Abstract
Gentamicin, an important aminoglycoside, is used to treat many types of bacterial infections, particularly those caused by Gram-negative organisms. It is a nephrotoxic antibiotic, which causes acute tubular necrosis, and its toxicity remains a major problem in clinical use. This study investigates the effect of pomegranate seed oil (PSO) on gentamicin-induced nephrotoxicity in adult male rats. Animals were randomly divided into four groups. Group one was treated with saline (1 ml/kg, i.p.), group 2 received gentamicin 80 mg/kg/day for 6 days and groups 3 and 4 received PSO 0.32 and 0.64 mg/kg/day i.p. respectively, 1 h before gentamicin. Serum urea, creatinine levels, urinary glucose and protein concentrations were evaluated as the markers of acute renal failure. Renal antioxidant indicators such as thiobarbituric acid-reactive substance (TBARS), and total thiol contents, were also determined. A significant elevation of serum creatinine and urea levels as well as urine glucose and protein concentrations were observed in gentamicin treated group. Gentamicin also caused a significant decrease in total thiol content and a significant increase in TBARS levels in kidney homogenate samples. PSO pretreatment resulted in a significant and dose-dependent decrease in serum creatinine and urea levels as well as urine glucose and protein concentrations when compared with gentamicin treated alone. PSO also significantly reversed the gentamicin-induced depletion in total thiol content and elevation in TBARS in kidney homogenate samples. The results of the present study indicate that PSO clearly attenuated gentamicin-induced nephrotoxicity, but elucidation of the mechanism(s) of this protection needs more investigation.
Keywords: Gentamicin, Nephrotoxicity, Pomegranate seed oil, Punica granatum, Lipid peroxidation
Introduction
Gentamicin (GM) is an important aminoglycoside antibiotic. Due to its high nephrotoxicity potential, the drug is only used in serious life-threatening gram-negative infections. It causes inhibition of protein synthesis, resulting in proximal tubule cell necrosis in renal cells in 13–30 % of treated patients. This mechanism specifically causes proximal tubule cells necrosis, resulting in acute tubular necrosis which can lead to acute renal failure (Sundin et al. 2001).
Pomegranate, Punica granatum L., is an ancient medicinal food plant which natively grows from the Himalayas in northern India to Middle East, but has also been cultivated and naturalized in many other places including Mediterranean region, Southeast Asia, tropical Africa, and American Southwest (Jurenka 2008). In addition to extensive uses of pomegranate in folk medicine of many cultures, pharmacological studies showed that pomegranate fruit preparations have antioxidant and anti-inflammatory (Lansky and Newman 2007) antimicrobial (Braga et al. 2005; Menezes et al. 2006) anticancer, and chemopreventive (Adams et al. 2006) effects.
Plants have formed the basis of sophisticated traditional medicine systems which have given rise to some important drugs still in use today. The search for new drug molecules, nowadays, guides the researchers across the globe toward natural products as an alternative source and class of medicinal compounds (Gurib-Fakim 2006). The main goal of present study was to evaluate the effect of pomegranate seed oil on gentamicin-induced nephrotoxicity in experimental animals as a potential new nephroprotective agent.
Materials and methods
DTNB (2,2′-dinitro-5,5′-dithiodibenzoic acid), TBA (2-thiobarbituric acid), n-butanol, NaOH (sodium hydroxide), NaCl (sodium chloride), Na2EDTA (ethylenediaminetetraacetic acid disodium salt), Trizma base (Tris (hydroxymethyl) aminomethane), phosphoric acid, HCl (hydrochloric acid), KCl (potassium chloride), ether, and TMP (tetramethoxypropane) were purchased from Merck (Darmstadt, Germany). Gentamicin was obtained from DarouPakhsh Co. (Tehran, Iran). Pomegranate seed oil (d = 0.81 g/ml at 25 °C) was a kind gift from Urom Narin Co. (Uromeya, I. R. Iran).
Adult male Wistar rats (Animal House, School of Medicine, Mashhad, I. R. Iran), weighing 250–300 g, were used for all experiments. Animals were housed in a pathogen-free facility on a 12-hour light/dark schedule and with ad libitum access to food and water. All animal procedures were approved by the university ethics committee and were in compliance with national laws and the National Institutes of Health guidelines for the use and care of laboratory animals. After acclimatization, animals were randomly divided into four groups (six each) and individually put in the metabolic cages. Group 1 (control group) was treated with saline (1 ml/kg, i.p.). Group 2 received gentamicin 80 mg/kg/day for 6 days (gentamicin sulfate powder, dissolved in saline to produce 80 mg/ml stock solution and about 0.25–0.3 ml was used for each animal) and groups 3 and 4 received pomegranate seed oil 0.32 mg/kg and 0.64 mg/kg, i.p. respectively, 1 h before gentamicin 80 mg/kg. All procedures were carried out between 10 and 12 AM. Animals were killed 24 h after the last injection of gentamicin using either anaesthesia; blood samples were taken out by cardiac puncture for measuring the level of serum urea and creatinine. 24-h urine samples, for measuring glucose and protein concentration were also collected, before scarifying animals. Both kidneys were removed, one was homogenized in cold KCl solution (1.5 %, pH = 7) to give a 10 % homogenate suspension and was used for measuring malondialdehyde (MDA) and thiol content, and the other one was fixed in 10 % formalin and sectioned for histopathological studies.
Urea concentration was determined colorimetrically using Autoanalyzer (Technicon RA-1000, England) and urea kit (Man Lab Company, Tehran, I. R. Iran). Creatinine concentration was measured by the Jaffe’s method (Masson et al. 1981). Glucose concentration was estimated by the enzymatic assay (glucose oxidase) and protein concentration was measured by the turbidimetric method (Lott and Turner 1975; Mc Elderry et al. 1982). The lipid peroxidation level of the kidney tissue was measured as malondialdehyde, which is the end product of lipid peroxidation and reacts with TBA as a thiobarbituric acid reactive substance (TBARS) to produce a red-colored complex which has peak absorbance at 532 nm (Fernandez et al. 1997). Briefly, 3 ml phosphoric acid (1 %) and 1 ml TBA (0.6 %) were added to 0.5 ml of homogenate in a centrifuge tube, and the mixture was heated for 45 min in a boiling water bath. After cooling, 4 ml of n-butanol was added to the mixture, vortexed for 1 min, and centrifuged at 20,000 rpm for 20 min. The organic layer was transferred to a fresh tube and its absorbance was measured at 532 nm. The standard curve of MDA was constructed over the concentration range of 0–40 μM (Hosseinzadeh et al. 2005). Total SH groups were measured using DTNB as the reagent. This reagent reacts with the SH groups to produce a yellow-colored complex with peak absorbance at 412 nm. Briefly, 1 ml Tris–EDTA buffer (pH = 8.6) was added to 50 μl kidney homogenate in 2 ml cuvettes and sample absorbance was read at 412 nm against Tris–EDTA buffer alone (A1). Then 20 μl DTNB reagent (10 mM in methanol) was added to the mixture, and after 15 min (stored in laboratory temperature), the sample absorbance was read again (A2). The absorbance of DTNB reagent was also read as a blank (B). Total thiol concentration (mM) was calculated from the following equation (Sedlak and Lindsay 1968):
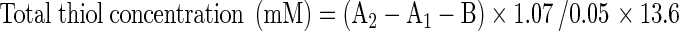 |
In a preliminary study, PSO alone did not significantly modify the biochemical parameters compared to the control group (data not shown).
Statistical analysis
Data were expressed as mean ± SEM. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey–Kramer post hoc test for multiple comparisons. The p-values less than 0.05 were considered to be statistically significant. The experiment was carried out as triplicate.
Results and discussion
As shown in Fig. 1, gentamicin caused a significant increase in the level of MDA (11.4 ± 1.49, p < 0.01), creatinine (1.7 ± 0.33, p < 0.001), protein (3.8 ± 0.16, p < 0.001), blood urea (88.7 ± 8.55, p < 0.001), urinary glucose (35.0 ± 4.14, p < 0.001) and a significant decrease in total thiol content (761.6 ± 69.85, p < 0.001) in kidney homogenate samples as compared with the control group. In treated group with 0.64 mg/kg PSO the concentrations of MDA (7.4 ± 0.41, p < 0.05), creatinine (0.93 ± 0.056, p < 0.01), protein (0.18 ± 0.054, p < 0.001) and blood urea (59.8 ± 2.65, p < 0.001) were significantly decreased compared with the gentamicin treated group, but thiol concentration (1197.1 ± 145.85, p < 0.05) showed a significant increase compared with gentamicin treated group. In treated group with 0.32 mg/kg PSO, the concentration of urinary protein (0.57 ± 0.210, p < 0.001) and glucose (15.7 ± 0.85, p < 0.001) revealed a significant decrease compared to gentamicin treated group. Although the function of kidneys in GM-treated group was different from PSO-treated and control groups, they did not show any significant differences in histopathological examinations (data not shown).
Fig. 1.
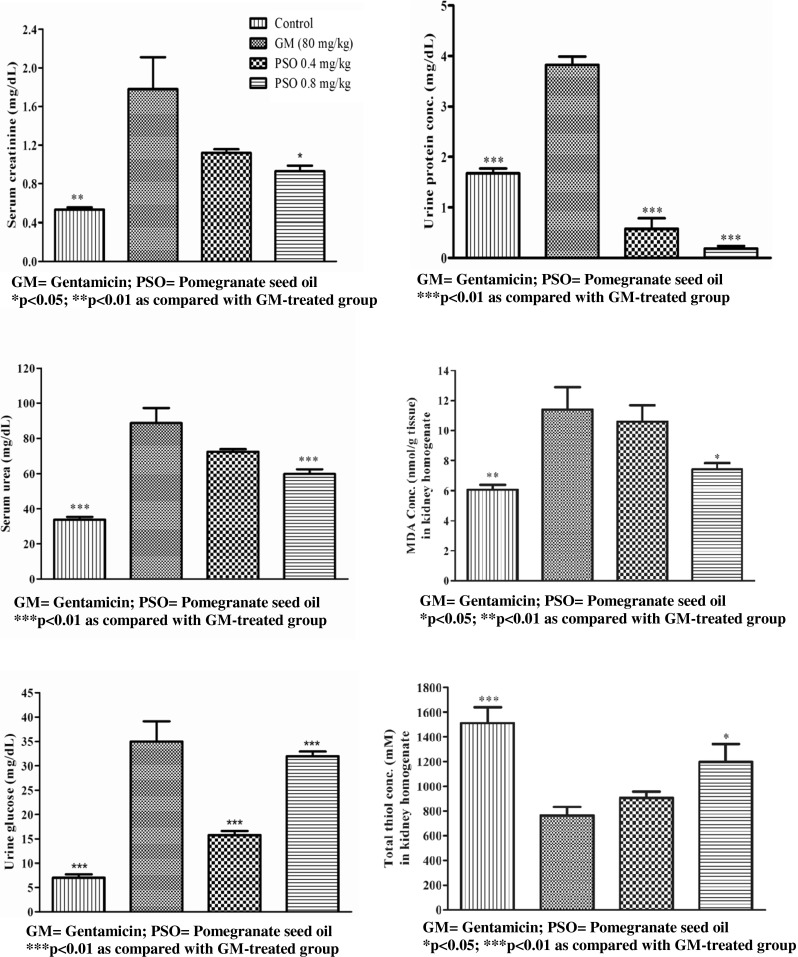
Effect of pomegranate seed oil on biochemical parameters in animals treated with gentamicin and pomegranate seed oil (PSO) (n = 6). Values are mean ± SEM
It is believed that natural compounds and their derivatives represent a source of potential chemotherapeutic agents. Dietary supplementation with these products rich in antioxidants is associated with inhibition of toxicity of many chemicals (Gurib-Fakim 2006). The results obtained in this study suggest that PSO has an overall protective effect against gentamicin-induced nephrotoxicity in rat model. The observed protective effects can be attributed to the antioxidant properties of PSO that has been shown in our previous study (Boroushaki et al. 2010). This study showed that gentamicin with dose of 80 mg/kg could induce renal dysfunction as revealed by increased urinary excretion of glucose and protein, and elevated levels of serum urea and creatinine. These data agreed with our previous findings (Boroushaki and Sadeghnia 2009). Also, the increase in MDA level and decrease in total thiol contents suggest enhanced oxidative stress causing tissue damage and renal functional failure in GM-treated animals. On the other hand, PSO pretreatment resulted in a significant improvement in the renal antioxidant status.
Sulfhydryl (SH) groups are highly reactive constituents of protein and non-protein molecules (e.g., glutathione or thioredoxin), and they participate in important biochemical and metabolic processes such as redox signaling, detoxification mechanisms, maintenance of protein systems, and function of metabolic enzymes. They are also important scavengers of oxygen-derived free radicals (Ziegler 1985). In this study, the thiol content, which is well known to be depleted following the toxicity of many nephrotoxins, was measured as an indicator of GM-induced nephrotoxicity and PSO pretreatment resulted in a significant improvement.
PSO is a rich source of conjugated fatty acids of which punicic acid is the most common (Kaufman and Wiesman 2007). Polyphenolic compounds are also present in the seed oil of the pomegranate (Kaufman and Wiesman 2007). These components have antioxidant and anti-inflammatory activities by inhibiting pro-inflammatory enzymes and expression of pro-inflammatory cytokines (Adams et al. 2006; Rasheed et al. 2009). PSO has also been shown in experimental studies to enhance B-cell function in vivo (Yamasaki et al. 2006) to suppress proliferation of several different tumor cell lines in vitro (Kim et al. 2002; Kawaii and Lansky 2004; Khan et al. 2007), and colon carcinogenesis in rats (Kohno et al. 2004). Pomegranate seed also contains coniferyl 9-O-[beta-D-apiofuranosyl(1–6)]-O-beta-Dglucopyranoside, sinapyl 9-O-[beta-D-apiofuranosyl (1–6)]-O-beta-D-glucopyranoside, sterols (daucosterol, beta-sitosterol), and hydroxybenzoic acids (gallic, ellagic and its derivatives) which exhibited antioxidant activity and decreased lipid peroxidation (Lansky and Newman 2007). Vivancos and Moreno (2005) showed that beta-sitosterol reverts impaired glutathione/oxidized glutathione ratio and modulates antioxidant enzyme response in RAW 264.7 macrophage.
Conclusion
In conclusion, the results of this study showed that PSO clearly attenuated GM-induced nephrotoxicity, via; a) improving kidney function by reducing urinary protein and glucose, b) reducing serum urea and creatinine, c) decreasing MDA, as an indicator of lipid peroxidation and d) increasing thiol content, as a protecting factor, but explanation of these mechanisms need further investigations.
Acknowledgments
This investigation was financially supported by the Vice Presidency in Research, Mashhad University of Medical Sciences, Mashhad, Iran.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- Adams L, Seeram NP, Aggarwal BB. Pomegranate juice, total pomegranate tannins and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54:980–985. doi: 10.1021/jf052005r. [DOI] [PubMed] [Google Scholar]
- Boroushaki MT, Sadeghnia HR. Protective effect of safranal against gentamicin-induced nephrotoxicity in rat. Iran J Med Sci. 2009;34:285–288. [Google Scholar]
- Boroushaki MT, Sadeghnia HR, Banihasan M. Protective effect of pomegranate seed oil on hexacholorobutadiene -induced nephrotoxicity in rat. J Ren Fail. 2010;32:612–617. doi: 10.3109/08860221003778056. [DOI] [PubMed] [Google Scholar]
- Braga LC, Shupp JW, Cummings C. Pomegranate extract inhibits staphylococcus aureus growth and subsequent enterotoxin production. J Ethnopharmacol. 2005;96:335–339. doi: 10.1016/j.jep.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Fernandez J, Perez-Alvarez JA, Fernandez-lopez JA. Thiobarbituric acid test for monitoring lipid oxidation in meat. J Food Chem. 1997;99:345–353. doi: 10.1016/S0308-8146(96)00114-8. [DOI] [Google Scholar]
- Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. J Mol Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H, Sadeghnia HR, Ziaee T. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion induced oxidative damage in rats. J Pharm Sci. 2005;8:387–393. [PubMed] [Google Scholar]
- Jurenka J. Therapeutic applications of pomegranate (Punicagranatum L.): a review. J Altern Med Rev. 2008;13:128–144. [PubMed] [Google Scholar]
- Kaufman M, Wiesman Z. Pomegranate oil analysis with emphasis on MALDI-TOF/MS triacylglycerol fingerprinting. J Agric Food Chem. 2007;55:10405–10413. doi: 10.1021/jf072741q. [DOI] [PubMed] [Google Scholar]
- Kawaii S, Lansky EP. Differentiation-promoting activity of pomegranate (Punica granatum) fruit extracts in HL-60 human promyelocytic leukemia cells. J Med Food. 2004;7:13–18. doi: 10.1089/109662004322984644. [DOI] [PubMed] [Google Scholar]
- Khan N, Hadi N, Afaq F, et al. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. J Carcinogenesis. 2007;28:163–173. doi: 10.1093/carcin/bgl145. [DOI] [PubMed] [Google Scholar]
- Kim ND, Mehta R, Yu W. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. J Breast Cancer Res Treat. 2002;71:203–217. doi: 10.1023/A:1014405730585. [DOI] [PubMed] [Google Scholar]
- Kohno H, Suzuk R, Yasui Y. Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. J Cancer Sci. 2004;95:481–486. doi: 10.1111/j.1349-7006.2004.tb03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansky P, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Lott JA, Turner K. Evaluation of Trinder’s glucose oxidase method for measuring glucose in serum and urine. J Clin Chem. 1975;21:1754–1760. [PubMed] [Google Scholar]
- Masson P, Ohlsson P, Bjorkhem I. Combined enzymatic-Jaffe’s method for determination of creatinine in serum. J Clin Chem. 1981;27:18–21. [PubMed] [Google Scholar]
- Mc Elderry LA, Tarbit IF, Cassells-Smith AJ. Six methods for urinary protein compared. J Clin Chem. 1982;28:356–360. [PubMed] [Google Scholar]
- Menezes SM, Cordeiro LN, Viana GS. Punica granatum (pomegranate) extract is active against dental plaque. J Herb Pharmacother. 2006;6:79–92. doi: 10.1080/J157v06n02_07. [DOI] [PubMed] [Google Scholar]
- Rasheed Z, Akhtar N, Anbazhagan AN. Polyphenol-rich pomegranate fruit extract (POMx) suppresses PMACI-induced expression of proinflammatory cytokines by inhibiting the activation of MAP kinases and NF-kappaB in human KU812 cells. J Inflamm. 2009;6:1–30. doi: 10.1186/1476-9255-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. J Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Sundin DP, Sandoval R, Molitoris BA. Gentamicin inhibits renal protein and phospholipid metabolism in rats: implications involving intracellular trafficking. J Am Soc Nephrol. 2001;12:114–123. doi: 10.1681/ASN.V121114. [DOI] [PubMed] [Google Scholar]
- Vivancos M, Moreno JJ. Beta-sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. J Free Radic Biol Med. 2005;39:91–97. doi: 10.1016/j.freeradbiomed.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Kitagawa T, Koyanagi N. Dietary effect of pomegranate seed oil on immune function and lipid metabolism in mice. J Nutrition. 2006;22:54–59. doi: 10.1016/j.nut.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Ziegler DM. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. J Ann Rev Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]


