± 0.30 | 4.0 ± 0.22 | 4.1 ± 0.22 | 4.1xy | |
| 7 | 4.5 ± 0.19 | 4.5 ± 0.25 | 4.4 ± 0.28 | 4.4 ± 0.19 | 4.57 ± 0.28 | 4.4yz | |
| 9 | 4.8 ± 0.26 | 4.7 ± 0.24 | 4.6 ± 0.42 | 4.6 ± 0.28 | 4.8 ± 0.24 | 4.7z | |
| Overall mean (Treatments) | 4.0 | 4.0 | 3.9 | 3.8 | 4.0 | ||
| APC (Cooked patties) | 0 | 2.6 ± 0.05 | 2.7 ± 0.09 | 2.7 ± 0.20 | 2.7 ± 0.17 | 2.7 ± 0.20 | 2.7w |
| 5 | 3.5 ± 0.17 | 3.4 ± 0.15 | 3.3 ± 0.16 | 3.5 ± 0.28 | 3.7 ± 0.28 | 3.5x | |
| 10 | 4.1 ± 0.19 | 4.0 ± 0.21 | 4.0 ± 0.25 | 3.9 ± 0.26 | 4.0 ± 0.27 | 4.0xy | |
| 15 | 4.7 ± 0.21 | 4.7 ± 0.27 | 4.6 ± 0.38 | 4.5 ± 0.32 | 4.7 ± 0.33 | 4.6z | |
| Overall mean (Treatments) | 3.7 | 3.7 | 3.6 | 3.6 | 3.8 | ||
| PPC (Cooked patties) | 0 | 1.5 ± 0.07 | 1.5 ± 0.08 | 1.4 ± 0.10 | 1.4 ± 0.06 | 1.6 ± 0.08 | 1.5w |
| 5 | 1.8 ± 0.04 | 1.9 ± 0.07 | 1.8 ± 0.11 | 1.9 ± 0.08 | 1.9 ± 0.10 | 1.9w | |
| 10 | 2.6 ± 0.08 | 2.5 ± 0.11 | 2.5 ± 0.18 | 2.4 ± 0.17 | 2.5 ± 0.06 | 2.5x | |
| 15 | 3.0 ± 0.09 | 3.0 ± 0.18 | 3.0 ± 0.21 | 2.9 ± 0.22 | 3.0 ± 0.22 | 3.0y | |
| Overall mean (Treatments) | 2.2 | 2.2 | 2.2 | 2.1 | 2.2 | ||
Overall means with same superscripts in a column (v,w,x,y,z) are not significantly different (P > 0.05) (n = 6). Control, no antioxidant; MLE 300, 300 μg M. oleifera leaves phenolics; MLE 450, 450 μg M. oleifera leaves phenolics; MLE 600, 600 μg M. oleifera leaves phenolics; BHT 200, 200 μg BHT
MLE M. oleifera leaves extract; BHT Butylated hydroxyl toluene; APC Aerobic plate count; PPC Psychrophilic count; CFU Colony forming unit
No change (P > 0.05) in sensory attributes of cooked pork patties due to incorporation of MLE or BHT was observed (Table 4). The addition of MLE and BHT did not produce appreciable colour, odour, flavour or texture changes and all the products were equally acceptable as evidenced by the overall acceptability scores. Devatkal et al. (2010) also reported that incorporation pomegranate rind and seed powder extracts did not affect the colour, flavourand overall acceptability of goat meat patties. However, Mitsumoto et al. (2005) found that tea catechins caused certain grey discolouration in cooked beef and chicken patties.
Table 4.
Sensory attributes of cooked pork patties incorporated with M. oleifera leaves extract (MLE) and butylated hydroxyl toluene (BHT) at different concentrations
| Parameters | Treatments | ||||
|---|---|---|---|---|---|
| Control | MLE 300 | MLE 450 | MLE 600 | BHT | |
| Appearance | 6.7 ± 0.13 | 6.6 ± 0.16 | 6.7 ± 0.13 | 6.6 ± 0.16 | 6.7 ± 0.13 |
| Flavour | 6.4 ± 0.18 | 6.3 ± 0.16 | 6.4 ± 0.18 | 6.4 ± 0.18 | 6.4 ± 0.18 |
| Texture | 6.6 ± 0.16 | 6.5 ± 0.16 | 6.6 ± 0.16 | 6.4 ± 0.18 | 6.6 ± 0.16 |
| Juiciness | 6.4 ± 0.18 | 6.5 ± 0.16 | 6.5 ± 0.16 | 6.4 ± 0.18 | 6.4 ± 0.18 |
| Overall acceptability | 6.5 ± 0.16 | 6.4 ± 0.18 | 6.5 ± 0.16 | 6.5 ± 0.16 | 6.4 ± 0.18 |
(n = 6). Control, no antioxidant; MLE 300, 300 μg M. oleifera leaves phenolics; MLE 450, 450 μg M. oleifera leaves phenolics; MLE 600, 600 μg M. oleifera leaves phenolics; BHT 200, 200 μg BHT
MLE M. oleifera leaves extract; BHT Butylated hydroxyl toluene
Conclusions
The present study showed that aqueous extract of M. oleifera leaves has substantial amounts of phenolic compounds with significant free radical scavenging activity and reducing power. The inclusion of MLE at a level of 600 mg equivalent phenolics/100 g meat inhibited (P < 0.05) lipid oxidation in raw and cooked pork patties to a much greater extent compared to control during refrigerated storage. However, synthetic antioxidant BHT had the best inhibitory effect on lipid oxidation in both raw and cooked pork patties compared to all other treatments. The eating quality of cooked pork patties was unaffected by addition of MLE. These results suggest that phytochemicals extracted from natural herbs like M. oleifera leaves may be used as a potential source of antioxidants to protect meat products against oxidative rancidity without any adverse effects on sensory attributes.
Acknowledgments
The authors wish to thank Indian Council of Agricultural Research (ICAR), India for financial support and Director, National Research Centre on Meat for providing necessary facilities to carry out the research work.
References
- An BJ, Kwak JH, Son JH, Park JM, Lee JY, Jo C. Biological and anti-microbial activity of irradiated green tea polyphenols. Food Chem. 2004;88:549–555. doi: 10.1016/j.foodchem.2004.01.070. [DOI] [Google Scholar]
- Annuk H, Hirmo S, Turi E, Mikelsaar M, Arak E, Wadström T. Effect on cell surface hydrophobicity and susceptibility of Helicobacter pylori to medicinal plant extracts. FEMS Microbiol Lett. 1999;172:41–45. doi: 10.1111/j.1574-6968.1999.tb13447.x. [DOI] [PubMed] [Google Scholar]
- Asghar A, Gray JI, Buckley DJ, Pearson AM, Booren AM. Perspectives on warmed-over flavour. Food Tech. 1988;42:102–108. [Google Scholar]
- Carpenter R, O’Grady MN, O’Callaghan YC, O’Brien NM, Kerry P. Evaluation of the antioxidant potential of grape seed and bearberry extracts in raw and cooked pork. Meat Sci. 2007;76:604–610. doi: 10.1016/j.meatsci.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Davidson PM. Chemical preservatives and natural antimicrobial compounds. In: Doyle MP, Beuchat LR, Montville TJ, editors. Food microbiology fundamentals and frontiers. Washington: ASM Press; 1997. [Google Scholar]
- Devatkal SK, Narsaiah K, Borah A. Anti-oxidant effect of extracts of kinnow rind, pomegranate rind and seed powders in cooked goat meat patties. Meat Sci. 2010;85:155–159. doi: 10.1016/j.meatsci.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Escarpa A, Gonzalez MC. Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Anal Chim Acta. 2001;427:119–127. doi: 10.1016/S0003-2670(00)01188-0. [DOI] [Google Scholar]
- Formanek Z, Lynch A, Galvin K, Farkas J, Kerry JP. Combined effects of irradiation and the use of natural antioxidants on the shelf life stability of over wrapped minced beef. Meat Sci. 2003;63:433–440. doi: 10.1016/S0309-1740(02)00063-3. [DOI] [PubMed] [Google Scholar]
- Garrido MD, Auqui M, Marti N, Linares MB. Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT-Food Sci Tech. 2011;44:2238–2243. doi: 10.1016/j.lwt.2011.07.003. [DOI] [Google Scholar]
- Gill CO (1983) Meat spoilage and evaluation of the potential storage life of fresh meat. J Food Prot 46:444–452 [DOI] [PubMed]
- Gordon MF. The mechanism of antioxidant action in vitro. In: Hudson BJF, editor. Food antioxidants. London: Elsevier Applied Science; 1990. [Google Scholar]
- Hazra S, Biswas S, Bhattacharyya D, Das SK, Khan A. Quality of cooked ground buffalo meat treated with the crude extracts of Moringa oleifera (Lam.) leaves. J Food Sci Technol. 2012;49:240–224. doi: 10.1007/s13197-011-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICMSF . International Commission of Microbiological Specifications for Foods. In: Elliott RP, Clark DS, Lewis KH, Lundbeck H, Olson JC, Simonsen B, editors. Microorganisms in foods. Their significance and methods of enumeration, Vol 1. Toronto: University of Toronto Press; 1983. [Google Scholar]
- Iqbal S, Bhanger MI. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J Food Compos Anal. 2006;19:544–551. doi: 10.1016/j.jfca.2005.05.001. [DOI] [Google Scholar]
- Jo C, Son JH, Sohn CB, Byun MW. Functional properties of raw and cooked pork patties with added irradiated, freeze-dried green tea leaf extract powder during storage at 4 °C. Meat Sci. 2003;64:13–17. doi: 10.1016/S0309-1740(02)00131-6. [DOI] [PubMed] [Google Scholar]
- John L, Cornforth D, Carpenter CE, Sorheim O, Pettee BC, Whittier DR. Color and thiobarbituric acid values of cooked top sirloin steaks packaged in modified atmospheres of 80 % oxygen, or 0.4 % carbon monoxide, or vacuum. Meat Sci. 2005;69:441–449. doi: 10.1016/j.meatsci.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Juntachote T, Berghofer E, Siebenhandl S, Baue F. The antioxidative properties of Holy basil and Galangal in cooked ground pork. Meat Sci. 2001;72:446–456. doi: 10.1016/j.meatsci.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Keeton JT. Effect of fat and NaCl/phosphate levels on the chemical and sensory properties of pork patties. J Food Sci. 1983;41:878–881. doi: 10.1111/j.1365-2621.1983.tb14921.x. [DOI] [Google Scholar]
- Kumudavally KV, Phanindrakumar HS, Tabassum A, Radhakrishna K, Bawa AS. Green tea- A potential preservative for extending the shelf life of fresh mutton at ambient temperature (25 ± 2 °C) Food Chem. 2008;107:426–433. doi: 10.1016/j.foodchem.2007.08.045. [DOI] [Google Scholar]
- McBride NTM, Hogan SA, Kerry JP. Comparative addition of rosemary extract and additives on sensory and antioxidant properties of retail packaged beef. Int J Food Sci Tech. 2007;42:1201–1207. doi: 10.1111/j.1365-2621.2006.01342.x. [DOI] [Google Scholar]
- McCarthy TL, Kerry JP, Kerry JF, Lynch PB, Buckley DJ. Evaluation of the antioxidant potential of natural food/plant extracts as compared with synthetic antioxidants and vitamin E in raw and cooked pork patties. Meat Sci. 2001;57:45–52. doi: 10.1016/S0309-1740(00)00129-7. [DOI] [PubMed] [Google Scholar]
- Mitsumoto M, O’grady MN, Kerry JP, Buckley DJ. Addition of tea catechins and vitamin C on sensory evaluation, colour and lipid stability during chilled storage in cooked or raw beef and chicken patties. Meat Sci. 2005;69:773–779. doi: 10.1016/j.meatsci.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Nair AGR, Subramanian SS. Pigments of the flowers of Moringa pterygosperma. Curr Sci. 1962;31:155–156. [Google Scholar]
- Naveena BM, Sen AR, Vaithiyanathan S, Muthukumar M, Babji Y. Effect of honey and vitamin C on quality of microwave cooked chicken patties. J Food Sci Technol. 2007;44:505–508. [Google Scholar]
- Naveena BM, Sen AR, Vaithiyanathan S, Babji Y, Kondaiah N. Comparative efficacy of pomegranate juice, pomegranate rind powder extract and BHT as antioxidants in cooked chicken patties. Meat Sci. 2008;80:1304–1308. doi: 10.1016/j.meatsci.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Negi PS, Jayaprakasha GK. Antioxidant and antibacterial activities of Punica granitum peel extracts. J Food Sci. 2003;68:1473–1477. doi: 10.1111/j.1365-2621.2003.tb09669.x. [DOI] [Google Scholar]
- O’ Sullivan A, O’ Sullivan K, Galvin K, Moloney AP, Troy DJ, Kerry JP. Influence of concentrate composition and forage type on retail packaged beef quality. J AniSci. 2004;82:2384–2391. doi: 10.2527/2004.8282384x. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction – antioxidant activities of products of browning reaction prepared from glucoamine. Jpn J Nutri. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Reddy V, Urooj A, Kumar A. Evaluation of antioxidant activity of some plant extracts and their application in biscuits. Food Chem. 2005;90:317–321. doi: 10.1016/j.foodchem.2004.05.038. [DOI] [Google Scholar]
- Rojas MC, Brewer MS. Effect of natural antioxidants on oxidative stability of frozen vacuum packaged beef and pork. J Food Qual. 2008;31:173–185. doi: 10.1111/j.1745-4557.2008.00196.x. [DOI] [PubMed] [Google Scholar]
- Salah N, Miller N, Paganga G, Tijburg L, Bolwell GP, Rice-Evans CA. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch Biochem Biophys. 1995;322:339–346. doi: 10.1006/abbi.1995.1473. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Gupta MP. Studies on seed fat composition of Moringaceae family. FetteSeifen Anstrichm. 1970;72:6–10. doi: 10.1002/lipi.19700720103. [DOI] [Google Scholar]
- Shahidi F, Ke PJ, Zhao X, Yang Z, Wanasundara PK (1992) Antioxidative activity of green and black tea in meat model systems. In Clermont-Ferrand, Proc. Thirty seventh International Congress of Meat Science and Technology, France. pp. 599–602
- Sherwin ER. Oxidation and antioxidants in fat and oil processing. J Am Oil Chem Soc. 1998;55:809–814. doi: 10.1007/BF02682653. [DOI] [Google Scholar]
- Singh RP, Murthy KNC, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in-vitro models. J Agric Food Chem. 2002;50:81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- Tang S, Kerry JP, Sheehan D, Buckley DJ, Morrissey PA. Antioxidative effect of added tea catechins on susceptibility of cooked red meat poultry and fish patties to lipid oxidation. Food Res Int. 2001;34:651–657. doi: 10.1016/S0963-9969(00)00190-3. [DOI] [Google Scholar]
- Warris PD. The extraction of haem pigments from fresh meat. J Food Tech. 1979;14:75–80. doi: 10.1111/j.1365-2621.1979.tb00849.x. [DOI] [Google Scholar]
- Warriss PD (2010) The chemical composition and structure of the meat. In Meat science: an introductory text, CABI Publishing, Cambridge
- Witte VC, Krauze GF, Bailey ME. A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. J Food Sci. 1970;35:582–585. doi: 10.1111/j.1365-2621.1970.tb04815.x. [DOI] [Google Scholar]